Asprosin-neutralizing antibodies as a treatment for metabolic syndrome
Figures
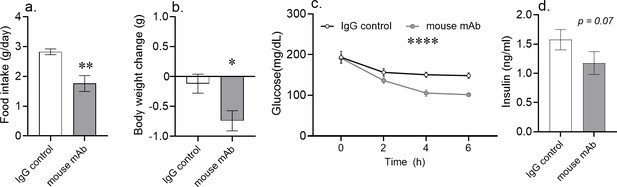
Acute asprosin neutralization reduces plasma glucose, appetite, and body weight in diet-induced obese mice.
(a–d) Cumulative food intake and body weight change (measured 24 hr post-treatment), baseline blood glucose (measured at hour 2, 4, and 6 post-mAb treatment) and plasma insulin (measured 6 hr post-treatment) were measured after a single dose of anti-asprosin mAb (250 µg/mouse) in 16-week-old male DIO (diet-induced obesity) mice, n = 5 or 6/group. Note that mice were without food for the duration of the experiment in (c, d), demonstrating that the glucose lowering effect was independent of the hypophagic effect of mAb treatment. Asterisk (*) indicate the range of alpha as determined by the t-test (two groups, one time point), or analysis of variance (ANOVA, sets involving multiple groups and time points. *p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001; Figure 1—source data 1).
-
Figure 1—source data 1
Raw data and statistical analysis values for Figure 1 and Figure 1—figure supplement 1.
- https://cdn.elifesciences.org/articles/63784/elife-63784-fig1-data1-v2.xlsx
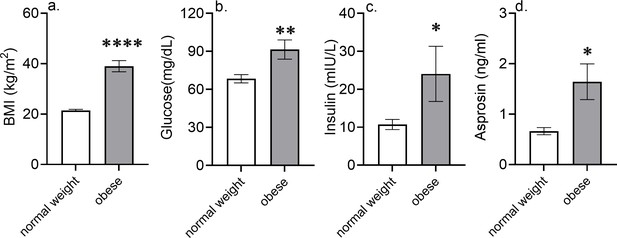
Higher blood glucose and insulin levels in metabolic syndrome patients are associated with elevated asprosin levels.
Blood glucose, plasma insulin, and asprosin levels were measured in metabolic syndrome male patients (n = 10; BMI > 25) and age-matched male subjects with normal BMI (<25). Asterisk (*) indicate the range of alpha as determined by Student’s two-tailed t-test. *p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001.

Asprosin neutralization does not cause glucosuria.
Urine glucose levels were measured at 0, 2, 4, and 6 hr after a single dose of anti-asprosin mAb or control, isotype-matched IgG (250 µg/mouse) in fasting 16-week-old male C57BL/6J diet-induced obese (DIO) mice (n = 7 or 8/group).
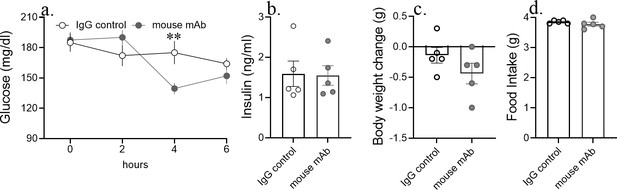
Acute asprosin neutralization reduces blood glucose, but not appetite and body weight in lean mice.
Cumulative food intake and body weight change (at 24 hr post-treatment), baseline blood glucose (at hour 2, 4, and 6 post-mAb treatment) and plasma insulin (at 6 hr post-treatment) was measured after a single dose of anti-asprosin mAb (250 µg/mouse) in 12-week-old male C57BL/6J lean mice, n = 5/group. Asterisk (*) indicate the range of alpha; *p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001, as determined by Student’s t-test.
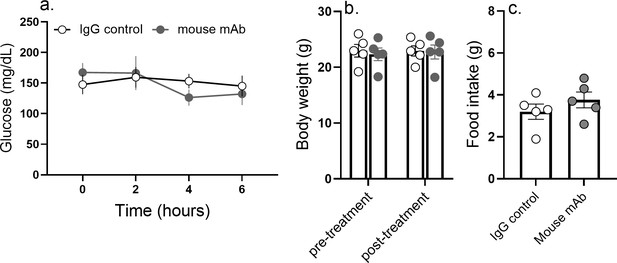
Blood glucose, appetite, and body weight remain unaltered in response to anti-asprosin treatment in Fbn1NPS/+ mice.
Cumulative food intake and body weight change (at 24 hr post-treatment) and baseline blood glucose (at hour 2, 4, and 6 post-mAb treatment) were measured after a single dose of anti-asprosin mAb (250 µg/mouse) in 35-week-old male Fbn1NPS/+ mice (neonatal progeroid syndrome), n = 5/group.
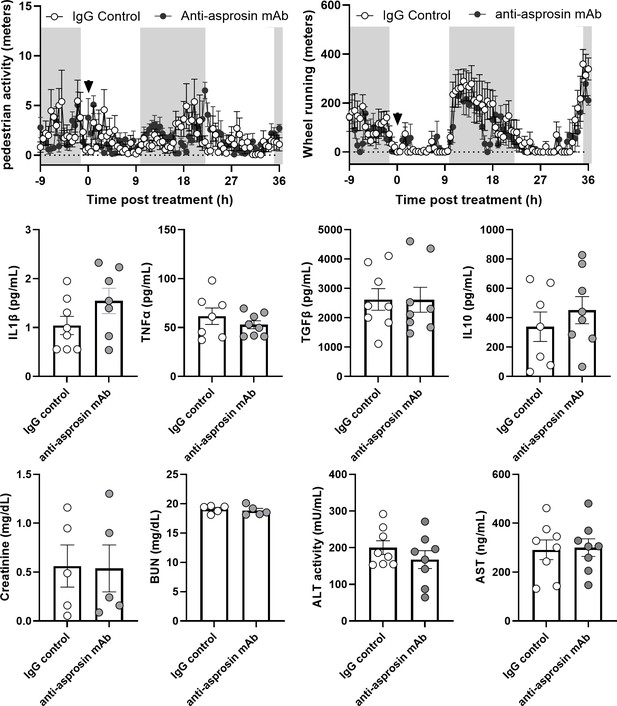
Anti-asprosin monoclonal antibody treatment does not lead to confounding side effects.
Markers of general sickness (half hourly pedestrian activity and wheel running activity), plasma levels of pro-inflammatory (IL1β and TNFα), and anti-inflammatory (IL10 and TGFβ) cytokines, markers of renal (plasma creatinine and blood urea nitrogen [BUN] levels) and hepatic health (plasma ALT, alanine aminotransferase and AST, aspartate aminotransferase levels) were measured in 16-week-old diet-induced obese (DIO) mice intra-peritoneally injected with control, isotype-matched IgG, or anti-asprosin mAb (250 µg/mouse). Downward arrow indicates the time of antibody treatment in the top panel.
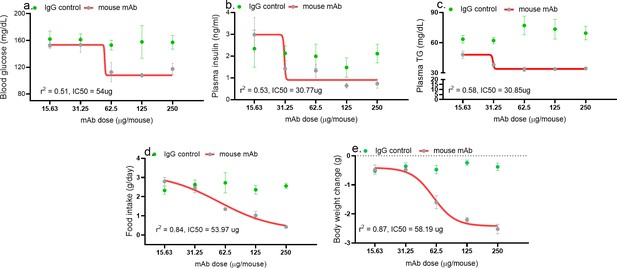
Asprosin neutralization corrects hyperglycemia, hyperphagia, and hypertriglyceridemia in a dose-dependent manner.
Baseline blood glucose (measured 4 hr post-treatment), plasma insulin and triglyceride (TG; at 24 hr post-treatment) levels, cumulative food intake, body weight change (measured 24 hr post-treatment), were measured upon injecting increasing dose of anti-asprosin mAb in 16-week-old male DIO (diet-induced obesity) mice, n = 5/group. The doses tested were 15.63, 31.25, 62.5, 125, and 250 µg/mouse, corresponding to 0.4, 0.85, 1.64, 3.28, and 6.88 mg/kg. Half maximal inhibitor concentration (IC50) was determined using a four- and three-parameter non-linear variable slope curve. r (Romere et al., 2016) value determined the goodness of fit (Figure 2—source data 1).
-
Figure 2—source data 1
Raw data and statistical analysis values for Figure 2 and Figure 2-supplements.
- https://cdn.elifesciences.org/articles/63784/elife-63784-fig2-data1-v2.xlsx
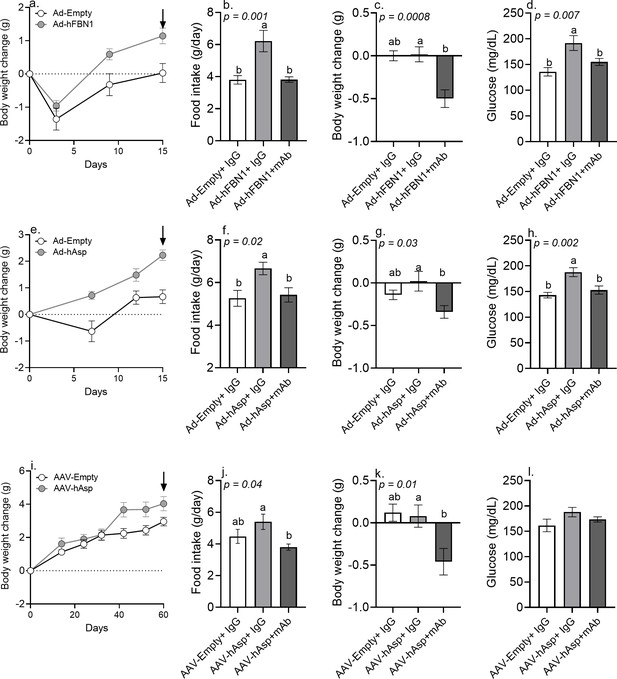
Immunologic neutralization fully rescues the metabolic effects of viral induction of plasma asprosin.
(a) Body weight change was measured over 15 days after 12-week-old male C57Bl/6 mice were tail-vein-injected with Ad-empty or Ad-FBN1 (3.6 × 109 pfu/mouse, n = 12/group) viruses. Downward arrow indicates the day of mAb treatment described below. (b–d) Cumulative food intake, body weight change, and blood glucose were measured 24 hr after intra-peritoneal injection of indicated control, isotype-matched IgG, or anti-asprosin mAbs (n = 6/group) in the above mice. (e) Body weight change was measured over 15 days after 12-week-old male C57Bl/6 mice were tail-vein-injected with Ad-empty or Ad-asprosin (5 × 1010 pfu/mouse, n = 12/group) viruses. Downward arrow indicates the day of mAb treatment described below. (f–h) Cumulative food intake, body weight change, and blood glucose were measured 24 hr after intra-peritoneal injection of indicated control, isotype-matched IgG or anti-asprosin mAb (n = 6/group) in the above mice. (i) Body weight change was measured over 60 days after 12-week-old male C57Bl/6 mice were tail-vein-injected with AAV8-empty or AAV8-asprosin (1 × 1012 GC/mouse, n = 10/group) viruses. Downward arrow indicates the day of mAb treatment described below. (j–l) Cumulative food intake, body weight change, and blood glucose were measured 24 hr after intra-peritoneal injection of indicated control, isotype-matched IgG, or anti-asprosin mAbs (n = 5/group) in the above mice. Different and same alphabets on bars indicate presence or absence of significant difference, respectively, between groups, as determined by one-way ANOVA followed by Dunnett’s multiple comparisons test. p<0.05 was considered statistically significant (Figure 3—source data 1).
-
Figure 3—source data 1
Raw data and statistical analysis values for Figure 3 and figure 3-supplements.
- https://cdn.elifesciences.org/articles/63784/elife-63784-fig3-data1-v2.xlsx

Viral overexpression of human asprosin results in a MS-like phenotype in lean mice.
(a–d) Body weight change, cumulative food intake, blood glucose, and plasma insulin were measured 15 days after 12-week-old male C57Bl/6 mice were tail-vein-injected with Ad-empty or Ad-FBN1 (3.6 × 109 pfu/mouse, n = 5/group) viruses. (f–i) Body weight change, cumulative food intake, blood glucose, and plasma insulin were measured 15 days after 12-week-old male C57Bl/6 mice were tail-vein-injected with Ad-empty or Ad-asprosin (5 × 1010 pfu/mouse, n = 12/group) viruses. (k–n) Body weight change, cumulative food intake, blood glucose, and plasma insulin were measured 57 days after 12-week-old male C57Bl/6 mice were tail-vein-injected with AAV8-empty or AAV8-asprosin (1 × 1012 GC/mouse, n = 10/group) viruses. (e, f, o) Human asprosin levels detected in plasma of Ad-FBN1-, Ad-asprosin-, and AAV8-asprosin-injected mice are plotted relative to the average background signal detected in Ad-empty and AAV8-empty injected mice. Asterisk (*) indicate the range of alpha; *p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001, as determined by Student’s t-test.
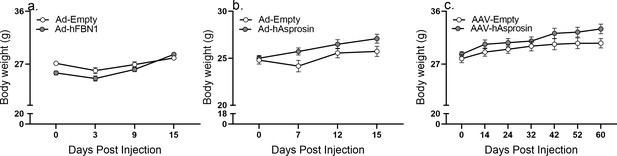
Raw body weight values of Ad5 and AAV experiments.
Body weight was measured after 12-week-old male C57Bl/6 mice were tail-vein-injected with Ad-empty or Ad-FBN1 (3.6 × 109 pfu/mouse, n = 12/group) virus, Ad-empty or Ad-asprosin (5 × 1010 pfu/mouse, n = 12/group) virus, and AAV8-empty or AAV8-asprosin (1 × 1012 GC/mouse, n = 10/group) viruses.
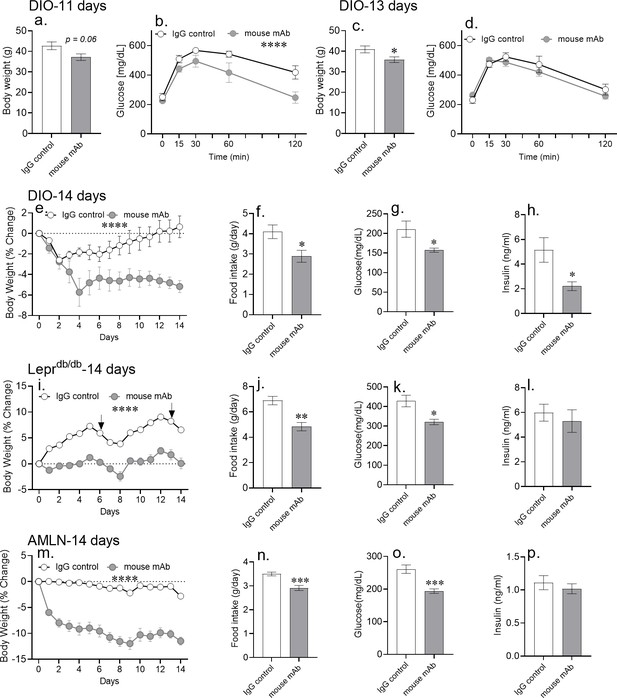
Chronic asprosin neutralization improves metabolic syndrome in three independent mouse models.
(a–d) Body weight and glucose tolerance were measured on day 11 (a, b) and day 13 (c, d) after 10 days of once daily intra-peritoneal injection of control, isotype-matched IgG, or anti-asprosin mAb in 16-week-old male DIO (diet-induced obesity) mice (n = 5/group). (e–p) Percent change in body weight, 24 hr cumulative food intake (measured on day 7), and blood glucose and plasma insulin (6 hr post-treatment on day 14) levels were measured after 14 days of once daily intra-peritoneal injection of control, isotype-matched IgG, or anti-asprosin mAb in 30-week-old male DIO mice (n = 8 or 9/group; e–h), 12-week-old male Leprdb/db mice (n = 5 or 6/group; i–l), and 30-week-old male mice on AMLN diet (n = 7/group; m–p). Asterisk (*) indicate the range of alpha for an effect of mAb treatment as determined by the t-test (two groups, one time point), or two-way analysis of variance (two-way ANOVA, sets involving multiple groups and time points. *p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001; Figure 4—source data 1). Downward arrows in (m) indicate the day of submandibular bleed in Leprdb/db mice.
-
Figure 4—source data 1
Raw data and statistical analysis values for figure and figure-supplements.
- https://cdn.elifesciences.org/articles/63784/elife-63784-fig4-data1-v2.xlsx
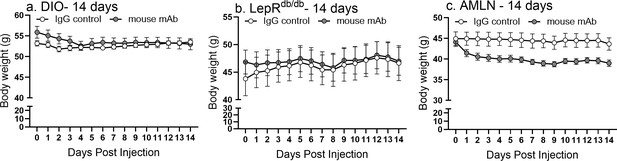
Raw body weight values of 14 day mAb experiments.
Body weight was measured daily with 14 days of once daily intra-peritoneal injection of control, isotype-matched IgG, or anti-asprosin mAb in 30-week-old male DIO mice (n = 8 or 9/group), 12-week-old male Leprdb/db mice (n = 5 or 6/group), and 30-week-old male mice on AMLN diet (n = 7/group).
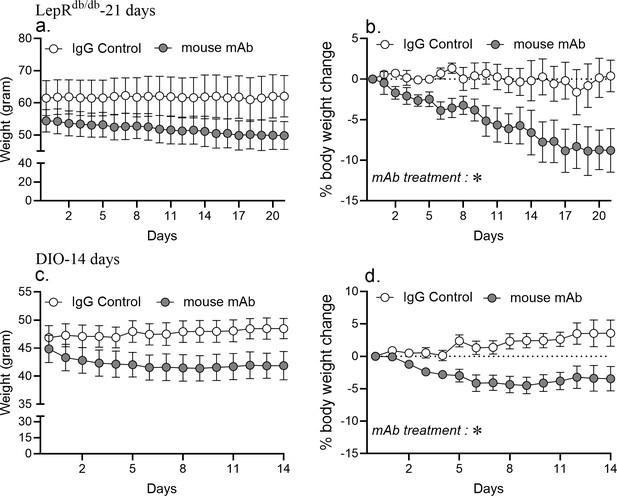
Chronic asprosin neutralization reduces body weight in LepRdb/db and DIO mice.
Body weight and % change in body weight were measured after once daily intra-peritoneal injection of control, isotype-matched IgG, or anti-asprosin mAb (250 µg/day) in 30-week-old male Leprdb/db mice (a,b; n = 5 or 6/group) and 16-week-old diet-induced obese (DIO) mice (c,d; n = 4/group). Asterisk (*) indicate the range of alpha for an effect of mAb treatment as determined by two-way analysis of variance (two-way ANOVA). *p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001.
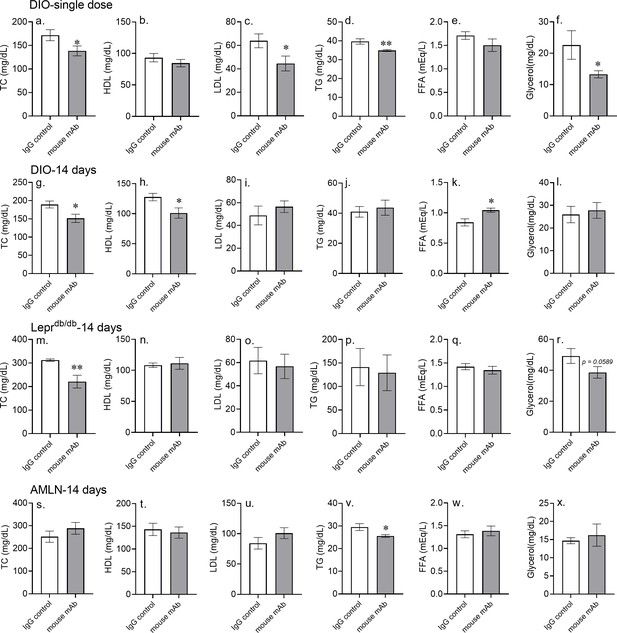
Asprosin neutralization improves dyslipidemia in MS mouse models.
(a–f) Plasma levels of total cholesterol (TC), low-density lipoproteins (LDL), high-density lipoprotein (HDL), triglycerides (TG), free fatty acids (FFA), and glycerol were measured 6 hr after intra-peritoneal injection of indicated control, isotype-matched IgG, or anti-asprosin mAb in 16-week-old male DIO mice (250 µg/mouse, n = 5/group; a–f), (g–x) plasma levels of TC, LDL, HDL, TG, FFA, and glycerol were measured 24 hr after 14 days of once daily intra-peritoneal injection of indicated control, isotype-matched IgG, or anti-asprosin mAb (250 µg/mouse) in 16-week-old male DIO mice (n = 6/ group; g–l), 12-week-old male Leprdb/db mice (n = 7/group; Db/Db; m–r) and 30-week-old male mice on NASH diet (n = 7/group; s–x). Asterisk (*) indicate the range of alpha; *p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001, as determined by Student’s t-test.
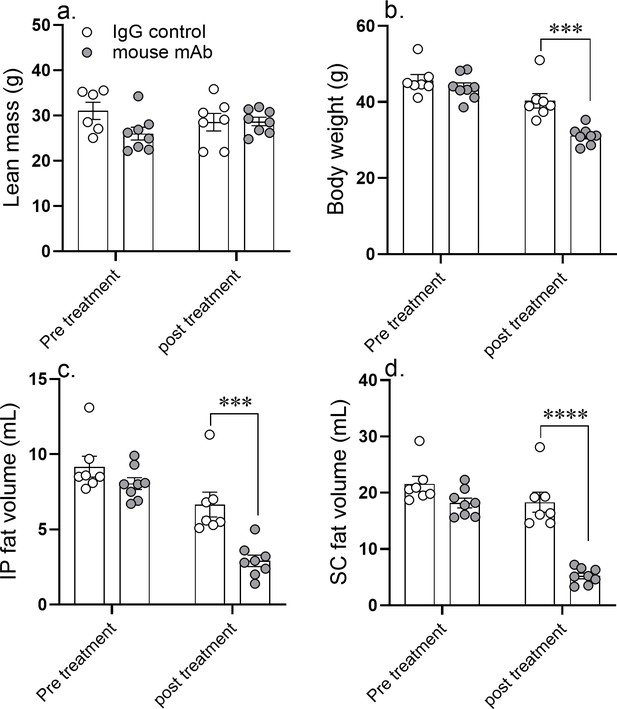
A 14 day course of anti-asprosin mAb treatment reduces adipose mass in diet-induced obese mice.
Lean mass (a), body weight (b), intra peritoneal fat volume (c), and subcutaneous fat volume (d) were measured pretreatment (day 0) and post-treatment (day 14) of once daily intra-peritoneal injection of control, isotype-matched IgG, or anti-asprosin mAb in 25-week-old male DIO (diet-induced obesity) mice (n = 7 or 8/group). Asterisk (*) indicate the range of alpha as determined by the two-tailed Student’s t-test (Figure 5—source data 1). *p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001.
-
Figure 5—source data 1
Raw data and statistical analysis values for Figure 5 and figure 5-supplements.
- https://cdn.elifesciences.org/articles/63784/elife-63784-fig5-data1-v2.xlsx
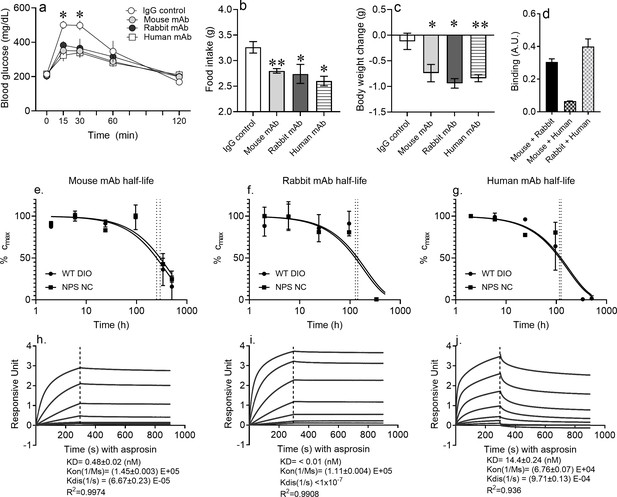
Pharmacokinetics of epitope-agnostic anti-asprosin mAbs from different sources.
(a–c) Neutralization of asprosin using mAbs from different sources is equally protective. mAb generated using mouse immunization with a 28-mer asprosin peptide (mouse mAb), immunization of rabbits with recombinant full-length human asprosin (rabbit mAb), and recombinant mAb generated by panning phages from a naïve human antibody library (human mAb) were injected IP in 16-week-old male DIO mice (250 µg/mouse, ~5 mg/kg) and the indicated end points measured (n = 5/group). (d) Epitope competition assay: In a sandwich ELISA, asprosin captured by each mAb (mouse, rabbit, or human mAb) was detected by each of the three mAbs in a 3 × 3 matrix to determine competition for their respective epitopes. (e–g) Half-life of asprosin-neutralizing mAbs. Mouse models of ‘high asprosin’ (16-week-old male mice with diet-induced obesity; DIO), and ‘low asprosin’ (10-week-old male NPS mice) were injected with mouse, rabbit, or human mAb against asprosin 250 µg mAb in 500 µl 0.9% saline; n = 2/group. mAb levels were determined in mouse plasma collected at 2, 6, 24, 96, 336, and 504 hr post-injection to determine in vivo half-life of mAbs. (h–j) Anti-asprosin mAb binding affinity. Equilibrium dissociation constant (KD) of recombinant asprosin binding to anti-asprosin mAb was determined by a 1:1 binding model and use of global fitting method on Pall ForteBio’s Octet RED96 system. Asterisk (*) indicate the range of alpha for an effect of mAb treatment as determined by t-test (two groups, one time point) or analysis of variance (ANOVA, sets involving multiple groups and time points; Figure 6—source data 1). *p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001.
-
Figure 6—source data 1
Raw data and statistical analysis values for figure 6 and figure-supplements.
- https://cdn.elifesciences.org/articles/63784/elife-63784-fig6-data1-v2.xlsx
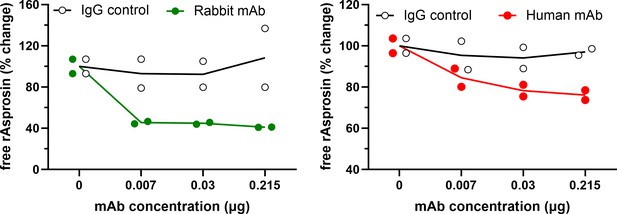
Characterization of the asprosin-neutralizing antibodies.
Free asprosin levels were detected by ELISA against recombinant asprosin (1 nM) preincubated with various concentrations of rabbit and fully human anti-asprosin mAbs or the IgG control antibody.
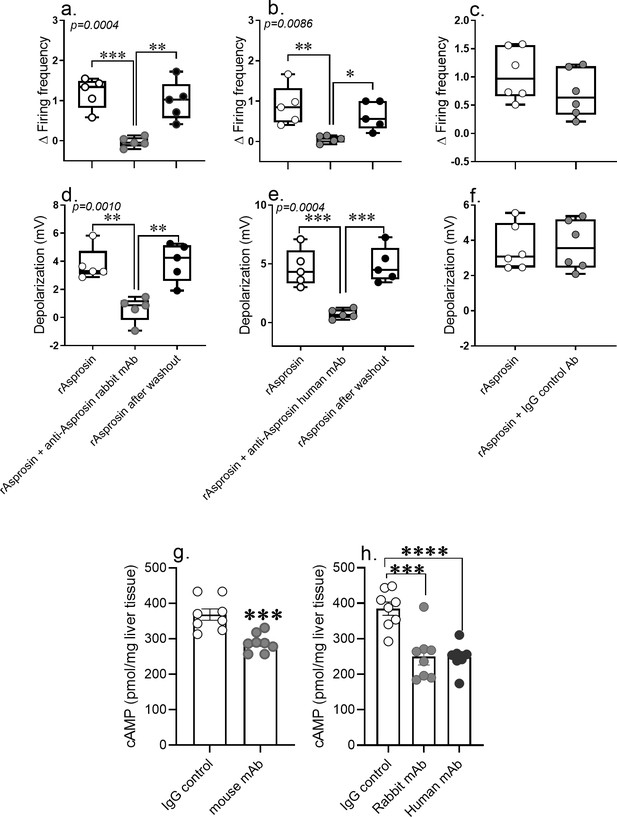
Anti-asprosin mAbs from different sources neutralize asprosin’s orexigenic and glucogenic function by blunting AgRP+ neuronal firing and hepatic cAMP signaling.
(a–f) The anti-asprosin neutralizing antibodies reversibly inhibit asprosin’s effect on AgRP+ neuronal activity. Firing frequency (a, b) and membrane potential (d, e) of AgRP+ neurons were recorded in response to bacterial recombinant asprosin, asprosin preincubated with anti-asprosin rabbit and human mAb, and asprosin after washout. (c, f) Firing frequency response and membrane potential response of AgRP+ neurons were recorded in response to bacterial recombinant asprosin and IgG control antibody. Asterisk (*) indicate the range of alpha as determined by Sidak post hoc test followed by one-way analysis of variance (ANOVA; Figure 7—source data 1). *p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001. (g, h) The anti-asprosin neutralizing antibodies blunt hepatic cAMP signaling. Sixteen-week-old male mice with diet-induced obesity (DIO) were injected with mouse, rabbit, or human mAb against asprosin (250 µg mAb in 500 µl 0.9% saline; n = 8/group). Three hour post-injection, hepatic cAMP levels were measured. Asterisk (*) indicate the range of alpha as determined by the two-tailed Student’s t-test (Figure 7—source data 1). *p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001.
-
Figure 7—source data 1
Raw data and statistical analysis values for Figure 7.
- https://cdn.elifesciences.org/articles/63784/elife-63784-fig7-data1-v2.xlsx
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Animal resource (Mus musculus) | C57BL/6J mice | Jackson Laboratory | RRID:IMSR_JAX:000664 | |
| Animal resource (Mus musculus) | C57BL/6-Fbn1em1Chop/J | Jackson Laboratory | RRID:IMSR_JAX:033548 | |
| Animal resource (Mus musculus) | C57BL/6J DIO | Jackson Laboratory | RRID:IMSR_JAX:380050 | |
| Animal resource (Mus musculus) | B6.BKS(D)-Leprdb/J | Jackson Laboratory | RRID:IMSR_JAX:000697 | |
| Research rodent food | Dustless pellet diet | Bio-Ser | F0173 | |
| Research rodent food | High-fat diet | Envigo Teklad | TD.06414 | |
| Research rodent food | AMLN diet | Research Diets | D09100301 | |
| Antibody | Mouse anti-asprosin mAb | This paper | ||
| Antibody | Rabbit anti-asprosin mAb | This paper | ||
| Antibody | Human anti-asprosin mAb | This paper | ||
| Antibody | Anti-mouse secondary antibody | Tonbo Biosciences | 72–8042 M001 | 1:10,000 |
| Antibody | Anti-rabbit secondary antibody | Cytiva’s Amersham ECL | NA934-1ML | 1:10,000 |
| Adenoviral vector | Ad5-hFBN1 | This paper | 3.6 × 109 pfu/mouse | |
| Adenoviral vector | Ad5-hAsprosin | This paper | 5 × 1010 pfu/mouse | |
| Adeno-associated viral vector | AAV8-hAsprosin | This paper | 1 × 1012 GC/mouse | |
| Adenoviral vector | Ad5-Empty | This paper | 3.6 × 109 pfu/mouse or 5 × 1010 pfu/mouse | |
| Adeno-associated viral vector | AAV8-Empty | This paper | 1 × 1012 GC/mouse | |
| Commercial kit | cAMP ELISA kit | Crystal Chemicals | Catalog # 581001 | |
| Commercial kit | Glucose quantitation kit | Cayman Chemicals | Catalog # 10009582 | |
| Commercial kit | Mouse insulin ELISA kit | Crystal Chemicals | Catalog # 90080 | |
| Commercial kit | Human insulin ELISA kit | Raybiotech | Catalog # ELH-Insulin | |
| Commercial kit | IL1β ELISA kit | Abcam | Catalog # ab197742 | |
| Commercial kit | TNF ELISA kit | Abcam | Catalog # ab208348 | |
| Commercial kit | IL10 ELISA kit | Thermo Scientific | Catalog # BMS614INST | |
| Commercial kit | TGFβ ELISA kit | R and D systems | Catalog # DB100B | |
| Commercial kit | ALT ELISA kit | Abcam | Catalog # ab105134 | |
| Commercial kit | AST ELISA kit | Abcam | Catalog # ab263882 |





