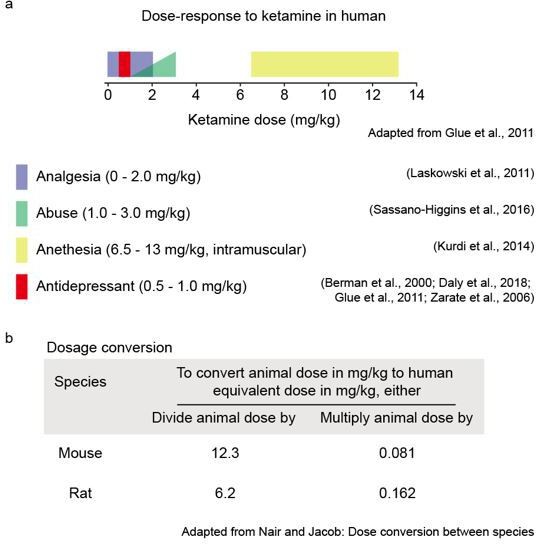
Attenuated dopamine signaling after aversive learning is restored by ketamine to rescue escape actions
Figures
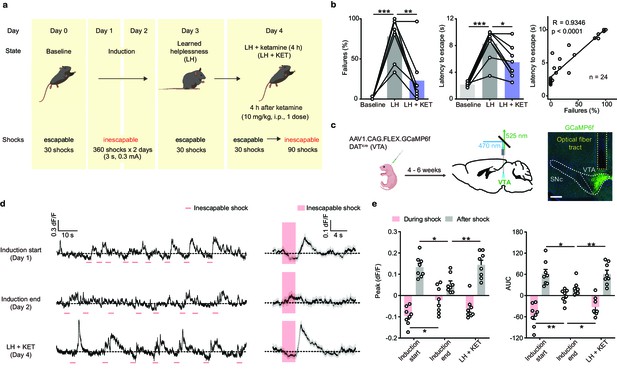
Ketamine rescues escape behavior and dampened DA neuronal activity after aversive learning.
(a). Schematic illustrating the timeline of behavioral and pharmacological manipulations in the LH paradigm. (b). Left, summary data showing the percentage of failures to escape an escapable aversive shock across phases of learning (Baseline, LH, and LH+ KET). Middle, same as left, but for latency to escape. Right, for all conditions, the correlation between percentage of failure to escape and escape latency (failure to escape trials scored as 10 sec latency). n = 24 trials from eight mice. % Failures: repeated measures one-way ANOVA, F (1.89, 13.23) = 27.9, p = 0.0001, Sidak’s multiple comparison test, Baseline vs LH, p = 0.0001, LH vs LH+ KET, p = 0.0037. Latency to escape: repeated measure one-way ANOVA, F (1.96, 13.72) = 29.63, p < 0.0001, Sidak’s multiple comparison test, Baseline vs LH, p = 0.0003, LH vs LH+ KET, p = 0.0141. Pearson correlation: R = 0.9346, p < 0.0001. (c). Left, schematic for viral transduction in the VTA and subsequent fiber implant. Right, fiber placement verification. Green, GCaMP6f; blue, Hoechst nuclear stain. Scale bar: 500 μm. (d). Left, baseline adjusted raw traces of VTA DA neuron Ca2+ responses to inescapable foot shocks (3 s, pink) in one animal, at the start of induction, at the end of induction, and 4 hr following a single dose of ketamine (LH+ KET, 10 mg/kg i.p.). Right, average traces in the same subject aligned to shock start time (20 trials/condition, mean ± SEM). (e). Left, quantification of peak Ca2+ transient amplitude during and after foot-shock stimuli across conditions. Right, same but for area under the curve (AUC). Both positive and negative values are quantified. n = 8 animals, repeated measures one-way ANOVA, Holm-Sidak’s multiple comparison test, Peak: During shock, F (1.823, 12.76) = 5.387, p = 0.0222, Induction start vs Induction end, p = 0.0458, Induction end vs LH + KET, p = 0.0788. After shock, F (1.693, 11.85) = 6.805, p = 0.0132, Induction start vs Induction end, p = 0.0230, Induction end vs LH + KET, p = 0.0068. AUC: During shock, F (1.705, 11.94) = 8.942, p = 0.0054, Induction start vs Induction end, p = 0.0058, Induction end vs LH + KET, p = 0.0258. After shock, F (1.437, 10.06) = 5.499, p = 0.0318, Induction start vs Induction end, p = 0.0257, Induction end vs LH + KET, p = 0.0069. *p < 0.05, **p < 0.01, ***p < 0.001. Error bars reflect SEM.
-
Figure 1—source data 1
Numerical data for the graphs in Figure 1.
- https://cdn.elifesciences.org/articles/64041/elife-64041-fig1-data1-v2.xlsx
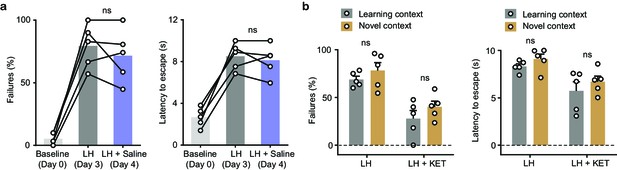
Characterization of behavior after learned helplessness.
(a). Left, summary data showing the percentage of failures to escape an escapable aversive shock across phases of learning (Baseline, LH, and LH+ Saline). Right, same as left, but for latency to escape. n = 5 animals. One-way ANOVA, Sidak’s multiple comparison test, LH vs LH+ KET, % Failures, = 0.5299, latency to escape, p = 0.7027. (b). Left, summary data showing the percentage of failures to escape an escapable aversive shock in learning and novel contexts (LH, and LH+ KET). Right, same as left, but for latency to escape. n = 5 animals. Two-way ANOVA, Sidak’s multiple comparison test, learning vs novel contexts, % Failures, LH, p = 0.5669, LH+ KET, p = 0.3924. Latency to escape, LH, p = 0.6409, LH+ KET, p = 0.5089. LH vs LH+ KET, % Failures, learning context, p** = 0.0013, novel context, p** = 0.0023. Latency to escape, learning context, p* = 0.0219, novel context, p* = 0.0336.
-
Figure 1—figure supplement 1—source data 1
Numerical data for the graphs in Figure 1—figure supplement 1.
- https://cdn.elifesciences.org/articles/64041/elife-64041-fig2-data2-v2.xlsx
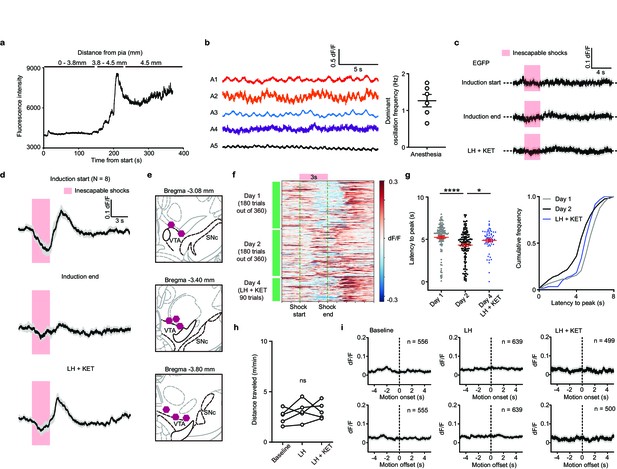
VTA DA neuron responses to anesthesia, foot shock, and motion transitions.
(a). Photometry-guided fiber implantation into the VTA. Recording began when the fiber traversed the pia. A gradual increase of fluorescence intensity was observed as the fiber tip approached GCaMP6f-expressing neurons, 3.8–4.5 mm from the pia, followed by a sharp signal increase and stabilization close to the VTA. (b). Oscillation of VTA DA Ca2+ transients under anesthesia. Approximately 1–2 Hz oscillations were observed in every animal. Summary data show the dominant oscillation frequencies. (c). Average neural activity-independent fluorescence transients illustrated for one EGFP-expressing animal in response to inescapable foot-shocks across learning phases. Traces are aligned to shock start time (20 trials/animal, n = 3 animals). (d). Average Ca2+ transients (mean ± SEM) in response to foot shocks for all animals across learning phases. Traces are aligned to shock start time (20 trials/animal, 8 animals). (e). Atlas locations showing fiber placements for data in Figure 1e. (f). Heatmap of single trial Ca2+ traces across learning in one mouse. Pink rectangle and green dashed lines mark the timing of shock stimuli. Colormap of dF/F, blue –0.3, red 0.3, saturated for values outside this range for illustration purposes. (g). Left, latencies from shock onset to dF/F peak (averages of sequential bins of 10 traces) for subjects across learning days. Right, cumulative frequency distribution of latencies to peak. n = 8 animals, one-way ANOVA, F (2, 258) = 9.387, p = 0.0001. Sidak’s multiple comparison test, Day 1 vs Day 2, p = 0.0001, Day 2 vs LH+ KET, p = 0.0342. (h). Open field locomotion (m/min) for n = 5 mice across learning phases. Repeated measures one-way ANOVA, F (1.038, 4.153) = 1.479, p = 0.2910. (i). Average Ca2+ transients around motion onset and offset for mice in the Baseline, LH, and LH+ KET conditions. Dashed black line marks motion onset/offset. The number of onsets/offsets, as noted. n = 5 animals. *p < 0.05, **** p < 0.0001. Error bars reflect SEM.
-
Figure 1—figure supplement 2—source data 1
Numerical data for the graphs in Figure 1—figure supplement 2.
- https://cdn.elifesciences.org/articles/64041/elife-64041-fig3-data3-v2.xlsx
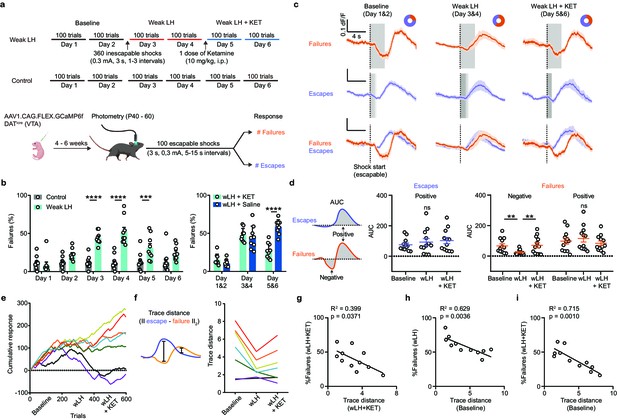
Weak learning analysis links DA activity, behavioral outcomes, and response to ketamine.
(a). Top, schematic of experimental timeline for weak LH (wLH). Bottom, schematic of viral transduction, test trial description, and timing of photometry recording. (b). Left, summary data showing the percentage of failures to escape an escapable aversive shock across 6 days for two groups (Gray bar, controls; Cyan, wLH). Right, summary of behavioral data for wLH mice with KET or Saline treatment. Left, responses across days, two-way ANOVA, Sidak’s multiple comparison test, Control vs wLH, Day 1, p = 0.9934, Day 2, p = 0.1147, Days 3 and 4, p < 0.0001, Day 5, p = 0.0007, and D6, p = 0.0691. Control, n = 12; wLH, n = 9 animals. Right, two-way ANOVA, Sidak’s multiple comparison test, wLH+ KET vs wLH+ Saline, Days 1 and 2, p = 0.6403, Days 3 and 4, p = 0.8715, Days 5 and 6, p < 0.0001. n = 9 animals/group. (c). Fiber photometry recordings of VTA DA Ca2+ transients, separated by behavioral response and aligned to shock start time (purple, escape; orange, failure; average dF/F across animals). Gray rectangles mark shock length in time, which is constant for failures to escape but variable for successful escapes and is shaded proportionally. Donut plots depict proportion of behavioral responses. n = 8 animals, baseline: 1405 trials, 25% failure 75% escape; wLH: 1459 trials, 60% failure 40% escape; wLH+ KET: 1403 trials, 36% failure, 64% escape. (d). Left, schematic illustration for measured variables. Middle, summary data for AUC of the positive Ca2+ transient peaks in escapes across learning phases. Right, same but AUC for both positive and negative peaks in failures, as shown in the schematic. n = 12 animals, AUC (Escapes), positive peak, repeated measures one-way ANOVA, F (1.897, 20.87) = 1.881, p = 0.1787. AUC (Failures), negative peak, repeated measures one-way ANOVA, F (1.852, 20.38) = 9.260, p = 0.0017, Holm-Sidak’s multiple comparison test, Baseline vs wLH, p = 0.0069, wLH vs wLH+ KET, p = 0.0069. Positive peak, repeated measures one-way ANOVA, F (1.540, 16.94) = 2.541, p = 0.1181. (e). Learning curves for individual animals. n = 7 animals, 600 trials/animal. (f). Left, schematic illustration for the measured variable, Euclidean norm of the difference between mean escape and failure-associated Ca2+ transients for each subject. Right, trace distances for each subject across learning phases with subject specific colors as in e. (g). Correlation between trace distance and the percentage of failures in wLH+ KET. n = 11 animals. (h). Correlation between trace distance in the baseline condition and the percentage of failures in wLH. n = 11 animals. (i). Correlation between trace distance in the baseline condition and the percentage of failures in wLH+ KET. n = 11 animals. ** p < 0.01, *** p < 0.001, **** p < 0.0001. Error bars reflect SEM.
-
Figure 2—source data 1
Numerical data for the graphs in Figure 2.
- https://cdn.elifesciences.org/articles/64041/elife-64041-fig2-data1-v2.xlsx
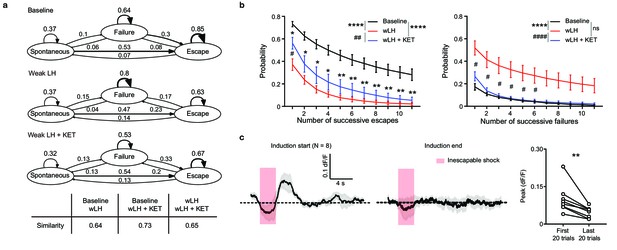
Characterization of behavioral sequences and dampened VTA DA activity in weak learned helplessness (wLH) paradigm.
(a). Graphs depicting transition probabilities between behavioral responses during baseline (top), wLH (middle), and wLH+ KET (bottom). Arrow sizes are proportional to transition probability, noted numerically. Table, Frobenius norms of differences between graphs for each graph pair. n = 7 animals, 1400 trials/condition. (b). Summary data showing the probability of successive responses of different lengths (1-11) for escape (left) and failure (right) across learning states. Two-way ANOVA, Sidak’s multiple comparison test. Escape main effect, Baseline vs wLH and Baseline vs wLH+ KET, p < 0.0001, wLH vs wLH+ KET, p = 0.0031. Failure main effect, Baseline vs wLH and wLH vs wLH+ KET, p < 0.0001, Baseline vs wLH+ KET, p = 0.3291. For comparison within specific length of responses, * p < 0.05 ** p < 0.01 vs Baseline, # p < 0.05 vs wLH, n = 7 animals. (c). Left, average Ca2+ transients in response to foot shocks during wLH induction (mean ± SEM). Traces are aligned to shock start time (20 trials/animal, 8 animals). Right, quantification of peak Ca2+ transient amplitude of first and last 20 trials during induction. Two-tailed paired t-test, p = 0.009. *p < 0.05, ** p < 0.01, **** p < 0.0001. #p < 0.05, ##p < 0.01, ####p < 0.0001. Error bars reflect SEM.
-
Figure 2—figure supplement 1—source data 1
Numerical data for the graphs in Figure 2—figure supplement 1.
- https://cdn.elifesciences.org/articles/64041/elife-64041-fig5-data5-v2.xlsx
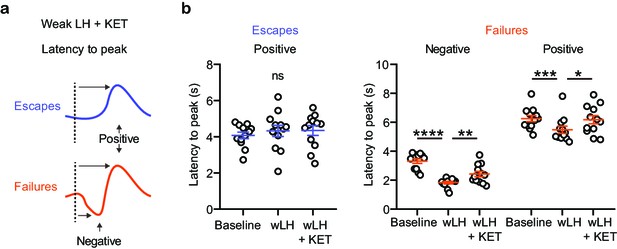
Outcome-specific VTA DA responses in wLH paradigm.
(a). Schematic illustration for the measured variables. (b). Left, summary data for latency to peak of the positive Ca2+ transient peaks in escapes across learning phases. Right, same but for both positive and negative peaks in failures, as shown in the schematic. n = 12 animals, Escapes, positive peak, repeated measures one-way ANOVA, F (1.902, 20.92) = 0.622, p = 0.5388. Failures, negative peak, repeated measures one-way ANOVA, F (1.784, 19.62) = 36.23, p < 0.0001, Holm-Sidak’s multiple comparison test, Baseline vs wLH, p < 0.0001, wLH vs wLH+ KET, p = 0.0025. Positive peak, repeated measures one-way ANOVA, F (1.301, 14.31) = 5.369, p = 0.0286, Holm-Sidak’s multiple comparison test, Baseline vs wLH, p = 0.0003, wLH vs wLH+ KET, p = 0.0446. *p < 0.05, ** p < 0.01, *** p < 0.001. Error bars reflect SEM.
-
Figure 2—figure supplement 2—source data 1
Numerical data for the graphs in Figure 2—figure supplement 2.
- https://cdn.elifesciences.org/articles/64041/elife-64041-fig6-data6-v2.xlsx
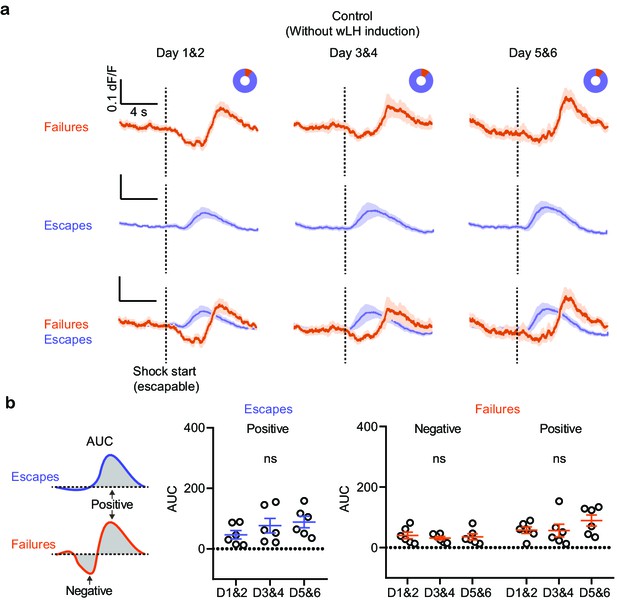
Outcome-specific VTA DA responses in control mice without wLH induction.
(a). Fiber photometry recordings of VTA DA Ca2+ transients, separated by behavioral response and aligned to shock start time (purple, escape; orange, failure; average dF/F across animals). Donut plots depict proportion of behavioral responses. n = 6 animals, Days 1 and 2: 11 % failure, 89% escape; Days 3 and 4: 12% failure, 88% escape; Days 5 and 6: 12% failure, 88% escape. (b). Left, schematic illustration for measured variables. Middle, summary data for AUC of the positive Ca2+ transient peaks in escapes across learning phases. Right, same but AUC for both positive and negative peaks in failures, as shown in the schematic. n = 6 animals, AUC (Escapes), positive peak, repeated measures one-way ANOVA, F (1.617, 8.084) = 2.103, p = 0.1858. AUC (Failures), negative peak, repeated measures one-way ANOVA, F (1.422, 7.112) = 0.2283, p = 0.7291, Positive peak, repeated measures one-way ANOVA, F (1.584, 8.918) = 1.191, p = 0.3391.
-
Figure 2—figure supplement 3—source data 1
Numerical data for the graphs in Figure 2—figure supplement 3.
- https://cdn.elifesciences.org/articles/64041/elife-64041-fig7-data7-v2.xlsx
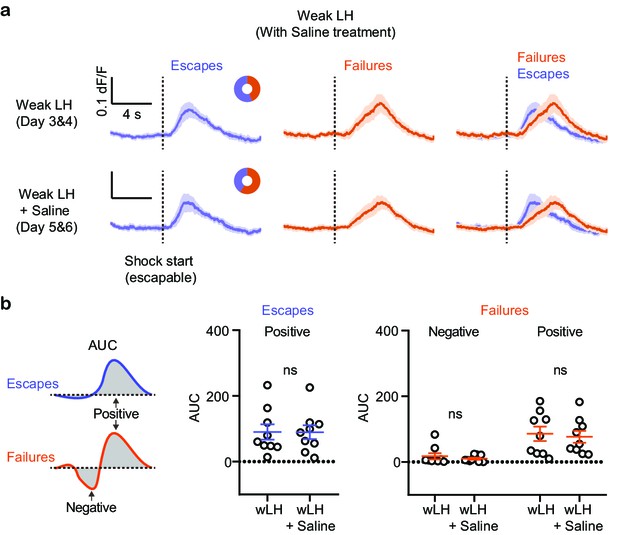
Outcome-specific VTA DA responses in wLH mice with saline treatment.
(a). Fiber photometry recordings of VTA DA Ca2+ transients, separated by behavioral response and aligned to shock start time (purple, escape; orange, failure; average dF/F across animals). Donut plots depict proportion of behavioral responses. n = 9 animals, wLH: 44% failure, 56% escape; wLH+ Saline: 59% failure, 41% escape. (b). Left, schematic illustration for measured variables. Middle, summary data for AUC of the positive Ca2+ transient peaks in escapes across learning phases. Right, same but AUC for both positive and negative peaks in failures, as shown in the schematic. n = 9 animals, AUC (Escapes), positive peak, two-tailed paired t-test, p = 0.9420. AUC (Failures), negative peak, two-tailed paired t-test, p = 0.3578, Positive peak, two-tailed paired t-test, p = 0.3988.
-
Figure 2—figure supplement 4—source data 1
Numerical data for the graphs in Figure 2—figure supplement 4.
- https://cdn.elifesciences.org/articles/64041/elife-64041-fig8-data8-v2.xlsx
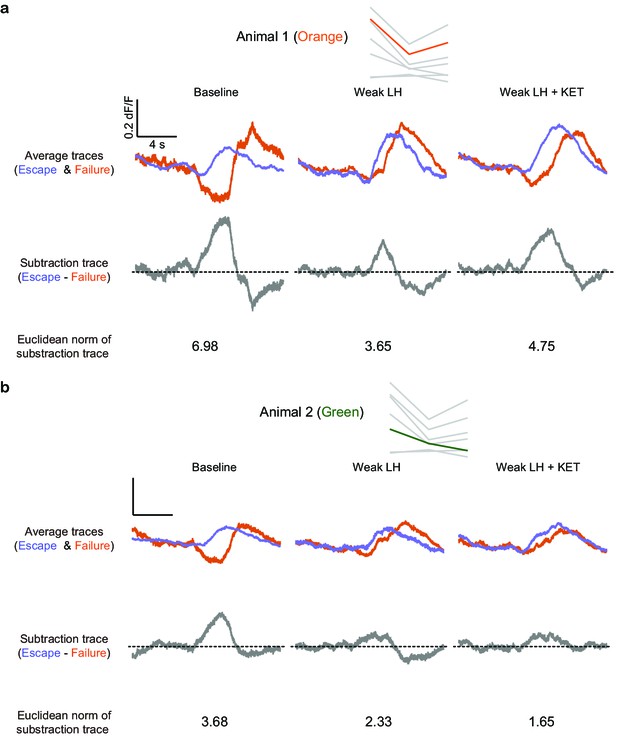
Trace distance of GCaMP transients between escape and failure trials.
(a–b). Example trace distance calculation for two animals. Top row, individual animals marked on the trace distance graph from Figure 2f. Second row, average traces of escapes and failures across learning states. Third row, difference between the average escape and failure traces. Bottom row, Euclidean norm of subtraction traces.
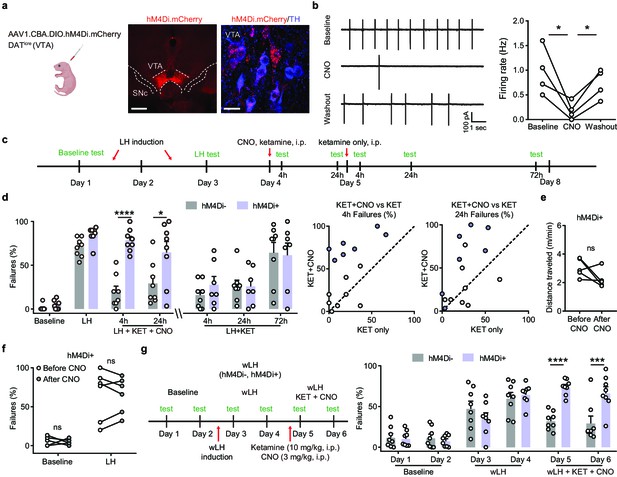
Ketamine behavioral effects in aversive learning require VTA DA activity.
(a). Schematic for viral transduction in the VTA and hM4Di expression in VTA DA neurons (red, hM4Di.mCherry; blue, tyrosine hydroxylase (TH) immunofluorescence). Scale bars, 500 μm and 20 μm. (b). Cell-attached recording of spontaneous activity in mCherry+ VTA neuron before, during, and after bath application of 1 µM clozapine-N-oxide (CNO). Example traces (left) and summary data (right). n = 4 cells from three mice, repeated measures one-way ANOVA, F (2, 6) = 9.634, p = 0.0134, Sidak’s multiple comparison test, Baseline vs CNO, p = 0.0104, CNO vs Washout, p = 0.0488. (c). Schematic of experimental timeline and pharmacological interventions in strong LH paradigm. (d). Left, summary data showing the percentage of failures to escape an escapable aversive shock across learning and treatment conditions for hM4Di AAV expressing DATiCre-positive and negative littermates. Middle, within subject summary data for behavioral responses after ketamine treatment only, compared with ketamine+ CNO, 4 hr after treatment. Right, same but for 24 hr after treatment. Two-way ANOVA, Sidak’s multiple comparison test, KET + CNO 4 hrs, p < 0.0001, KET + CNO 24 hr, p = 0.0107, KET only, following 4, 24, and 72 hr, p > 0.9, n = 7–8 animals. (e). Total distance traveled per minute in an open field locomotion assay for hM4Di+ animals before and after CNO treatment. Two-tailed paired t-test, p = 0.1473, n = 5 animals. (f). Summary data showing the percentage of failures to escape an escapable aversive shock in the baseline condition, for hM4Di-expressing mice before and after CNO treatment. n = 5–6 animals, Two-way ANOVA, Sidak’s multiple comparison test, Baseline, p = 0.9538, LH, p = 0.9947. (g). Left, schematic of experimental timeline and pharmacological interventions in weak LH paradigm. Right, summary data showing the percentage of failures to escape an escapable aversive shock across 6 days for two groups (white bar, hM4Di- controls; purple, hM4Di+). Two-way ANOVA, Sidak’s multiple comparison test, hM4Di + vs hM4Di-, D1-D4, p > 0.5, D5, p < 0.0001, D6, p = 0.0001, n = 8 animals. *p < 0.05, *** p < 0.001, **** p < 0.0001. Error bars reflect SEM.
-
Figure 3—source data 1
Numerical data for the graphs in Figure 3.
- https://cdn.elifesciences.org/articles/64041/elife-64041-fig3-data1-v2.xlsx
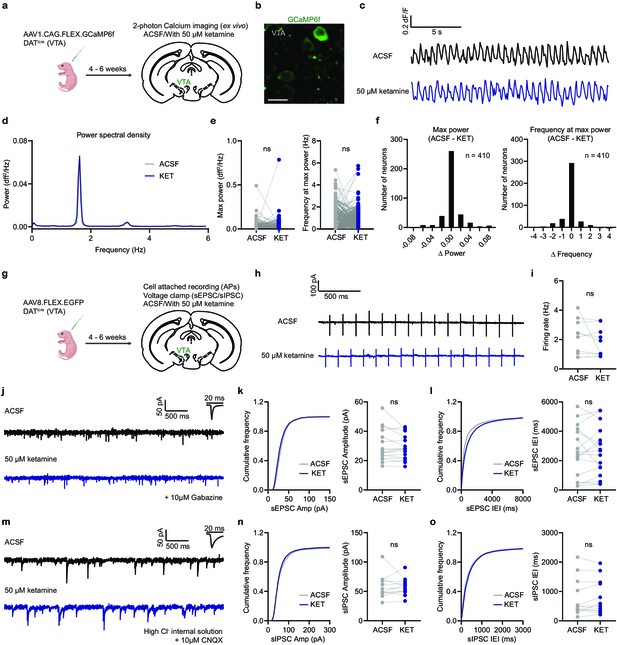
Ex vivo ketamine application does not alter GCaMP6f transients, neuronal firing, and synaptic inputs of VTA DA neurons.
(a). Schematic illustrating viral transduction strategy and two-photon Ca2+ imaging of VTA DA neurons in acute brain slices. (b). Example 2PLSM image of VTA DA neurons expressing GCaMP6f. Scale bar, 20 µm. (c). Spontaneous Ca2+ oscillations in one neuron with and without ketamine bath application (50 µM). Black, ACSF; blue, with ketamine. (d). Power spectral density of Ca2+ transients for the neuron in (c). (e). Left, quantification of max power with and without ketamine treatment (n = 410 neurons). Right, quantification of frequency at max power. Paired two-tailed t test, ACSF vs KET, Max power, p = 0.8865, Frequency at max power, p = 0.3779. (f). Left, histogram showing the distribution of changes in max power with ketamine application. Right, same but for frequency at max power. n = 410 neurons. (g). Schematic illustrating viral transduction strategy and electrophysiological recording of VTA DA neurons in acute brain slices. (h). Spontaneous action potentials recorded in one neuron with and without ketamine bath application (50 µM). Black, ACSF; blue, with ketamine. (i). Quantification of neuronal firing rate with and without ketamine treatment (n = 9 neurons from three animals). Paired two-tailed t test, ACSF vs KET, p = 0.2561. (j). Spontaneous EPSCs recorded in one neuron with and without ketamine bath application (50 µM). Black, ACSF; blue, with ketamine. Holding membrane potential at –70 mV, 10 µM Gabazine in ASCF for both conditions. (k). Left, cumulative frequency distribution of sEPSCs amplitudes. Right, quantification of sEPSC amplitude in recorded neurons. Paired two-tailed t test, ACSF vs KET, p = 0.1958. n = 17 neurons from three animals. (l). Same as (k), but for sEPSCs inter-event intervals (IEI). Paired two-tailed t test, ACSF vs KET, p = 0.8413. (m). Spontaneous IPSCs recorded in one neuron with and without ketamine bath application (50 µM). Black, ACSF; blue, with ketamine. Holding membrane potential at –70 mV with high chloride internal solution, 10 µM CNQX in ASCF for both conditions. (n). Left, cumulative frequency distribution of sIPSCs amplitudes. Right, quantification of sIPSC amplitude in recorded neurons. Paired two-tailed t test, ACSF vs KET, p = 0.9164. n = 12 neurons from two animals. (o). Same as (n), but for sIPSCs inter-event intervals (IEI). Paired two-tailed t test, ACSF vs KET, p = 0.5675.
-
Figure 4—source data 1
Numerical data for the graphs in Figure 4.
- https://cdn.elifesciences.org/articles/64041/elife-64041-fig4-data1-v2.xlsx
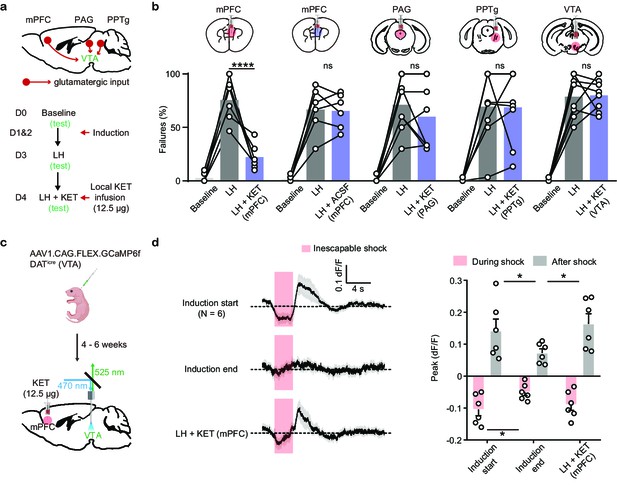
Local ketamine infusion in mPFC rescues escape behavior and VTA DA activity after LH.
(a). Top, schematic for selected glutamatergic input regions to VTA. Bottom, experimental timeline. (b). Summary data showing the percentage of failures after local infusion of ketamine or ACSF in mPFC, and ketamine in PAG, PPTg, and VTA. Two-way ANOVA, Sidak’s multiple comparison test, LH vs LH+ KET, mPFC, p< 0.0001, PAG, p = 0.4965, PPTg, p = 0.9998, VTA, p = 0.9986. LH vs LH+ ACSF (mPFC), p = 0.9993. (c). Schematic for viral transduction and VTA photometry recording of Ca2+ transients with local ketamine delivery in mPFC. (d). Left, average Ca2+ transients (mean ± SEM) in response to foot shocks at the start of induction, at the end of induction, and following local ketamine infusion. Traces are aligned to shock start time (20 trials/animal, 6 animals). Right, quantification of peak Ca2+ transient amplitude during and after foot shock stimuli across conditions. Both positive and negative values are quantified. n = 6 animals, repeated measures one-way ANOVA, Holm-Sidak’s multiple comparison test, Peak: During shock, F (1.948, 9.739) = 5.547, p = 0.0252, Induction start vs Induction end p = 0.0433, Induction end vs LH + KET, p = 0.0823. After shock, F (1.468, 7.341) = 10.05, p = 0.0105, Induction start vs Induction end, p = 0.0462, Induction end vs LH + KET, p = 0.0147. *p < 0.05, *** p < 0.001, **** p < 0.0001. Error bars reflect SEM.
-
Figure 5—source data 1
Numerical data for the graphs in Figure 5.
- https://cdn.elifesciences.org/articles/64041/elife-64041-fig5-data1-v2.xlsx
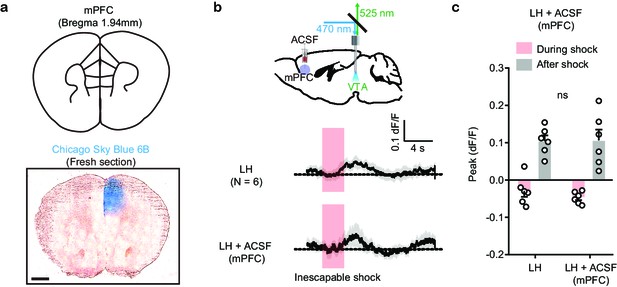
VTA DA activity after local ASCF infusion in mPFC.
(a). Atlas location and dye infusion in mPFC. Blue: Chicago Sky Blue 6B. Scale bar: 500 µm. (b). Top, schematic for VTA photometry recording of Ca2+ transients with local ACSF delivery in mPFC. Bottom, average Ca2+ transients (mean ± SEM) in response to foot shocks in LH mice before and after local ACSF infusion. Traces are aligned to shock start time (20 trials/animal, 6 animals). (c). Quantification of peak Ca2+ transient amplitude during and after foot shock stimuli across conditions. Both positive and negative values are quantified. n = 6 animals, two-tailed paired t-test, Peak: During shock, p = 0.3615; After shock, p = 0.9509.
-
Figure 5—figure supplement 1—source data 1
Numerical data for the graphs in Figure 5—figure supplement 1.
- https://cdn.elifesciences.org/articles/64041/elife-64041-fig12-data12-v2.xlsx
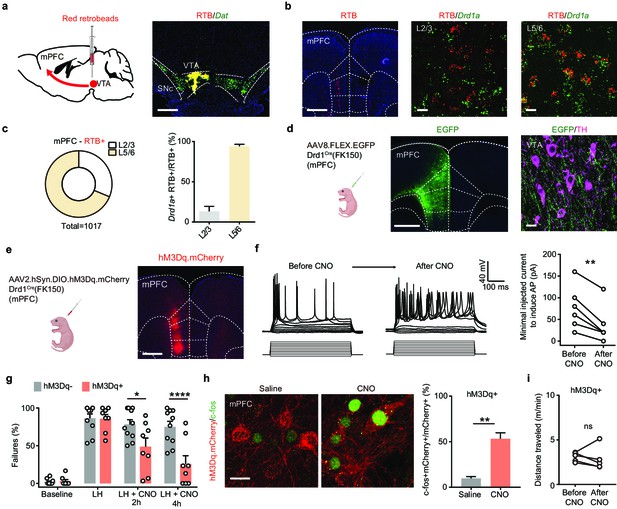
Activation of mPFC Drd1+ neurons rescues escape actions.
(a). Left, schematic illustrating retrograde labeling strategy. Right, coronal image of retrobead (RTB) injection site. Red, retrobeads; green, Dat mRNA; blue, DAPI. Scale bar, 500 μm. (b). Left, retrobead fluorescence in mPFC (atlas overlay, dashed line). Middle and right, colocalization of RTB and Drd1 mRNA in superficial and deep layers of mPFC. Scale bars, 500 μm (left), 20 μm (middle and right). (c). Left, the proportional distribution of RTB+ neurons across superficial and deep cortical layers. Right, quantification of the percentage of Drd1a + cells among RTB+ cells across layers. n = 2 animals, 31.3% RTB+ neurons in layer 2/3, 68.7% RTB+ in layer 5/6. Among those RTB+ neurons, 13.2% ± 6.2% are Drd1+ in layer 2/3, + ± 2.5 are Drd1+ in layer 5/6. (d). Left, schematic illustrating viral transduction strategy. Right, a coronal image showing the expression of EGFP (green) in the mPFC Drd1+ neurons. Scale bar, 500 μm. (e). Left, schematic illustrating viral transduction strategy. Right, epifluorescent image of hM3Dq-mCherry expression in mPFC. Scale bar, 500 μm. (f). Whole-cell recording of action potentials evoked by different amplitude current injections in one mCherry+ mPFC neuron before, during, and after bath application of 1 µM CNO. Left, example traces. Right, summary data for minimal injected current sufficient to evoke action potential firing before and after CNO application. n = 6 cells from two animals, two-tailed paired t-test, p = 0.0028. (g). Summary data showing the percentage of failures to escape an escapable aversive shock in Drd1-Cre+ and Drd1-Cre- mice expressing hM3Dq across phases of learning and after CNO treatment (Baseline, LH, LH+ CNO 2 hrs, and LH+ CNO 4 hrs). n = 8 animals for Cre-, n = 10 mice for Cre+, two-way ANOVA, Sidak’s multiple comparison test, LH+ CNO 2 hr, p = 0.0157, LH+ CNO 4 hr, p < 0.0001, Baseline/LH, p > 0.9. (h). Left, colocalization of c-fos immunolabeling in hM3Dq.mCherry+ mPFC neurons in saline and CNO-treated Drd1 Cre+, hM3Dq-expresing mice. Right, the quantification of percentage of c-fos+ cells among mCherry+ cells. Scale bar, 20 μm. n = 3 mice/condition, two-tailed unpaired t-test, p = 0.0031. (i). Total distance traveled per minute in an open-field locomotion assay for hM3Dq+ animals before and after CNO treatment. Two-tailed paired t-test, p = 0.6289, n = 5 animals. *p < 0.05, ** p < 0.01, **** p < 0.0001. Error bars reflect SEM.
-
Figure 6—source data 1
Numerical data for the graphs in Figure 6.
- https://cdn.elifesciences.org/articles/64041/elife-64041-fig6-data1-v2.xlsx
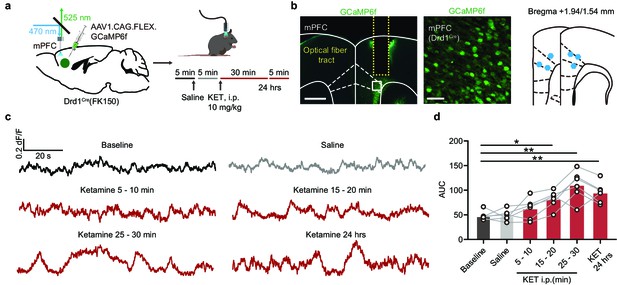
Activity of Drd1+ neurons in mPFC after in vivo ketamine treatment.
(a). Left, schematic for viral transduction of GCaMP6f in the mPFC with subsequent fiber implant in Drd1Cre(FK150) mice. Right, timeline of photometry recording with saline and ketamine treatment (10 mg/kg, i.p.). (b). Left, fiber placement illustration on a coronal section through mPFC, with a close-up image of Drd1+ (white dashed lines, Paxinos atlas overlay; yellow dashed lines, fiber track). Green, GCaMP6f; blue, Hoechst nucleic stain. Scale bars: 500 μm and 50 μm. Right, atlas location of fiber placement for each subject. (c). Example traces showing Ca2+ transients of Drd1+ neurons in the mPFC from one mouse in baseline, following saline treatment, and after ketamine treatment (5–10, 15–20, 25–30 min, and 24 hr). Black, baseline; gray, saline; red, after ketamine. (d). Summary data showing area under the curve (AUC) of Ca2+ transients in 5 min bins across conditions (Baseline, Saline, 5–10, 15–20, 25–30 min, and 24 hr after ketamine). n = 6 animals, repeated measures one-way ANOVA, F (2.498, 12.49) = 16.94, p = 0.0002. Sidak’s multiple comparison test vs Baseline, Saline, p > 0.9, KET 5–10 min, p = 0.3605, KET 15–20 min, p = 0.0131, KET 25–30 min , p = 0.0063, KET 24 hr, p = 0.0031. *p < 0.05, ** p < 0.01.
-
Figure 6—figure supplement 1—source data 1
Numerical data for the graphs in Figure 6—figure supplement 1.
- https://cdn.elifesciences.org/articles/64041/elife-64041-fig14-data14-v2.xlsx
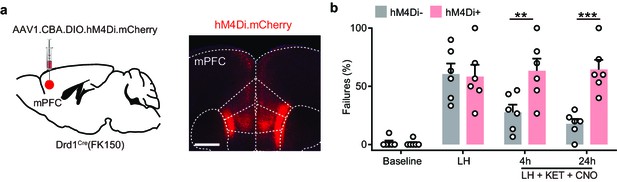
Inhibition of Drd1+ neurons in mPFC blocks the behavioral effects of ketamine.
(a). Left, schematic illustrating viral transduction strategy. Right, epifluorescent image of hM3Dq-mCherry expression in mPFC. Scale bar, 500 μm. (b). Summary data showing the percentage of failures to escape an escapable aversive shock in Drd1-Cre+ and Drd1-Cre- mice expressing hM4Di across phases of learning and after CNO treatment (Baseline, LH, LH+ CNO 4 hr, and LH+ CNO 24 hr). n = 6 animals, two-way ANOVA, Sidak’s multiple comparison test, LH+ CNO 4 hr, p = 0.0056, LH+ CNO 24 hr, p = 0.0002, Baseline/LH, p > 0.9.
-
Figure 6—figure supplement 2—source data 1
Numerical data for the graphs in Figure 6—figure supplement 2.
- https://cdn.elifesciences.org/articles/64041/elife-64041-fig15-data15-v2.xlsx
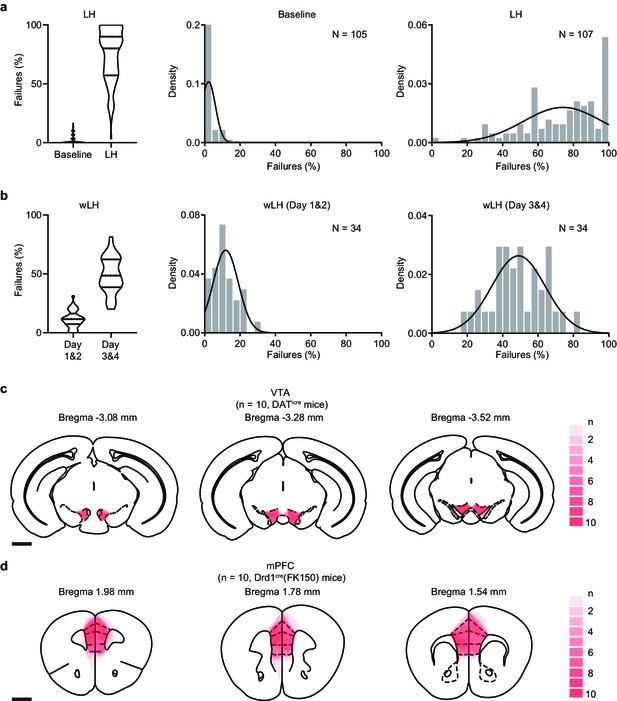
Behavioral outcomes and viral expression distributions.
(a). Left, violin plot showing the percentage of failures to escape an escapable aversive shock before and after LH induction. The width of the box indicates relative number of data points and the crossbars indicate three quartiles (Q1, Q2, Q3). Middle and right, density histograms fitted with the Gaussian distribution. Parameter estimated (Mean, SD± error): Baseline (2.19 ± 0.38, 3.844 ± 0.27), LH (74.02 ± 2.15, 22.23 ± 1.52). n = 105, 107 mice for baseline and LH. (b). Same as (a) but for wLH. Days 1 and 2 (11.88 ± 1.22, 7.10 ± 0.86), Days 3 and 4 (48.91 ± 2.60, 15.14 ± 1.84). n = 34 mice/condition. (c). Heatmap showing viral expression in the VTA of DATiCre mice. n = 10 mice. Scale bar = 1 mm. (d). Same but for the mPFC of Drd1Cre(FK150) mice. n = 10 mice.
-
Figure 6—figure supplement 3—source data 1
Numerical data for the graphs in Figure 6—figure supplement 3.
- https://cdn.elifesciences.org/articles/64041/elife-64041-fig16-data16-v2.xlsx
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (M. musculus) | C57BL/6 | Jackson Laboratory | Cat#000664;RRID: IMSR_JAX:000664 | |
| Strain, strain background (M. musculus) | B6.SJL-Slc6a3tm1.1(cre)Bkmn/J(DATiCre) | Jackson Laboratory | Cat#006660;RRID: IMSR_JAX:006660 | |
| Strain, strain background (M. musculus) | B6.FVB(Cg)-Tg(Drd1a-cre)FK150Gsat/Mmucd(Drd1Cre (FK150)) | Jackson Laboratory | RRID:MMRRC_036916-UCD | |
| Recombinant DNA reagent | AAV1.CAG.Flex.GCaMP6f.WPRE.SV40 | Chen et al., 2013, Dr. Douglas Kim | Addgene viral prep; # 100835-AAV1 | |
| Recombinant DNA reagent | AAV8.CAG.Flex.EGFP | UNC Vector Core, (Dr. Boyden) | N/A | |
| Recombinant DNA reagent | AAV1.CBA.DIO.hM4Di.mCherry | Hou et al., 2016, Packaged by Vigene, (Plasmid, Dr. Sabatini) | Addgene plasmid # 81,008 | |
| Recombinant DNA reagent | AAV2.hSyn.DIO.hM3Dq.mCherry | Krashes et al., 2011, Dr. Bryan Roth | Addgene viral prep; # 44361-AAV2 | |
| Antibody | Rabbit polyclonal anti-Tyrosine Hydroxylase | Millipore | Cat#AB152; RRID: AB_390204 | (1:1000) |
| Antibody | Mouse monoclonal anti-Tyrosine Hydroxylase | Abcam | Cat#AB129991; RRID: AB_11156128 | (1:1000) |
| Antibody | Rabbit polyclonal anti-RFP | Rockland | Cat#600-401-379; RRID:AB_2209751 | (1:500) |
| Antibody | Rabbit polyclonal anti-c-Fos | Synaptic systems | Cat# 226 003; RRID:AB_2231974 | (1:5000) |
| Chemical compound, drug | Ketamine | Vedco | 217-484-6; CAS: 1867-66-9 | |
| Chemical compound, drug | Clozapine-N-oxide | Sigma-Aldrich | C0823; CAS: 34233-69-7 | |
| Chemical compound, drug | Red retrobeads (RRBs) | Lumafluor | Cat#R180 | |
| Chemical compound, drug | SR95531 hydrobromide | Tocris | Cat#1262; CAS 104104-50-9 | |
| Chemical compound, drug | CNQX disodium salt | Tocris | Cat#1045; CAS 479347-85-8 | |
| Chemical compound, drug | Chicago Sky Blue 6B | Tocris | Cat#0846; CAS 2610-05-1 | |
| Commercial assay or kit | RNAscope Fluorescence Multiplex Assay | ACDBio | Cat No. 320,850 | |
| Commercial assay or kit | RNAscope Probe-Mm-drd1a(Drd1) | ACDBio | Cat No. 406491-C2 | |
| Commercial assay or kit | RNAscope Probe-Mm-slc6a3(Dat) | ACDBio | Cat No. 315,441 | |
| Software, algorithm | GraphPad Prism 7 | GraphPad | RRID: SCR_002798 | |
| Software, algorithm | FIJI | Schindelin et al., 2012 | http://fiji.sc/; RRID: SCR_002285 | |
| Software, algorithm | MATLAB | MathWorks | RRID: SCR_001622 | |
| Software, algorithm | Toxtrac | Rodriguez et al., 2018 | N/A | |
| Software, algorithm | Python | Python Software Foundation | RRID:SCR_008394 | |
| Software, algorithm | Clampfit 11.2 | Molecular Devices | SCR_011323 | |
| Other | Active/Passive Avoidance Shuttle Box | MazeEngineers | https://mazeengineers.com/portfolio/active-passive-avoidance-shuttle-box/#description | |
| Other | Raspberry Pi | Raspberry Pi Foundation | https://www.raspberrypi.org/ |