Synaptotagmin-7 places dense-core vesicles at the cell membrane to promote Munc13-2- and Ca2+-dependent priming
Figures
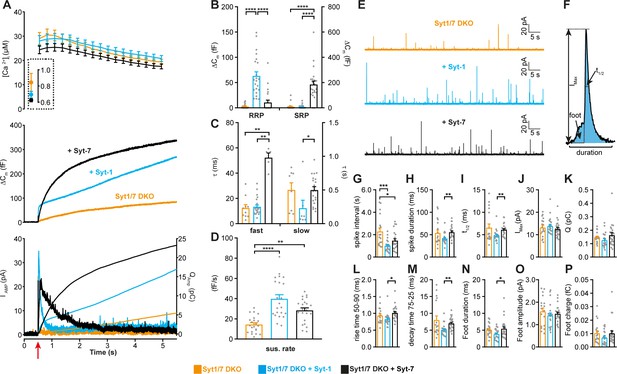
Syt-1 and Syt-7 are stand-alone calcium-sensors with different kinetics.
(A) Calcium uncaging experiment in Syt-1/Syt-7 DKO cells (orange) and in DKO cells overexpressing Syt-7 (black) or Syt-1 (blue). Top panel: [Ca2+] before (insert) and after calcium uncaging (uncaging flash at red arrow, bottom panel). Middle panel: capacitance traces (mean of all cells) show that the secretion is potentiated more (higher amplitude) by Syt-7 expression, but the kinetics of the secretory burst is faster after Syt-1 expression. Bottom panel: Mean amperometry (left ordinate axis) and mean integrated amperometry (right ordinate axis). Note that the integrated amperometric traces agree very well with the capacitance traces. (B) Sizes of the RRP and SRP. (C) Time constants, τ, of fusion for fast (i.e. RRP) and slow (i.e. SRP) secretion. (D) Sustained rates of secretion. Data information: In (A–D), data with error bars are presented as mean ± SEM; in (A), the traces are the mean of all cells. *: p<0.05; **: p<0.01; ***: p<0.001; ****: p<0.0001. Kruskal-Wallis test with Dunn’s post-hoc test. Number of cells, DKO: N = 23 cells; DKO + Syt-1: N = 22 cells; DKO + Syt-7: N = 21 cells. (E) Amperometric currents induced by infusion of ~5 μM Ca2+ into the cell via a patch pipette. Syt-1/Syt-7 DKO cells, DKO cells expressing either Syt-1 (blue trace) or Syt-7 (black trace). (F) Single amperometric spike, indicating measurement of peak current (Imax), total charge (Q, by integration), duration at half maximum (t1/2), and total duration of spike. The foot signal, which reports on the fusion pore before it expands, is indicated. (G) The spike interval. (H) Spike duration. (I) Duration at half maximum (t1/2). (J) Peak current (Imax). (K) Total charge of foot and spike (Q). (L) Spike 50–90% rise time. (M) Spike 75–25% decay time. (N) Duration of foot signal. (O) Amplitude of foot signal. (P) Charge of foot signal. The spike interval was significantly decreased by expression of either Syt-1 or Syt-7 in DKO cells. The shape parameters show that spikes have faster dynamics in the presence of Syt-1 than with Syt-7. Data information: In (G–P), data are presented as mean ± SEM. *: p<0.05; **: p<0.01; ***p<0.001. In (G, L, N): One-way ANOVA with post-hoc Tukey’s test. In (H, I, M): Kruskal-Wallis test with post-hoc Dunn's test. The spike interval (G) and the duration of foot signal (N) were log-transformed before statistical testing. Number of cells: DKO: N = 18 cells DKO + Syt-1: N = 21 cells; DKO + Syt-7: N = 24 cells.
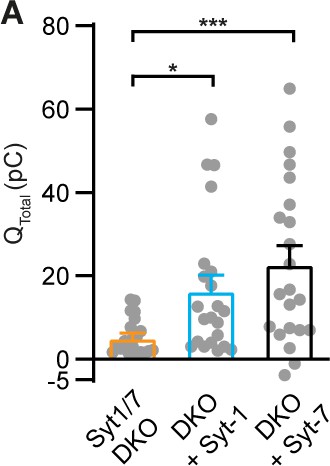
Amperometric charge quantification.
Integrated amperometric current 5 s after Ca2+-uncaging (mean ± SEM) of Syt-1/Syt-7 double KO (DKO), and DKO cells overexpressing Syt-1 (DKO + Syt-1) or Syt-7 (DKO + Syt-7). *: p<0.05; ***: p<0.001. Kruskal-Wallis test with post-hoc Dunn’s test.
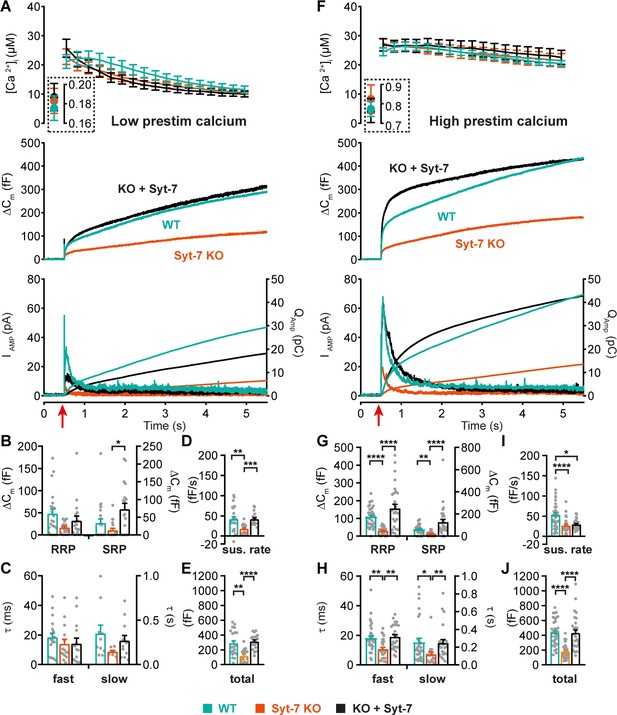
Syt-7 potentiates primed vesicle pool sizes at higher prestimulation [Ca2+].
(A) Calcium uncaging experiment from low prestimulation [Ca2+] in WT cells (persian green), Syt-7 KO cells (vermilion) and in Syt-7 KO cells overexpressing Syt-7 (black traces). Panels are arranged as in Figure 1A. (B) Sizes of the RRP and SRP. (C) Time constants of fusion for fast (i.e. RRP) and slow (i.e. SRP) secretion. (D) Sustained rates of secretion. (E) Total capacitance increase. (F) Calcium uncaging experiment from high prestimulation [Ca2+] in WT cells (green), Syt-7 KO cells (vermilion) and in Syt-7 KO cells overexpressing Syt-7 (black traces). Panels arranged as in Figure 1A. (G) Sizes of the RRP and SRP. (H) Time constants of fusion for fast (i.e. RRP) and slow (i.e. SRP) secretion. (I) Sustained rates of secretion. (J) Total capacitance increase. When stimulated from high prestimulation [Ca2+], Syt-7 expression potentiated RRP and SRP size. Data information: In (A–J) data with error bars are presented as mean ± SEM; in (A and F), the traces are the mean of all cells. *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001. Kruskal-Wallis test with Dunn’s post-hoc test. Number of cells in (A–E): Syt-7 WT: N = 22 cells; Syt-7 KO: N = 19 cells; Syt-7 KO + Syt-7: N = 18 cells, in (F–J) Syt-7 WT: N = 36 cells; Syt-7 KO: N = 27 cells; Syt-7 KO + Syt-7: N = 28 cells. Note that in cases where a cell did not have a given pool (SRP or RRP), the size of that pool was set to zero, and no time constant was estimated.
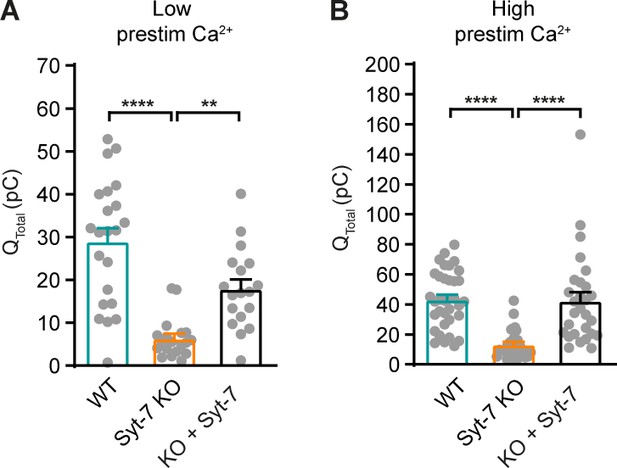
Amperometric charge quantification.
(A) Integrated amperometry (mean ± SEM) of WT, Syt-7 KO, and Syt-7 KO overexpressing Syt-7 (KO + Syt-7) stimulated from low prestimulation [Ca2+]. **: p<0.01; ****: p<0.0001. Kruskal-Wallis test with post-hoc Dunn’s test. (B) Integrated amperometry (mean ± SEM) of WT, Syt-7 KO, and KO + Syt-7 stimulated from high prestimulation [Ca2+]. ****: p<0.0001. Kruskal-Wallis test with post-hoc Dunn’s test.
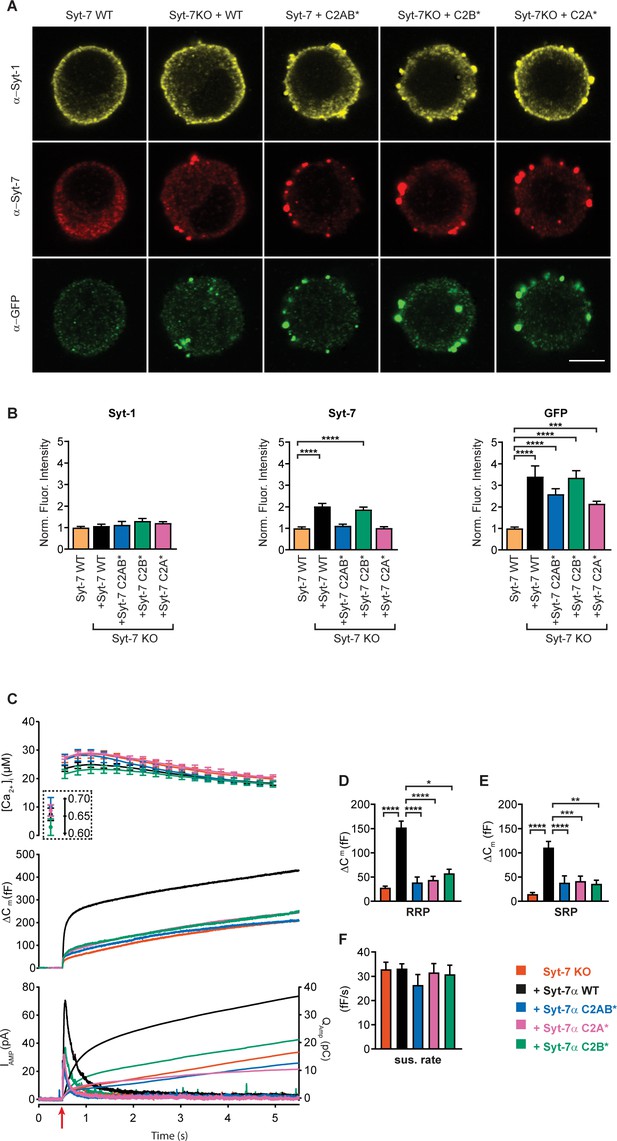
Mutation of Ca2+-binding sites in Syt-7 abolishes rescue function.
(A) Single confocal slices of new-born mouse chromaffin cells stained against Syt-1 (α-Syt-1, mouse monoclonal α-Syt1 Synaptic Systems 105011), Syt-7 (α-Syt-7, rabbit polyconal α-Syt7, Synaptic Systems 105173) and GFP (α-GFP, chicken polyclonal Abcam ab13970) in WT cells, Syt-7 KO cells and Syt-7 KO cells overexpressing Syt-7 WT, a Syt-7 C2A-mutation (C2A*)(D225,227,233A), a Syt-7 C2B-mutation (C2B*) (D357,D359A), and a Syt-7 mutated in both C2A and C2B (C2AB*) (D225,227,233,357,359A). (B) Quantification (mean ± SEM) of staining against Syt-1, Syt-7 and GFP normalized to Syt-7 WT cells. ***: p<0.001; ****: p<0.0001. In B, middle panel: one-way ANOVA with post-hoc Dunnett’s test; B, left and right panels: Kruskal-Wallis test with post-hoc Dunn’s test. Number of cells: Syt-7 WT: N = 20 cells; Syt-7 KO overexpressing Syt-7: N = 21; C2A* mutation: N = 20 cells; C2B* mutation: N = 20 cells; C2AB* mutation: N = 20 cells. (C) Calcium uncaging experiment from a relatively high prestimulation [Ca2+] in Syt-7 KO cells (vermilion), in Syt-7 KO cells overexpressing Syt-7 WT (black traces), a Syt-7 C2A-mutation (C2A*, pink traces, D225,227,233A), a Syt-7 C2B-mutation (C2B*, green traces D357,D359A), and a Syt-7 mutated in both C2A and C2B (C2AB*, blue traces, D225,227,233,357,359A). Panels are arranged as in Figure 1A. (D) Sizes (mean ± SEM) of the RRP. (E) Sizes (mean ± SEM) of the SRP. (F) Sustained rate (mean ± SEM) of secretion. *: p<0.05; **: p<0.01; ***: p<0.001; ****: p<0.0001. Kruskal-Wallis with post-hoc Dunn's Multiple Comparison test. Syt-7 KO: N = 50 cells; Syt-7 KO + Syt-7 WT: N = 52 cells, Syt-7 KO + Syt-7 C2A*: N = 30 cells, Syt-7 KO + Syt-7 C2B*: N = 22 cells, Syt-7 KO + Syt-7 C2AB*: 20 cells.
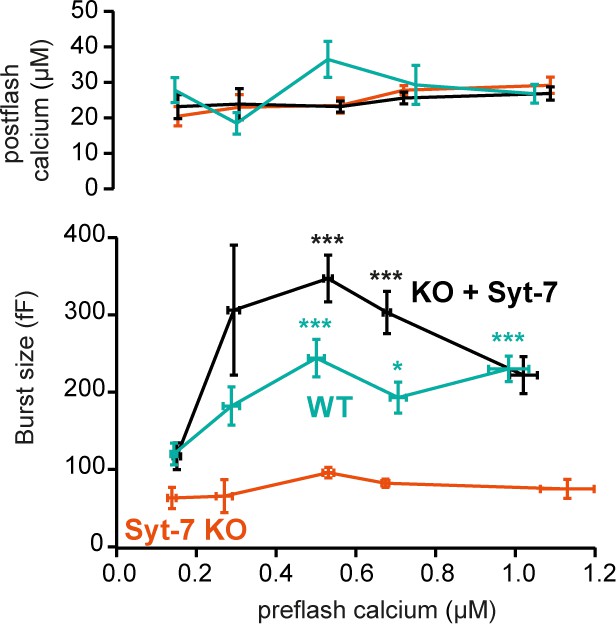
Calcium-dependent steady-state priming depends on Syt-7 expression.
Titration of burst of secretion (i.e. secretion within the first 0.5 s of the uncaging flash, approximately corresponding to the fusion of the RRP and SRP) against pre-stimulation [Ca2+]. Top panel: post-stimulation [Ca2+], bottom panel: Burst size against pre-stimulation [Ca2+]. Syt-7 KO cells (vermilion) displayed no strong dependence on [Ca2+]. Calcium-dependent priming was strong in cells overexpressing Syt-7 (black), and intermediate in WT cells expressing Syt-7 at endogenous levels (persian green). Data information: Data are presented as mean ± SEM. *: p<0.05; ***p<0.001. Testing was by Kruskal-Wallis test with Dunn’s post-hoc test. Cells were pooled in 0.2 µM [Ca2+] bins from 0.0 µM to 0.8 µM and a final bin 0.8–1.2 µM for a total of 5 bins. Statistical testing is relative to the burst size at the lowest [Ca2+] bin for the same genotype. The number of cells in each bin from low to high [Ca2+]: WT: N = 29, 9, 9, 10, 22 cells; Syt-7 KO: N = 10, 12, 16, 43, 15 cells; Syt-7 KO + Syt-7: N = 12, 10, 28, 28, 20 cells.
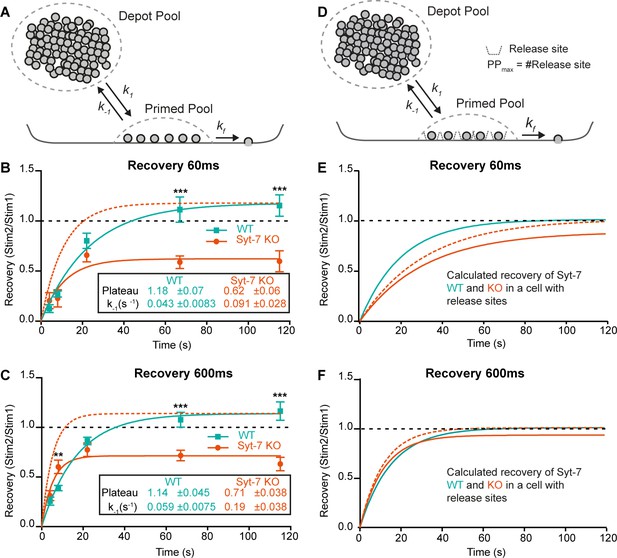
Syt-7 increases forward priming and decreases depriming.
(A) Simple model (Model I) featuring a single primed vesicle pool, a reversible priming reaction (forward rate: k1; reverse rate: k-1), and a fusion rate kf. (B) Recovery in WT cells (persian green) and Syt-7 KO cells (vermilion) of secretion at 60 ms after an uncaging stimulus, approximately corresponding to the RRP. Stim1, Stim2 = amplitude of secretion after first, or second, stimulus. Shown are mean ± SEM, plus a fit of a mono-exponential recovery curve (lines). The fit to the Syt-7 KO is also shown after scaling to the same amplitude as the WT curve (vermilion broken line), to show the faster kinetics. The fitted parameters: Plateau and the rate constant of recovery, which is the rate constant for depriming, k-1, under simplified assumptions (see text). Both Plateau and k-1 are significantly different from each other (Extra sum-of-squares F test for comparison of models, p<0.0001). (C) Same as B, but secretion at 600 ms after uncaging was used, approximately corresponding to the fusion of both RRP and SRP. (D) Model (Model II) featuring a single primed vesicle pool, limited by a fixed number of release sites, PPmax, a reversible priming reaction (forward rate: k1; reverse rate: k-1), and a fusion rate kf. (E) Recovery (at 60 ms) in the WT (green, Model II) with parameters recalculated from the fit of Model I to the data (panel B, see Materials and methods), and Syt-7 KO curve (vermilion), with the same change in priming and depriming rate as observed experimentally, now translated to a release site model, and after scaling to the WT amplitude (vermilion broken line). Under these circumstances, recovery in the Syt-7 KO trails the WT. (F) Recovery (at 600 ms) in the WT (Model II) with parameters recalculated from panel C, and in the KO (vermilion), when introducing the same changes as observed experimentally, translated to a release site model, and after scaling to the WT amplitude (vermilion broken lines). Under these circumstances, the Syt-7 KO leads the WT trace, but the differences are small. Data information: Data are presented as mean ± SEM. **: p<0.01; ***: p<0.001. In (B, C) Student’s t-test: test between genotypes (WT vs. KO) at the same inter stimulus intervals; Mann Whitney test: (600 ms: WT22s vs. KO22s).
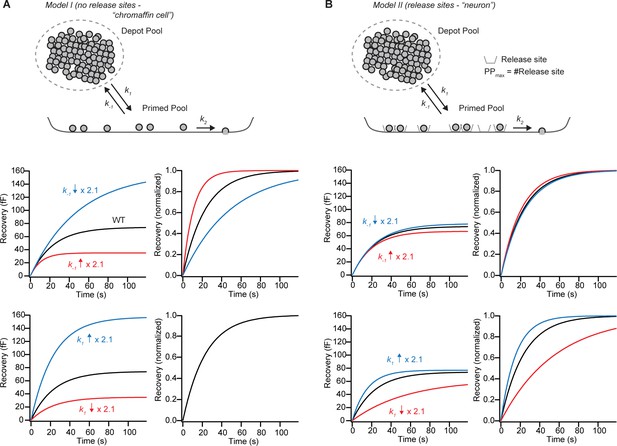
Differences in unpriming rate can be distinguished during pool recovery, if the Primed Pool is not limited by release sites.
(A) In Model I, we assume that vesicles from a very large Depot Pool prime reversibly (priming rate: k1; unpriming rate: k-1) into the Primed Pool, which is free to change is size. Fusion happens from the primed pool with rate kf. The bottom left panel shows the recovery of the primed pool (black line) with parameters from a fit to WT data (Figure 4B), k1⋅ DP = 3.21 fF/s, k-1 = 0.043 s−1, and after increasing (red line, k-1 = 0.091 s−1) or decreasing (blue line, k-1 = 0.020 s−1) the k-1 by a factor of 2.12. The red line corresponds closely to the fit of the Syt-7 KO condition. Right-hand panel: after normalization, it is appreciated that the high-k-1 condition recovers with faster kinetics than the WT condition, which recovers faster than the low-k1 condition. (B) In Model II, we assume that the Primed Pool is limited by a fixed number of release sites, which sets an upper limit (PPmax) to the Primed Pool size. The bottom panel shows the recovery of the primed pool (black line) with parameters fitting the Syt-7 WT condition (black curve, see Materials and methods), and after increasing (red curve) or decreasing (blue curve) the unpriming rate (k-1) by a factor of 2.1. Right-hand panel: normalized traces. In the presence of release sites, recovery becomes less sensitive to k-1.
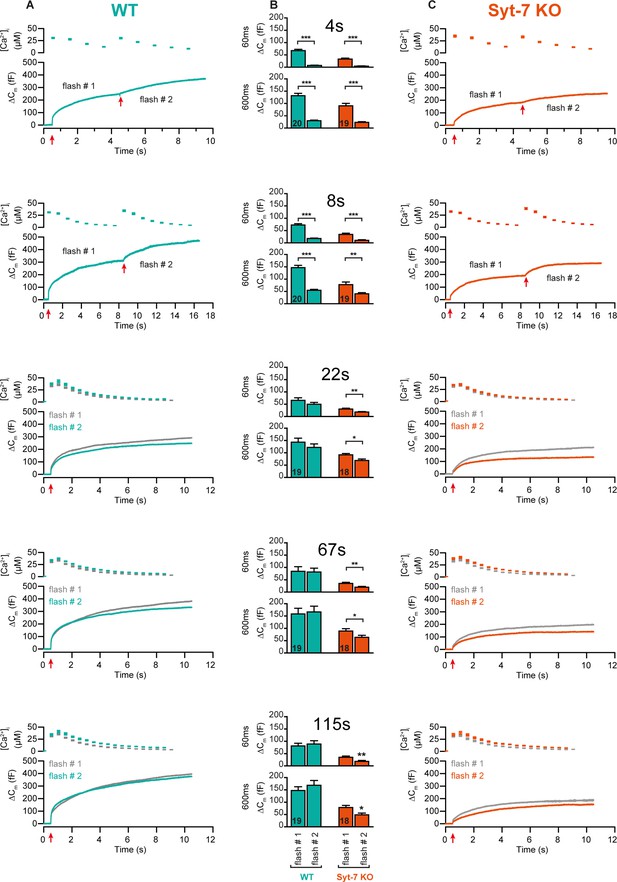
Double stimulation experiments in Syt-7 WT and KO cells.
(A) Mean [Ca2+]i and capacitance traces of Syt-7 WT cells during double uncaging experiments. Time intervals are indicated in panel B. For time intervals 4 and 8 s, the two sequential stimulations are shown on a continuous time axis, for longer intervals they are shown overlaid. (B) Quantification of capacitance at 60 and 600 ms after flash uncaging, for the first and second stimulation. Number of cells (N) are shown on bar diagrams. (C) Mean [Ca2+]i and capacitance traces of Syt-7 KO cells during double uncaging experiments. Arranged as in A. *: p<0.05; **: p<0.01; ***: p<0.001. Mann-Whitney test.
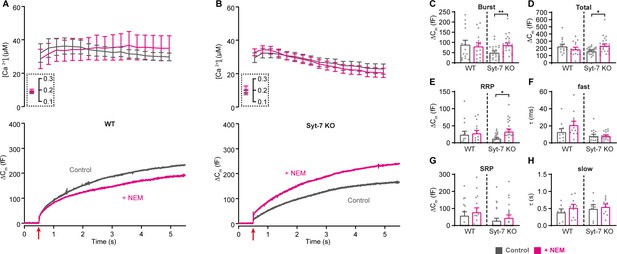
Blocking NSF-dependent de-priming occludes the effect of Syt-7 at low prestimulation [Ca2+].
(A,B) Calcium uncaging experiment from low prestimulation [Ca2+] in WT (A) and in Syt-7 KO (B) control cells (Control, gray) and in cells infused with 200 µM N-Ethylmaleimide (+NEM, magenta). Panels are arranged as in Figure 1A, except that amperometric measurements were not included. (C) Size of the burst (secretion within 0.5 s after the flash). (D) Total release (secretion within 5 s after the flash). (E) Size of the RRP. (F) Time constant, τ, of fusion for fast (i.e. RRP). (G) Size of the SRP. (H) Time constant, τ, of fusion for slow (i.e. SRP). Data information: Data are presented as mean ± SEM. *: p<0.05; **: p<0.01, Mann Whitney test comparing control cells to cells infused with NEM from the same genotype. Number of cells: WT control: N = 15 cells; WT + NEM: N = 15 cells; Syt-7 KO control: N = 24 cells; Syt-7 KO + NEM: N = 23 cells.
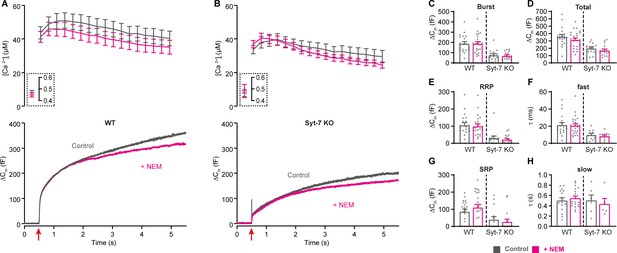
Blocking NSF had no effect in WT and Syt-7 KO cells when stimulated from high prestimulation [Ca2+].
(A,B) Calcium uncaging experiment from high prestimulation [Ca2+] in WT (A) and in Syt-7 KO (B) control cells (Control, gray) and in cells infused with 200 µM N-Ethylmaleimide (+NEM, magenta). Panels are arranged as in Figure 1A, except that amperometric measurements are not included. (C) Size of the burst (secretion within 0.5 s after the flash). (D) Total release (secretion within 5 s after the flash). (E) Size of the RRP. (F) Time constant, τ, of fusion for fast (i.e. RRP). (G) Size of the SRP. (H) Time constant, τ, of fusion for slow (i.e. SRP). Data information: Data are presented as mean ± SEM. *: p<0.05; **: p<0.01, Mann-Whitney test comparing control cells to cells infused with NEM from the same genotype. Number of cells: WT control: N = 20 cells; WT + NEM: N = 24 cells; Syt-7 KO control: N = 15 cells; Syt-7 KO + NEM: N = 17 cells.
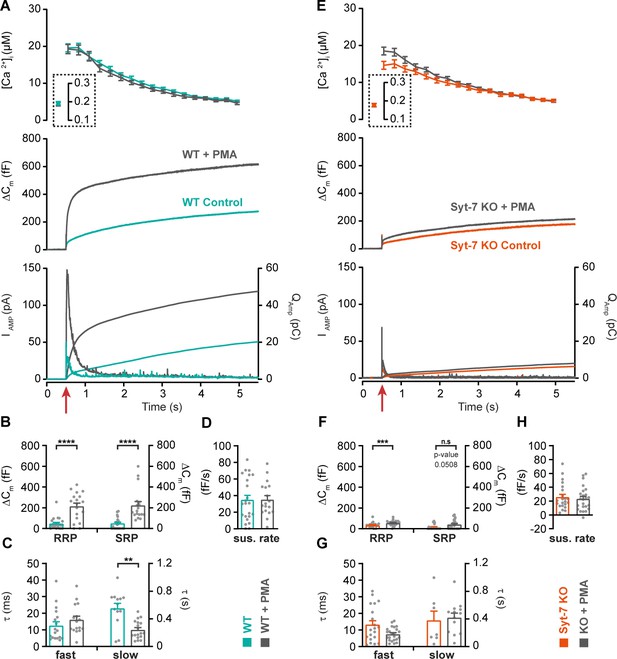
Syt-7 stimulates PMA-induced potentiation of release at low prestimulation [Ca2+].
(A) Calcium uncaging experiment from low prestimulation [Ca2+] in Syt-7 WT cells (persian green) and in Syt-7 WT cells perfused with 100 nM phorbol 12-myristate 13-acetate (PMA) (WT + PMA) (gray). Panels are arranged as in Figure 1A. PMA treatment strongly augmented the primed pool size in WT cells. (B) Sizes of the RRP and SRP. (C) Time constants, τ, of fusion for fast (i.e. RRP) and slow (i.e. SRP) secretion. (D) Sustained rates of secretion. (E) Calcium uncaging experiment from low prestimulation [Ca2+] in Syt-7 KO cells (vermilion) and in Syt-7 KO cells perfused with 100 nM PMA (KO + PMA) (gray). PMA-induced potentiation of release was much weaker in Syt-7 KO cells. (F) Size of the RRP and SRP. (G) Time constants, τ, of fusion for fast (i.e. RRP) and slow (i.e. SRP) secretion. (H) Sustained rate of secretion. Data information: Data with error bars (A–H) are presented as mean ± SEM; in (A, E), the traces are the average of all cells. Statistics: *: p<0.05; **p<0.01; ***p<0.001; ****p<0.0001. Analysis was performed with Mann-Whitney test. Number of cells: WT: N = 23 cells; WT + PMA: N = 18 cells; Syt-7 KO: N = 22 cells; Syt-7 KO + PMA = 24 cells.
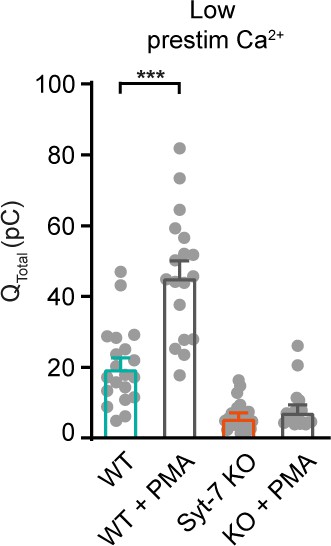
Integrated amperometry (mean ± SEM) of WT, WT cells treated with 100 nM phorbol 12-myristate 13-acetate (PMA) (WT + PMA), Syt-7 KO and Syt-7 KO with 100 nM PMA (Syt-7 KO + PMA) stimulated from low prestimulation [Ca2+].
***: p<0.001. Student’s t-test.
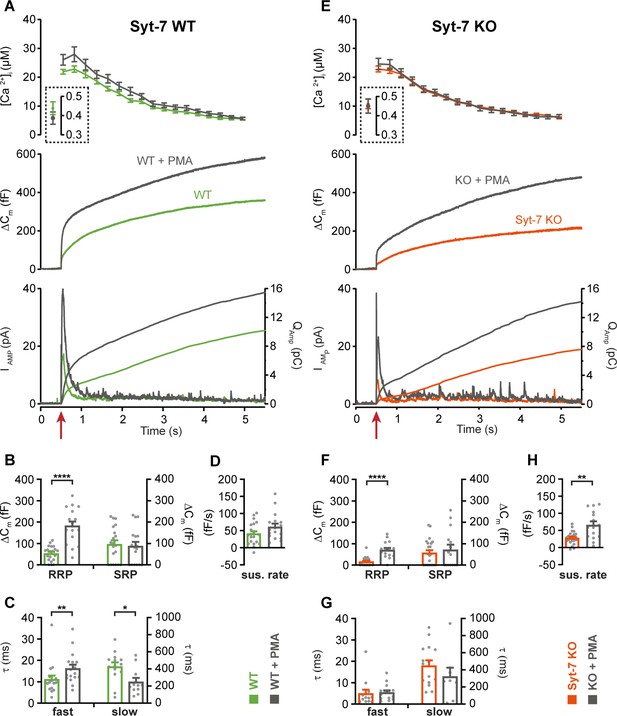
Application of phorbol esters to Syt-7 WT and KO at higher prestimulation [Ca2+].
(A) Calcium uncaging experiment from high prestimulation [Ca2+] in Syt-7 WT cells (green traces) and in Syt-7 WT cells perfused with 100 nM PMA (WT + PMA) (gray traces). Panels are arranged as in Figure 1A. PMA treatment strongly augmented the primed pool size in WT cells. (B) Sizes of the RRP and SRP. (C) Time constants, τ, of fusion for fast (i.e. RRP) and slow (i.e. SRP) secretion. (D) Sustained rates of secretion. (E) Calcium uncaging experiment from high prestimulation [Ca2+] in Syt-7 KO cells (vermilion traces) and in Syt-7 KO cells perfused with 100 nM PMA (Syt-7 KO + PMA) (gray traces). PMA-induced potentiation of release was robust when Syt-7 KO cells were stimulated from higher prestimulation [Ca2+]. (F) Sizes of the RRP and SRP. (G) Time constants, τ, of fusion for fast (i.e. RRP) and slow (i.e. SRP) secretion. (H) Sustained rates of secretion. *: p<0.05; **: p<0.01; ****: p<0.0001, Mann-Whitney test. Number of cells: Syt-7 WT: N = 20 cells; Syt-7 WT + PMA: N = 17 cells; Syt-7 KO: N = 23 cells, Syt-7 KO + PMA: N = 15 cells.
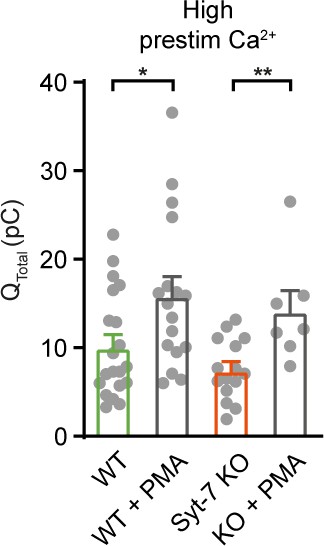
Integrated amperometry (mean ± SEM) of WT, WT cells perfused with 100 nM phorbol 12-myristate 13-acetate (PMA) (WT + PMA), Syt-7 KO and Syt-7 KO treated with 100 nM PMA (syt-7 KO + PMA) stimulated from high prestimulation [Ca2+].
*: p<0.05; **: p<0.01. Student’s t-test.
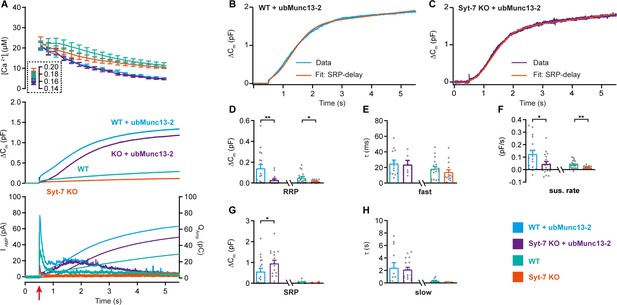
Syt-7 stimulates ubMunc13-2-dependent priming at low prestimulation [Ca2+].
(A) Calcium uncaging experiment in WT overexpressing ubMunc13-2 (WT + ubMunc13-2) (cyan traces), and Syt-7 KO overexpressing ubMunc13-2 (KO + ubMunc13-2) (purple traces) stimulated from a low prestimulation [Ca2+]. Data from Syt-7 WT and Syt-7 KO are the same as in Figure 2A. Panels are arranged as in Figure 1A. The overexpression of ubMunc13-2 potentiated the release in Syt-7 KO cells after a remarkable delay of the SRP. (B) An example capacitance trace (‘Data’) from a WT cell overexpressing ubMunc13-2 (cyan trace) with a function taking into account the SRP-delay (‘Fit’, red trace). (C) An example capacitance trace (‘Data’) from a Syt-7 KO cell overexpressing ubMunc13-2 (‘Fit’, purple trace) fitted with a function taking into account the SRP-delay (red trace). (D–H) In the Syt-7 WT + ubMunc13-2 or Syt-7 KO + ubMunc13-2, for each cell recorded, the chi-square values between fit and data were used to judge whether the standard sum of two exponentials and a line function or the function including the SRP delay (see Materials and methods) fitted the traces better. Values from the best fit were averaged to obtain the RRP, SRP sizes and time constants. (D, G) Sizes of the RRP and SRP. (E, H) Time constant, τ, of fusion for fast (i.e. RRP) and slow (i.e. SRP) secretion. Note that the τ for the SRP in the Syt-7 WT + ubMunc13-2 and Syt-7 KO + ubMunc13-2 groups include both the secretory delay and the fusion kinetics (Equation 7). Due to the slower τ for the SRP in this data set, we assumed that a τ originated from the RRP if τ ≤60 ms and from the SRP when 60 ms ≤τ ≤1600 ms (se Materials and methods). (F) Sustained rate of secretion. Note that in some cases when fitting with the function taking into account the SRP-delay, a negative sustained rate resulted from the fit. Data information: In (A–F) data with error bars are presented as mean ± SEM; in (A), the traces are the mean of all cells. WT (persian green) and Syt-7 KO (vermilion) were not obtained in parallel experiments, but are displayed here only to illustrate the increase upon ubMunc13-2 overexpression; statistical tests are only conducted for Syt-7 KO + ubMunc13-2 vs Syt-7 WT + ubMunc13-2 and Syt-7 WT vs Syt-7 KO. Note that the RRP size of WT vs Syt-7 KO is significantly differient, due to the two-group comparison, which was not the case in Figure 2A (three-group comparison). Statistics: *: p<0.05; **: p<0.01, Mann Whitney test. Number of cells: WT + ubMunc13-2: N = 17; Syt-7 KO + ubMunc13-2: N = 17 cells; WT: N = 22; Syt-7 KO: N = 19.
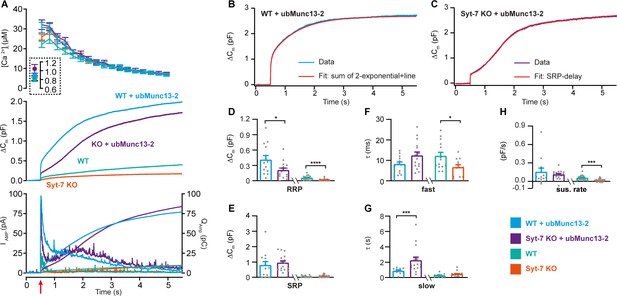
Overexpressing ubMunc13-2 in Syt-7 WT and KO cells at higher prestimulation [Ca2+].
(A) Calcium uncaging experiment from high prestimulation [Ca2+] in WT cells (persian green), WT overexpressing ubMunc13-2 (WT + ubMunc13-2) (cyan), Syt-7 KO (vermilion) and Syt-7 KO overexpressing ubMunc13-2 (KO + ubMunc13-2) (purple). Panels are arranged as in Figure 1A. The overexpression ubMunc13-2 potentiated the release in both WT and Syt-7 KO cells with a remarkable delay of the SRP in the Syt-7 KO. (B) A capacitance trace (‘Data’, blue) from a WT cell overexpressing ubMunc13-2 (cyan) fitted with a sum of two exponentials and a line (‘Fit’, red). (C) A capacitance trace (‘Data’) from a Syt-7 KO cell overexpressing ubMunc13-2 (‘Fit’, purple) fitted with a modified function, taking into account the SRP-delay (red trace). (D, E) Sizes of the RRP and SRP. (F, G) Time constant, τ, of fusion for fast (i.e. RRP) and slow (i.e. SRP) secretion. Note that the τ for the SRP in the Syt-7 KO + ubMunc13-2 group includes both the secretory delay and the fusion kinetics (Equation 7) and is therefore not directly comparable to the τ from other groups. (H) Sustained rate of secretion. Data information: In (A–H) data with error bars are presented as mean ± SEM; in (A), the traces are the mean of all cells. WT (green) and Syt-7 KO (vermilion) are displayed here only to illustrate the massive increase upon ubMunc13-2 overexpression; statistical tests are only conducted for Syt-7 KO + ubMunc13-2 vs Syt-7 WT + ubMunc13-2 and Syt-7 WT vs Syt-7 KO. Statistics: *: p<0.05; **: p<0.01; ***: p<0.001; ****: p<0.0001. In D, E, G, and H: Mann-Whitney test. Number of cells: WT: N = 15 cells; WT + ubMunc13-2: N = 13 cells; Syt-7 KO: N = 25 cells; Syt-7 KO + ubMunc13-2: N = 16 cells.
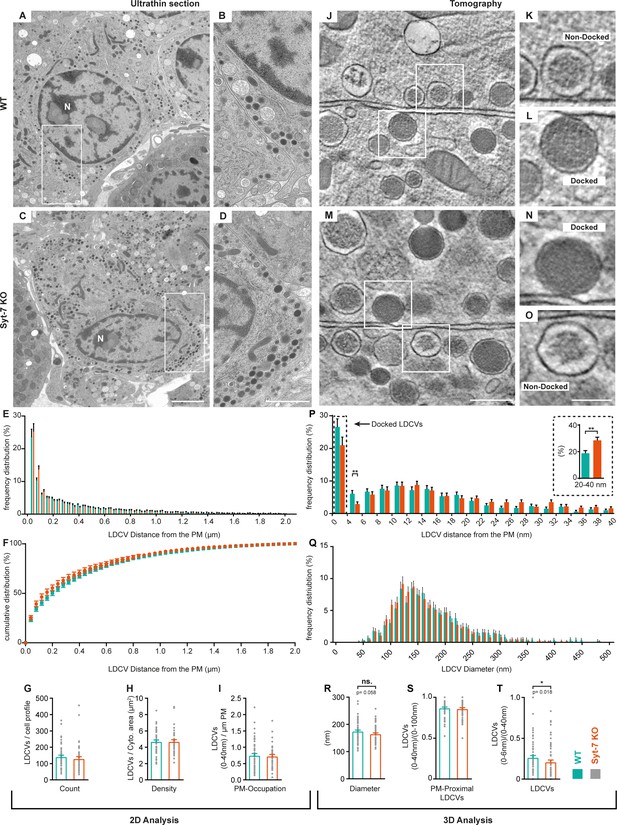
Syt-7 induces membrane-apposition of LDCVs.
(A, C) 2D-EM micrographs of ultrathin adrenal sections from WT (A) and Syt-7 KO (C) newborn mice. Nucleus is designated (N). Scale bar: 2 µm. (B, D) Magnification of selection in (A) and (C), respectively. Scale bar: 1 µm. (E) Frequency distribution of large dense core vesicles (LDCVs) within 2 µm from the plasma membrane (PM) in WT (persian green) and Syt-7 KO (vermilion) cells. (F) Cumulative frequency plot of (E). (G) Total number of LDCV per cell profile. (H) Number of LDCVs per cytosolic area (Density = LDCVs/µm2). (I) Number of PM-Proximal LDCVs per µm of PM circumference. 2D analysis revealed normal cell morphology and LDCV distribution in the absence of Syt-7. (J, M) 3D-EM reconstructed tomogram subvolume from WT (J) and Syt-7 KO (M) showing two cells with opposing membranes. Scale bar: 300 nm. K, L, N, O Magnifications of selected regions in (J) and (M); showing docked (L, N) and non-docked (K, O) LDCVs. Scale bar: 150 nm. (P) Frequency distribution of PM-proximal LDCVs where docked vesicles are accumulated in the 0–4 nm bin. Insert is a summation of 20–40 nm into single bins. (Q) Frequency distribution of LDCV diameter. (R) Diameter of LDCVs within 100 nm from the PM. (S) PM-Proximal LDCVs (0–40 nm) normalized to (0–100 nm) LDCVs (0–40 nm LDCVs/0–100 nm LDCVs). (T) Vesicles within 6 nm of the membrane (bin 0–6 nm) normalized to PM-Proximal LDCVs (0–6 nm LDCVs/0–40 nm LDCVs). Overall, the 3D analysis showed that LDCVs 0–6 nm from the PM are markedly reduced in the absence of Syt-7. Non-docked LDCVs in the Syt-7 KO accumulated at 20–40 nm from the PM. Data information: Values are mean ± SEM.*: p<0.05; **: p<0.01. Student’s t-test: (G): p=0.4240; (H): p=0.9711; (I): p=0.9241; (Insert in (P): 20–40 nm): p=0.0025; (R): p=0.0581; (S): p=0.6127. Mann Whitney test: (P): 4–6 nm: p=0.0039; (T): p=0.0184. Number of cells, 2D analysis: (WT) N = 60 cells, (Syt-7 KO) N = 46 cells; 3D analysis: (WT) N = 74 cells, (Syt-7 KO) N = 74 cells.
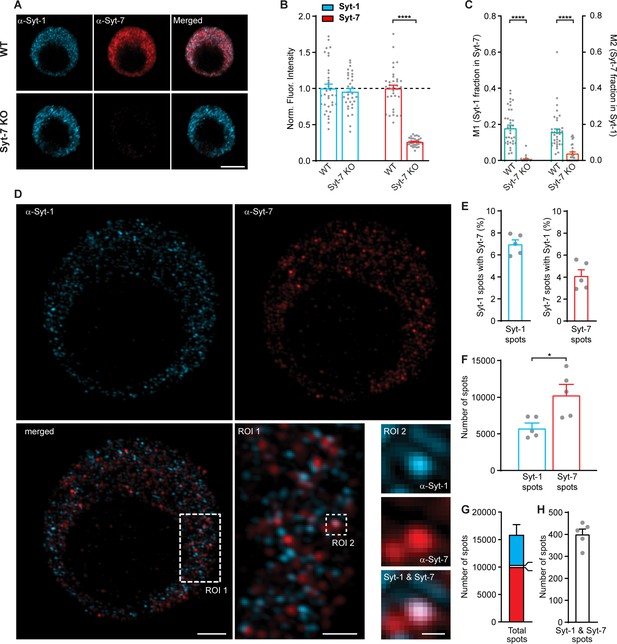
Syt-1 and Syt-7 displays limited colocalization.
(A) Single confocal slices of new-born mouse chromaffin cells stained against Syt-1 (α-Syt-1) and Syt-7 (α-Syt-7) in WT cells and in Syt-7 KO cells, and merged images. Scale bar: 5 µm. (B) Quantification of staining against Syt-1 and Syt-7 in WT and Syt-7 KO cells. For staining with other antibodies: see Figure 9—figure supplement 1D–F. (C) Manders’ coefficients M1 and M2 (mean ± SEM) for co-localization analysis of Syt-1 and Syt-7 in WT and Syt-7 KO cells. (D) Single optical slices of new-born WT mouse chromaffin cells stained against Syt-1 (α-Syt-1) and Syt-7 (α-Syt-7) acquired with 3D-structured illumination microscopy (3D-SIM). Scale bar: 2 µm. Bottom right: Magnified ROIs. ROI 1: A section of the cell from the merged-channel image showing that the majority of the spots are identified either as Syt-1 -or Syt-7-positive and few are positive for both isoforms. Scale bar: 1 µm. ROI 2: An example of a spot where Syt-1 and Syt-7 appear co-localized (top panel: α-Syt-1; middle panel: α-Syt-7; bottom panel: A merged image of α-Syt-1 and α-Syt-7 channels). Scale bar: 0.2 µm. (E) Quantification of the percentage of Syt-1 spots that were costained for Syt-7 and vice versa. (F) Number of Syt-1 and Syt-7 spots per cell, while analyzing every third optical slice (thickness of each 0.11 µm) from the top to the bottom of the cells using the ComDet plugin for ImageJ. (G) The total number of spots per cell. Bar colors indicate the proportions of Syt-1 (cyan), Syt-7 (red) and Syt-1/Syt-7 (white) spots. (H) Number of Syt-1/Syt-7 spots where the two isoforms are considered to be colocalized to the same vesicle as shown in ROI two example (D). Data information: Values are mean ± SEM. *: p<0.05; ****: p<0.0001, Student’s t-test. Number of cells in (B, C): Syt-7 WT: N = 22 cells; Syt-7 KO: N = 22 cells. Number of cells in (E–H): Syt-7 WT, N = 5 cells.
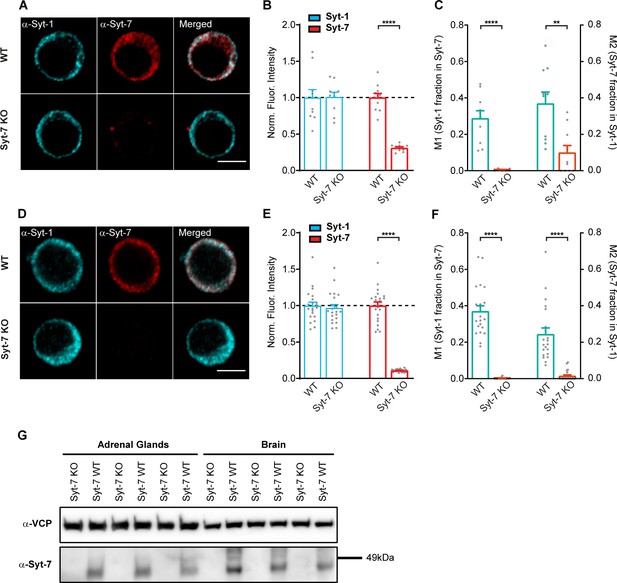
Syt-1 and Syt-7 limited colocalization.
(A–C) Immunostaining and quantification of Syt-1 and Syt-7 expression in the absence of glutaraldehyde during the fixation step. Antibodies are a α-Syt-7 rabbit polyclonal antibody and a α-Syt-1 mouse monoclonal antibody (105173 and 105011 from Synaptic Systems; see Materials and methods). (A) Single confocal slices of newborn mouse chromaffin cells stained against Syt-1 (α-Syt-1) and Syt-7 (α-Syt-7) in WT cells and in Syt-7 KO cells, and merged images. Scale bar: 5 μm. (B) Quantification of Syt-1 and Syt-7 staining in WT and Syt-7 KO cells. Some residual unspecific staining was detected in the Syt-7 KO cells. (C) Manders’ coefficients (mean ± SEM) for the colocalization analysis of Syt-1 fraction in Syt-7 and Syt-7 fraction in Syt-1 in WT and Syt-7 KO cells. (D–F) Immunostaining and quantification of Syt-1 and Syt-7 expression using a mouse monoclonal Syt-7 antibody (clone 275/14, MABN665, Sigma-Aldrich) and a rabbit polyclonal Syt-1 antibody (W855; T.C. Südhof). (D) Single confocal slices of newborn mouse chromaffin cells stained against Syt-1 (α-Syt-1) and Syt-7 (α-Syt-7) in WT cells and in Syt-7 KO cells, and merged images. Scale bar: 5 μm. (E) Quantification of the staining against Syt-1 and Syt-7 in WT and Syt-7 KO cells. (F) Manders’ coefficients (mean ± SEM) for the colocalization analysis of Syt-1 fraction in Syt-7 and Syt-7 fraction in Syt-1 in WT and Syt-7 KO cells. (G) Protein immunoblot probed with antibodies against Syt-7 (α-Syt-7) and VCP (α-VCP) as a loading control in Syt-7 KO or WT adrenal glands and brains obtained from three new-born mice (one lane per animal).
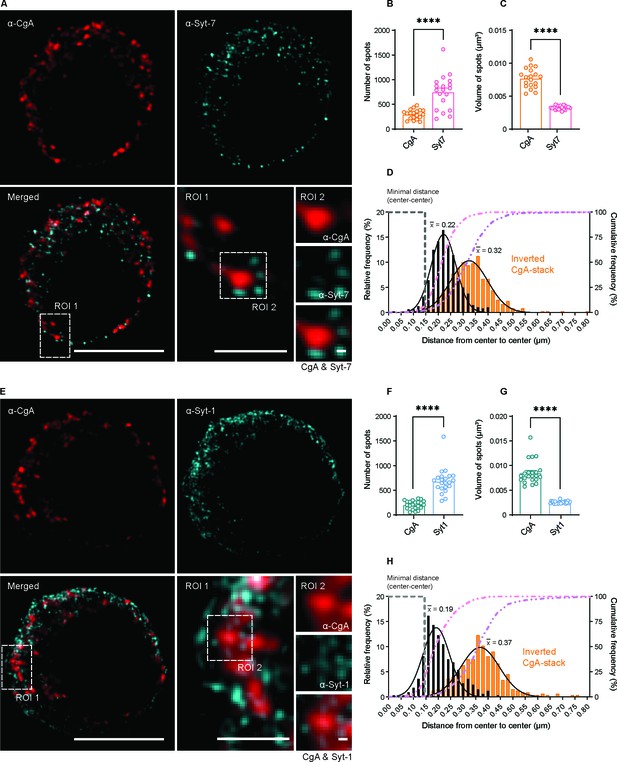
Syt-1 and Syt-7 are found on the outside of Chromogranin-A-positive vesicles.
(A, E) Single optical slices of WT mouse chromaffin cells stained against CgA (α-CgA) and Syt-7 (α-Syt-7) or Syt-1 (α-Syt-1) acquired with 3D-structured illumination microscopy (3D-SIM). Scale bar: 5 µm. Bottom right: Magnified ROIs. ROI 1: a region of the cell from the merged-channel image. Scale bar: 2 µm. ROI 2: An example of a CgA-vesicles with Syt-7 or Syt-1 spots localized adjacent to it (top panels: α-CgA; middle panels: α-Syt-7 or α-Syt-1; bottom panels: merged image of CgA and Syt-7 or Syt-1 channels). Scale bar: 0.2 µm. (B, F) Quantification of spot numbers (mean ± SEM) for Syt-1, Syt-7 and CgA in WT and Syt-7 KO cells using the DiAna plugin for ImageJ. The number of spots was quantified in a 3D-volume with z-length of 2.1 µm around the middle of the cell (note that the numbers in Figure 9 are for the entire cell and estimated using another plugin). (C, G) Volumes of Syt-1, Syt-7 and CgA spots (mean ± SEM) calculated using the DiAna plugin. (D, H) Center-to-center distances between segmented spots from the CgA-channel (CgA) and their closest neighbor in the Syt-7 or Syt-1 channel. The histogram of distance distribution (black) shows that the center of the nearest Syt-7 and Syt-1 spot is localized at 0.22 and 0.19 µm from the center of CgA vesicles, respectively (mean of Gaussian fits). The histogram of partially randomized distance distributions (orange) obtained after inversion of the CgA-stack shows that the center of the nearest Syt-7 and Syt-1 spot are localized at a larger distance (0.32 and 0.37 µm, respectively, mean of Gaussian fits). A gray dashed line identifies the minimal distance center-to-center between two spots localized side-by-side, calculated by adding the mean radii of CgA- and Syt-spots. A pink dashed line represents the cumulative distribution of the center-to-center distances between spots from the two images. A purple dashed line represents the cumulative distribution of the randomized distances between spots. Data information: Data are presented as mean ± SEM. ****p<0.0001 (Mann-Whitney test). Number of cells: CgA/Syt-7: N = 19 cells; CgA/Syt-1: N = 22 cells.
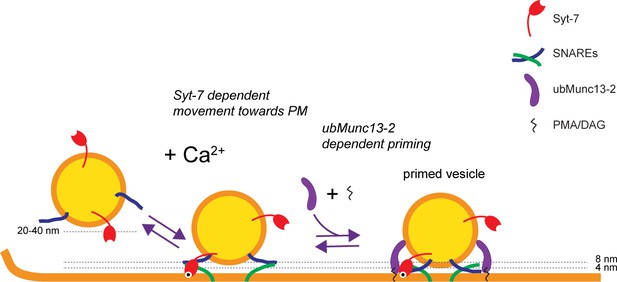
Proposed role of Syt-7 and ubMunc13-2 in dense-core vesicle priming.
Syt-7 recruits vesicles into a critical distance (6–10 nm), where ubMunc13-2 can bridge vesicle and plasma membrane and stimulate SNARE-complex formation. The ubMunc13-2-dependent priming-step is promoted by phorbol esters (PMA/DAG) and can be stabilized by blocking NSF-dependent de-priming. At high prestimulation Ca2+, other molecular calcium-sensors likely contribute to Ca2+-dependent recruitment of vesicles to the plasma membrane.
Tables
Secretion parameters for Syt-7 WT and KO.
Estimated parameters for Syt-7 WT and KO when secretion is measured 60 ms or 600 ms after Ca2+-uncaging, which corresponds approximately to the fusion of the RRP, or the RRP + SRP, respectively. Syt-7 elimination resulted in an increase in the reverse priming rate (k-1), and a reduction in forward priming rate (k1*DP, where DP is the size of the Depot Pool, and k1 is the rate constant for priming) following stimulation.
| 60 ms (approx. RRP) | 600 ms (approx. RRP+SRP) | |||
|---|---|---|---|---|
| Syt-7 WT | Syt-7 KO | Syt-7 WT | Syt-7 KO | |
| Pool size (fF) | 74.2 ± 3.3 | 33.6 ± 0.77 | 145 ± 4 | 85.4 ± 2.7 |
| k-1 (s−1) | 0.043 ± 0.0083 | 0.091 ± 0.028 | 0.059 ± 0.0075 | 0.19 ± 0.038 |
| k1 (Before Stim) (fF/s) | 3.21 ± 0.63 | 3.07 ± 0.95 | 8.60 ± 1.12 | 15.8 ± 3.32 |
| k1 (After Stim) (fF/s) | 3.78 ± 0.78 | 1.90 ± 0.62 | 9.8 ± 1.3 | 11.29 ± 2.44 |
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (M. musculus) | C57BL/6 | Experimental Medicine, Panum Stable, University of Copenhagen. | ||
| Strain, strain background (M. musculus) | CD1 | Experimental Medicine, Panum Stable, University of Copenhagen. | ||
| Genetic reagent (M. musculus) | Synaptotagmin-7 (syt7) null allele | Maximov A, Lao Y, Li H, Chen X, Rizo J, Sørensen JB, Südhof TC. Genetic analysis of synaptotagmins-7 function in synaptic vesicle exocytosis. Proc Natl Acad Sci U S A. 2008 Mar 11;105(10):3986–3991. | PMID:18308933 | |
| Genetic reagent (M. musculus) | Synaptotagmin-1 (syt1) null allele | Geppert M, Goda Y, Hammer RE, LI C, Rosahl TW, Stevens CF, Südhof TC. 1994. Synaptotagmins I: a major Ca2+ sensor for transmitter release at a central synapse. Cell 79(4): 717–727. | PMID:7954835 | |
| Transfected construct (Rattus norwegicus) | p156rrl-pCMV- pH(ecliptic GFP)-TEV-rnSyt1 | This paper, Syt-1 WT | Local reference: Lenti #94 | |
| Transfected construct (Rattus norwegicus) | p156rrl-pCMV- pH(ecliptic GFP)-TEV-rnSyt7S | This paper, Syt-7 WT | Local reference: Lenti #96 | |
| Transfected construct (Rattus norwegicus) | p156rrl-pCMV- pH(ecliptic GFP)-TEV-rnSyt7S-C2AB/D225,227,233,357,359A | This paper, Syt-7 C2AB* | Local reference: Lenti #133 | |
| Transfected construct (Rattus norwegicus) | p156rrl-pCMV- pH(ecliptic GFP)-TEV-rnSyt7S-C2B/D357,359A | This paper, Syt-7 C2B* | Local reference: Lenti #135 | |
| Transfected construct (Rattus norwegicus) | p156rrl-pCMV- pH (ecliptic GFP)-TEV-rnSyt7S-C2A/D225,227,233A | This paper, Syt-7 C2A* | Local reference: Lenti #136 | |
| Transfected construct (Rattus norwegicus) | pSFV1-EGFP-ubMunc13-2 | Zikich D, Mezer A, Varoqueaux F, Sheinin A, Junge HJ, Nachiel E, Melamed R, Brose N, Gutman M, Ashery U. 2008. J. Neurosci. 28:1949–1960. | PMID:18287511 | Local reference: Semliki #486 |
| Antibody | Chicken anti-GFP | Abcam | Ab13970 RRID:AB_300798 | 1:500; 2 hr at room temperature |
| Antibody | Rabbit anti-Chromogranin A | Abcam | Ab15160 RRID:AB_301704 | 1:500; Overnight at four degrees |
| Antibody | Rabbit anti-synaptotagmin-1 | Gift from T. C. Südhof, Stanford, CA | W855 | 1:2000; 2 hr at room temperature |
| Antibody | Mouse anti-synaptotagmin-1 | Synaptic System | SySy: 105011 RRID:AB_887832 | 1:500; Overnight at 4 degrees or 2 hr at room temperature |
| Antibody | Rabbit anti-synaptotagmin-7 | Synaptic System | SySy: 105173 RRID:AB_887838 | ICC: 1:500; Overnight at 4 degrees or 2 hr at room temperature WB: 1:500; Overnight at four degrees. |
| Antibody | Mouse anti-synaptotagmin-7 | Sigma-aldrich | MABN665 RRID:AB_2888943 | 1:200; Overnight/4 degrees |
| Antibody | anti-VCP | Abcam | Ab11433 RRID:AB_298039 | 1:10000; 1 hr at room temperature |
| Antibody | Goat anti-rabbit HRP | Agilent | Dako-P0448 RRID:AB_2617138 | 1:2000; 1 hr and30 min at room temperature |
| Antibody | Goat anti-mouse HRP | Agilent | Dako-P0447 RRID:AB_2617137 | 1:10000; 30 mins at room temperature |
| Antibody | Goat anti-mouse Alexa 546 | ThermoFisher Scientific | A11003 AB_2534071 | 1:500; 30 min at room temperature |
| Antibody | Goat anti-rabbit Alexa 647 | ThermoFisher Scientific | A21245 RRID:AB_2535813 | 1:500; 30 min at room temperature |
| Antibody | Goat anti-mouse Alexa 488 | Invitrogen | A11029 RRID:AB_2534088 | 1:500; 30 min at room temperature |
| Antibody | Goat anti-chicken Alexa 488 | Abcam | Ab150169 RRID:AB_2636803 | 1:500; 30 min at room temperature |
| Commercial assay or kit | BCA Protein assay kit | Pierce | Pierce: 23227 | |
| Chemical compound, drug | NaCl | Sigma-aldrich | Sigma-aldrich: S9888 | |
| Chemical compound, drug | KCl | Sigma-aldrich | Sigma-aldrich: P5405 | |
| Chemical compound, drug | NaH2PO4 | Sigma-aldrich | Sigma-aldrich: S8282 | |
| Chemical compound, drug | Glucose | Sigma-aldrich | Sigma-aldrich: G8270 | |
| Chemical compound, drug | DMEM | Gibco | Gibco: 31966047 | |
| Chemical compound, drug | L-cysteine | Sigma-aldrich | Sigma-aldrich: C7352 | |
| Chemical compound, drug | CaCl2 | Sigma-aldrich | Sigma-aldrich: 499609 | |
| Chemical compound, drug | EDTA | Sigma-aldrich | Sigma-aldrich: E5134 | |
| Chemical compound, drug | Papain | Worthington Biochemical | Worthington Biochemical: LS003126 | |
| Chemical compound, drug | Albumin | Sigma-aldrich | Sigma-aldrich: A3095 | |
| Chemical compound, drug | Trypsin-inhibitor | Sigma-aldrich | Sigma-aldrich: T9253 | |
| Chemical compound, drug | Penicillin/streptomycin | Invitrogen | Invitrogen: 15140122 | |
| Chemical compound, drug | Insulin-transferrin-selenium-X | Invitrogen | Invitrogen: 51500056 | |
| Chemical compound, drug | Fetal calf serum | Invitrogen | Invitrogen: 10500064 | |
| Chemical compound, drug | MgCl2 | Sigma-aldrich | Sigma-aldrich: 449172 | |
| Chemical compound, drug | HEPES | Sigma-aldrich | Sigma-aldrich: H3375 | |
| Chemical compound, drug | Nitrophenyl-EGTA (NPE) | Synthesized at the Max-Planck-Institut for biophysical chemistry, Göttingen. | ||
| Chemical compound, drug | Fura-4F | Invitrogen | Invitrogen: F14174 | |
| Chemical compound, drug | Furaptra | Invitrogen | Invitrogen: M1290 | |
| Chemical compound, drug | Mg-ATP | Sigma-aldrich | Sigma-aldrich: A9187 | |
| Chemical compound, drug | GTP | Sigma-aldrich | Sigma-aldrich: G8877 | |
| Chemical compound, drug | Ascorbic acid | Sigma-aldrich | Sigma-aldrich: A5960 | |
| Chemical compound, drug | EGTA | Sigma-aldrich | Sigma-aldrich: E4378 | |
| Chemical compound, drug | Ethylmaleimide (NEM) | Sigma-aldrich | Sigma-aldrich: 04259 | |
| Chemical compound, drug | Phorbol 12-myristate 13-acetate (PMA) | Sigma-aldrich | Sigma-aldrich: P8139 | |
| Chemical compound, drug | Paraformaldehyde | Sigma-aldrich | Sigma-aldrich: P6148 | |
| Chemical compound, drug | PIPES | Sigma-aldrich | Sigma-aldrich: 80635 | |
| Chemical compound, drug | Triton X-100 | Sigma-aldrich | Sigma-aldrich: T8787 | |
| Chemical compound, drug | BSA | Sigma-aldrich | Sigma-aldrich: A4503 | |
| Chemical compound, drug | Prolong Gold | Invitrogen | Invitrogen: P36934 | |
| Chemical compound, drug | Protease cocktail inhibitor | Invitrogen | Invitrogen: 87785 | |
| Chemical compound, drug | RIPA buffer | Invitrogen | Invitrogen: R0278 | |
| Chemical compound, drug | ECL plus western blotting substrate | Pierce | Pierce: 32132 | |
| Software, algorithm | Igor 8.0 | Wavemetrics | ||
| Software, algorithm | ImageJ | NIH software |





