Linking mPFC circuit maturation to the developmental regulation of emotional memory and cognitive flexibility
Figures
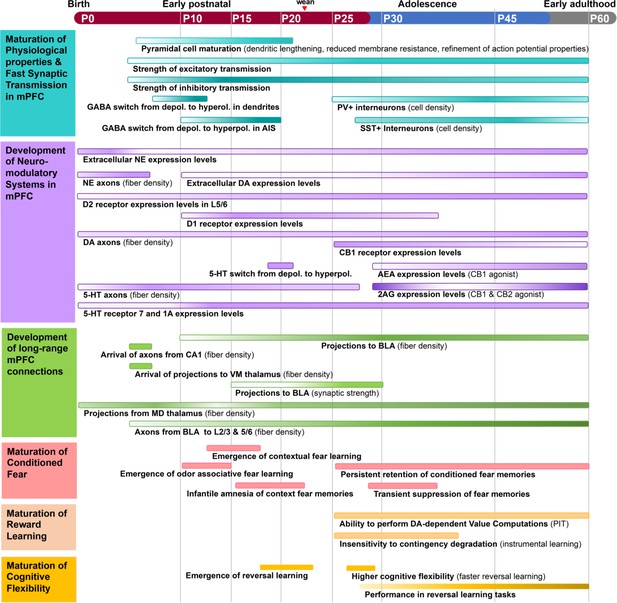
Timeline of major events during rodent medial prefrontal cortex (mPFC) development.
The structural and functional organization of mPFC circuitry is largely established in early postnatal development and then refines into early adulthood. Adolescence is marked by enhanced bidirectional innervation of mPFC, amygdala, and neuromodulatory centers. Inhibitory neurotransmission increases from P15–P60, with major changes in the excitatory/inhibitory ratio of synaptic transmission in adolescence. Aspects of mPFC circuit development align with the maturation of cognitive behaviors. The ability to perform reversal learning and contextual fear learning emerges just prior to adolescence and remains highly malleable until at least mid-adolescence. Each process is represented as a colored bar, with the gradient of color intensity (low to high) marking initiation, peak, and decline of the process where applicable. Of note, bars representing the magnitude of axonal innervation usually begin at the earliest point reported in the literature, but do not remove the possibility of earlier innervation.
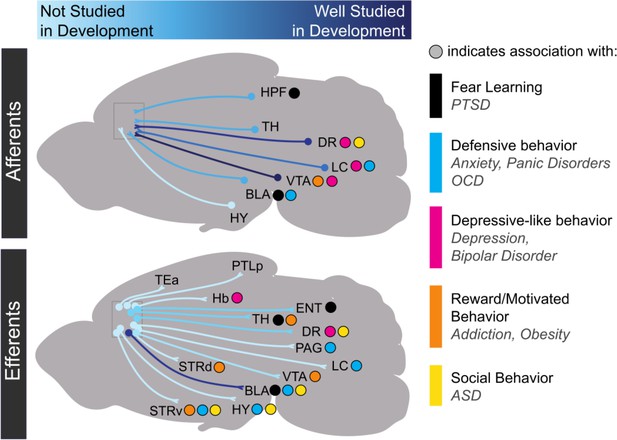
Progress in research on the development of medial prefrontal cortex long-range connectivity.
Light blue indicates that a particular projection has not been studied in development while dark blue indicates that it has been relatively well-studied. Dots indicate behavioral repertoires and diseases associated with particular connections. HPF: hippocampal formation; TH: thalamus; DR: dorsal raphe nucleus; VTA: ventral tegmental area; LC: locus coeruleus; BLA: basolateral amygdala; HY: hypothalamus; TEa: temporal association area; PTLp: posterior parietal association area; Hb: habenula; ENT: entorhinal cortex; PAG: periaqueductal gray; STRd: dorsal striatum; STRv: ventral striatum.
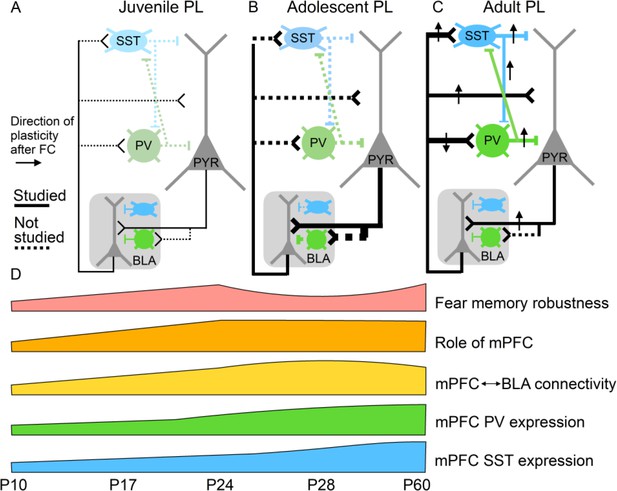
Potential relationships between prelimbic cortex-basolateral amygdala (PL–BLA) circuit assembly and the development of persistent fearful memories.
(A) During juvenile period, weak connections between PL and BLA may contribute to infantile amnesia. (B) During adolescence, BLA axons continue to innervate PL, and there is a major increase in feed-forward inhibition in the PL projection to the BLA. In addition, parvalbumin-positive and somatostatin inhibitory interneurons, which are known to receive direct synaptic input from BLA, undergo physiological changes. Changes in inhibitory dynamics may contribute to the temporary suppression of fearful memories during adolescence. (C) In the adult, when fearful memories are robust and long-lasting, PL–BLA circuitry has stabilized in its mature form, with a slight refinement in the strength of the descending projection from PL to BLA, and the ascending projection from BLA to PL exhibiting stronger connections onto local interneurons than onto pyramidal cells.
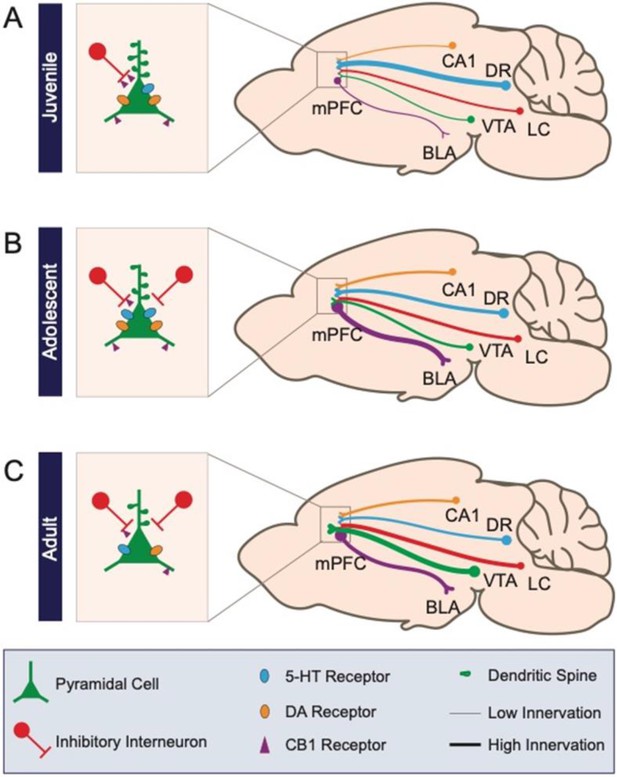
Schematic of prefrontal cellular and circuit changes throughout development.
(A) The juvenile period is characterized by low-density anatomical connections and elevated spine density. (B) During adolescence, long-range connectivity strengthens along with local inhibitory circuits in medial prefrontal cortex. (C) In the adult, aspects of circuitry refine, including the density of dendritic spines and neuromodulatory receptors. Long-range axonal innervation density continues to increase between some regions. Numbers CA1: CA1 region of the hippocampus; DR: dorsal raphe nucleus; VTA: ventral tegmental area; LC: locus coeruleus; BLA: basolateral amygdala.
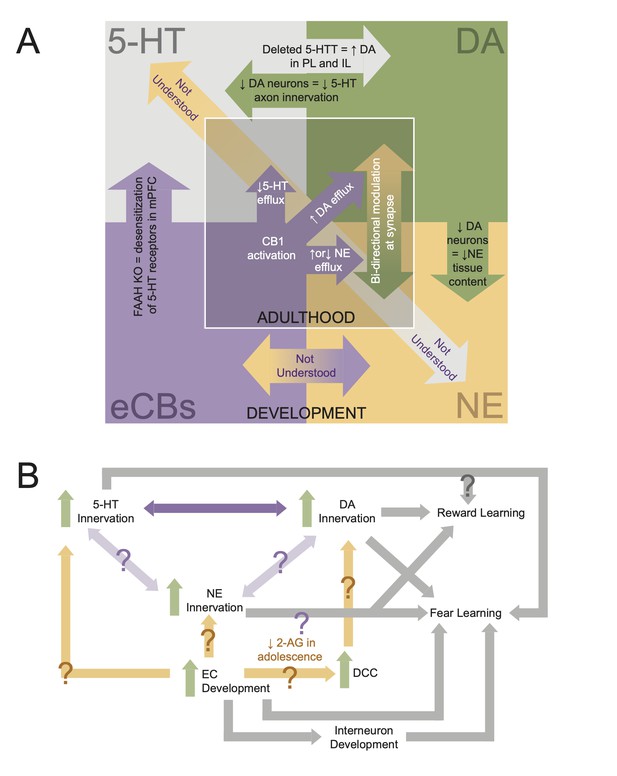
Interdependencies during neuromodulatory system development.
(A) Schematic showing known interactions between neuromodulatory systems in medial prefrontal cortex (mPFC). Inner square displays phenomena shown in adults, while the outer square displays developmental interactions. (B) Flowchart of how development of mPFC neuromodulations converges to give rise to behavior. Arrows with question marks indicate unstudied interactions.
Tables
Summary of phenotypes in four mouse models.
PYR: pyramidal cell; IN: interneuron; MGE: median ganglionic eminence; Pr: release probability; PSD: postsynaptic density; E/I: excitatory/inhibitory.
| Phenotypes | |||||
|---|---|---|---|---|---|
| Gene | Protein function | Cell types | Cellular | Circuit | Behavior |
| CNTNAP2 | Axonal transmembrane protein | PYR, INs | Reduced spine density, reduced excitatory and inhibitory synaptic input to PYR cells | Altered phase modulated spiking to delta and theta rhythms, reduced long-range cortico-cortical connectivity, and reduced local connectivity | Repetitive behaviors and cognitive inflexibility |
| Disc1 | Intracellular scaffold | PYR, INs, glia | Reduced PV expression, change in Pr in INs, and reduced inhibitory input to PYR cells | Reduced feed-forward inhibition in thalamocortical circuits and elevated E/I ratio | Impairments in working memory, latent inhibition, and pre-pulse inhibition, and increased immobility in forced swim test |
| Dlx5/6 | Transcription factor | MGE INs | Deficits in IN migration and reduced IN number | Altered gamma rhythms | Anxiety and congnitive inflexibility |
| Shank3 | Excitatory synaptic scaffold | PYR | Reduced dendritic complexity, reduced spine density and PSD length, and reduced excitatory synaptic transmission | Reduced frontostriatal connectivity, reduced local and long-range cortical connectivity, and reduced prefrontal gray matter | Social deficits, anxiety, and repetitive behaviors |





