Capping protein regulates endosomal trafficking by controlling F-actin density around endocytic vesicles and recruiting RAB5 effectors
Figures
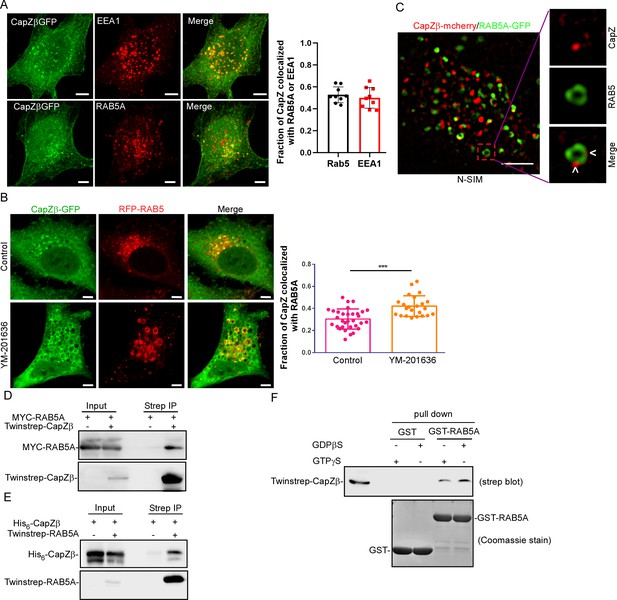
CapZ is associated with early endosomes.
(A) CapZβ-GFP-expressing HeLa cells were immunostained with antibodies against EEA1 (top panel) or RAB5A (lower panel). Confocal images taken along the z-axis were projected (Maximum Intensity Projection). The colocalization coefficients (MCC) of CapZβ-GFP and EEA1 or CapZβ-GFP and RAB5A were quantified. (B) CapZβ-GFP/RFP-RAB5A-expressing HeLa cells were treated with or without YM-201636 (5 μM) for 8 hr, followed by confocal imaging. The colocalization coefficients (MCC) of mRFP-RAB5A and CapZβ-GFP were quantified. (C) The N-SIM S Super-resolution imaging of CapZβ-mCherry/RAB5A-GFP-expressing HeLa cells. (D) HEK293 cells were transiently transfected with MYC-RAB5A, and/or Twinstrep-CapZβ, and the cell lysates were incubated with Strep-Tactin Sepharose. The pulldowns were subjected to immunoblotting analysis against CapZβ and RAB5A. (E) HEK293 cells were transiently transfected with His6-CapZβ and/or Twinstrep-RAB5A, and the cell lysates were then incubated with Strep-Tactin Sepharose. The pulldowns were subjected to immunoblot analysis against CapZβ and RAB5A. (F) The lysates of Twinstrep-CapZβ-expressing HEK293 cells were incubated with GST or GST-RAB5A recombinant proteins in the presence of GDPβS or GTPγS, followed by GST pulldown and strep immunoblotting. The blots, images, and graphs represent data from at least three independent experiments. The data are expressed as mean ± SD. The difference between the two groups was analyzed using two-tailed Student’s t-test, p<0.05 was considered statistically significant. *** p<0.001. Scale bar, 5 μm. MCC, Manders colocalization coefficient.
-
Figure 1—source data 1
Original immunoblots.
- https://cdn.elifesciences.org/articles/65910/elife-65910-fig1-data1-v2.xlsx
-
Figure 1—source data 2
Data for Figure 1A, B.
- https://cdn.elifesciences.org/articles/65910/elife-65910-fig1-data2-v2.xlsx
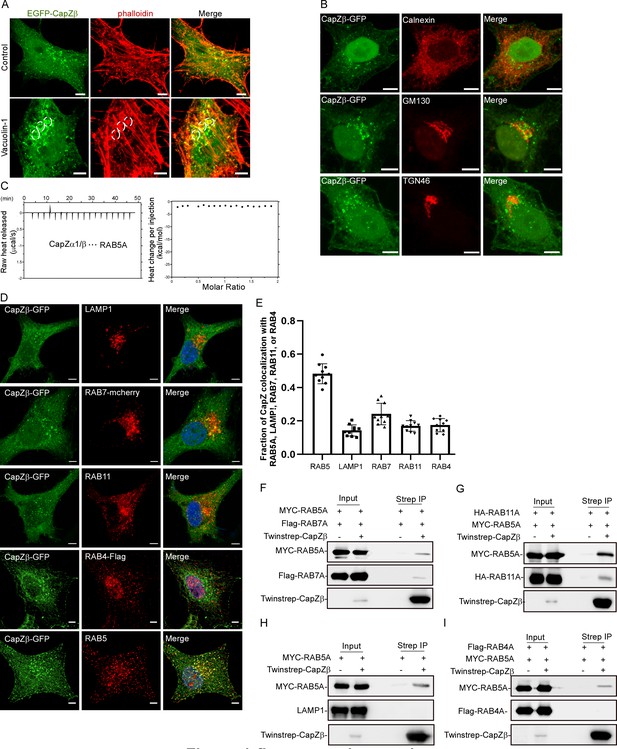
CapZ is associated with early endosomes.
(A) CapZβ-GFP-expressing HeLa cells were treated with or without vacuolin-1 (1 μM) for 12 hr, and were then immunostained with Red-X Phalloidin; the white dashed circles indicate the vacuolin-1-induced vesicular structures. Confocal images taken along the z-axis were projected (Maximum Intensity Projection). (B) CapZβ-EGFP-expressing HeLa cells were immunostained with antibodies against Calnexin, GM130, or TGN46. Confocal images taken along the z-axis were projected (Maximum Intensity Projection). (C) The interaction between CapZ (CapZα1-CapZβ) and RAB5A was assessed by ITC. (D, E) CapZβ-EGFP-expressing HeLa cells were transfected with RAB7-mcherry or RAB4-Flag, followed by immunostaining with antibodies against LAMP1, RAB11, or Flag (D). The colocalization coefficients (MCC) of RAB5A, LAMP1, RAB7-mcherry, RAB4, RAB11 over CapZβ-GFP were quantified (E). (F) HEK293 cells were transiently transfected with MYC-RAB5A, Flag-RAB7A, and/or Twinstrep-CapZβ, and the cell lysates were incubated with Strep-Tactin Sepharose. The pulldowns were subjected to immunoblotting analysis against CapZβ, RAB5A, and RAB7A. (G) HEK293 cells were transiently transfected with MYC-RAB5A, HA-RAB11A, and/or Twinstrep-CapZβ, and the cell lysates were incubated with Strep-Tactin Sepharose. The pulldowns were subjected to immunoblotting analysis against CapZβ, RAB5A, and RAB11A. (H) HEK293 cells were transiently transfected with MYC-RAB5A, and/or Twinstrep-CapZβ, and the cell lysates were incubated with Strep-Tactin Sepharose. The pulldowns were subjected to immunoblotting analysis against CapZβ, RAB5A, and LAMP1. (I) HEK293 cells were transiently transfected with MYC-RAB5A, Flag-RAB4A, and/or Twinstrep-CapZβ, and the cell lysates were incubated with Strep-Tactin Sepharose. The pulldowns were subjected to immunoblotting analysis against CapZβ, RAB5A, and RAB4A. Scale bar, 5 μm. ITC, isothermal titration calorimetry; MCC, Manders colocalization coefficient.
-
Figure 1—figure supplement 1—source data 1
Original immunoblots.
- https://cdn.elifesciences.org/articles/65910/elife-65910-fig1-figsupp1-data1-v2.xlsx
-
Figure 1—figure supplement 1—source data 2
Data for 1C, 1E.
- https://cdn.elifesciences.org/articles/65910/elife-65910-fig1-figsupp1-data2-v2.xlsx
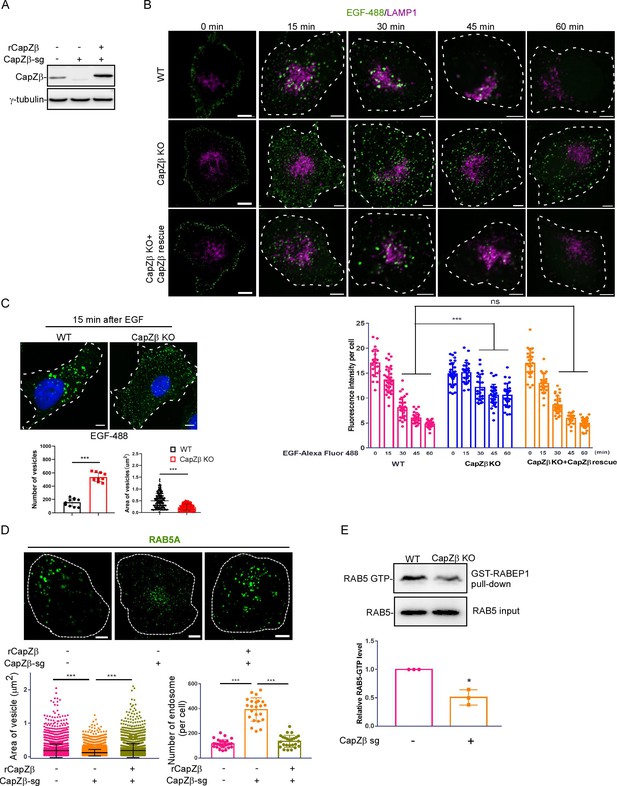
CapZ is required for early endosome maturation.
(A) The expression of CapZβ in control, CapZβ-knockout, or CapZβ-reconstituted HeLa cells. (B) Control, CapZβ-knockout, or CapZβ-reconstituted HeLa cells were treated with EGF-488 for the indicated times, and then processed for immunofluorescence analysis. (C) Control or CapZβ-knockout HeLa cells were treated with EGF-488 for 15 min, and the number and size of EGF-positive vesicles in these cells were quantified. (D) Control, CapZβ-knockout, or CapZβ-reconstituted HeLa cells were immunostained with an anti-RAB5A antibody. The number and size of the early endosomes in these cells were quantified. (E) Active RAB5 in control or CapZβ knockout cells was examined with a GST-R5BD pulldown assay. The images and graphs represent data from three independent experiments. The data are expressed as mean ± SD. The difference between the two groups was analyzed using two-tailed Student’s t-test, p<0.05 was considered statistically significant. *** p<0.001. Scale bar, 5 μm.
-
Figure 2—source data 1
Original immunoblots.
- https://cdn.elifesciences.org/articles/65910/elife-65910-fig2-data1-v2.xlsx
-
Figure 2—source data 2
Data for 2B–2E.
- https://cdn.elifesciences.org/articles/65910/elife-65910-fig2-data2-v2.xlsx
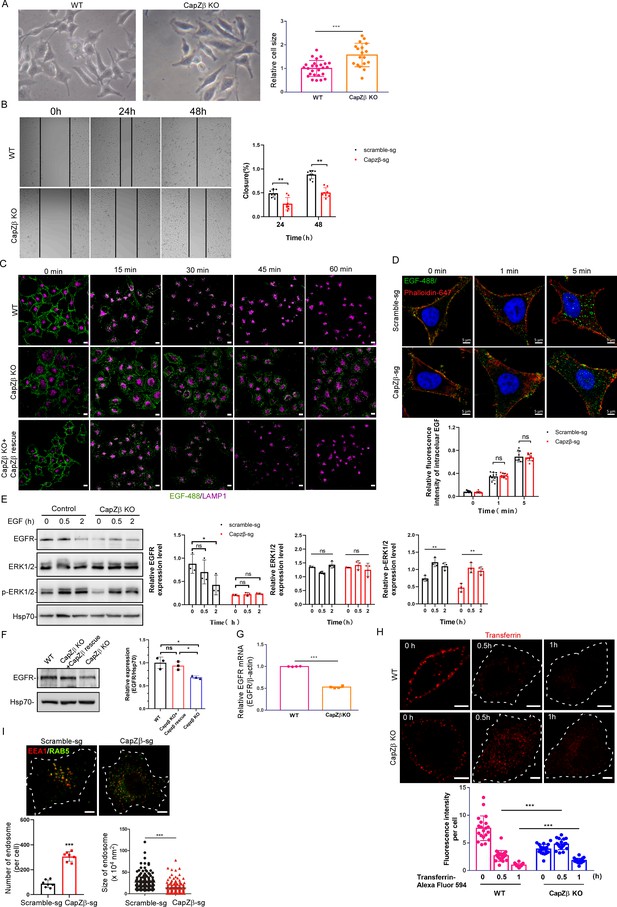
CapZ is required for early endosome maturation.
(A) Phase-contrast images of control or CapZβ-knockout HeLa cells, and the size of the cells was measured by Image J. (B) Control or CapZβ-knockout HeLa cells were plated in 24-well plates, and were then scratch-wounded 24 hr later. The borders of the scratch-wounds were imaged, and the percentage of closure was quantified at 0, 24, and 48 hr post-scratching. (C) Control, CapZβ-knockout, or CapZβ-reconstituted HeLa cells were treated with EGF-488 for the indicated times, and processed for immunofluorescence analysis. (D) Control or CapZβ-knockout HeLa cells were treated with EGF-488 for the indicated times before fixation and staining with phalloidin-647 and DAPI (without permeabilization), and then processed for immunofluorescence analysis. (E) Control or CapZβ-knockout HeLa cells were treated with EGF (100 ng/ml). They were then collected at the indicated time points and subjected to Western blot analysis against EGFR, ERK, pERK, and Hsp70. (F) The expression levels of EGFR in Control, CapZβ-knockout, or CapZβ-reconstituted HeLa cells were determined by immunoblot analysis. (G) The expression levels of EGFR mRNA in control or CapZβ-knockout HeLa cells were determined by RT-qPCR analysis. (H) Control or CapZβ-knockout HeLa cells were incubated with Transferrin-594 (25 μg/ml) on ice for 0.5 hr, and then in complete medium at 37°C in a CO2 incubator for the indicated times, followed by confocal imaging. (I) Control or CapZβ-knockout HeLa cells were immunostained with anti-EEA1 and anti-RAB5A antibodies. The number and size of the early endosomes in these cells were quantified. The blots, images, and graphs represent data from three independent experiments. The data are expressed as mean ± SD. The difference between the two groups was analyzed using two-tailed Student’s t-test, p<0.05 was considered statistically significant. *** p<0.001. Scale bar, 5 μm.
-
Figure 2—figure supplement 1—source data 1
Original immunoblots.
- https://cdn.elifesciences.org/articles/65910/elife-65910-fig2-figsupp1-data1-v2.xlsx
-
Figure 2—figure supplement 1—source data 2
Data for 1B, 1D-1F, 1 H, 1I.
- https://cdn.elifesciences.org/articles/65910/elife-65910-fig2-figsupp1-data2-v2.xlsx
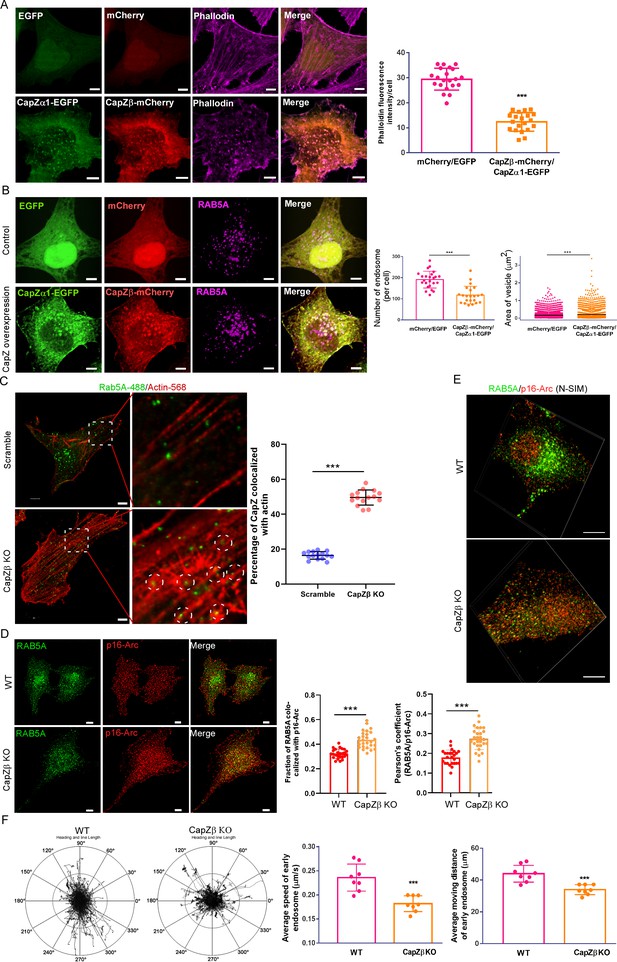
CapZ controls the actin density around early endosomes.
(A, B) HeLa cells were stably transfected with EGFP/mCherry or CapZα1-EGFP/CapZβ-mCherry constructs. These cells were either immunostained with phalloidin (A), or with an anti-RAB5A antibody (B). The number and size of the early endosomes in these cells were quantified (B). (C) Control or CapZβ-knockout HeLa cells were immunostained with antibodies against actin and RAB5A. The single plane whole-cell confocal images were then captured with a resolution of 2048×2048 pixels (left panels). The selected area in the cells was further scanned with a resolution of 4096×4096 pixels (right panels). The percentage of RAB5 puncta that are actin positive against total RAB5 puncta (MCC) in control or CapZβ-knockout cells was quantified. (D, E) The control or CapZβ-knockout HeLa cells were stained with antibodies against RAB5 and p16-Arc, followed by confocal imaging and colocalization coefficients (MCC or PCC) analysis of RAB5/p16-Arc (D) or N-SIM S Super-resolution imaging (E). (F) Control or CapZβ-knockout HeLa cells were transiently transfected with RFP-RAB5A, and cells were then imaged without delay for 3 min by time-lapse Nikon high-speed confocal microscopy. 2D track analysis was performed using NIS-Elements AR software. The images and graphs represent data from at least three independent experiments. The data are presented as mean ± SD. The difference between the two groups was analyzed using two-tailed Student’s t-test. Differences were considered statistically significant when p<0.05. * p<0.05, ** p<0.01, and *** p<0. 001. Scale bar, 5 μm. MCC, Manders colocalization coefficient; PCC, Pearson’s correlation coefficient.
-
Figure 3—source data 1
Data for 3A–3D, 3F.
- https://cdn.elifesciences.org/articles/65910/elife-65910-fig3-data1-v2.xlsx
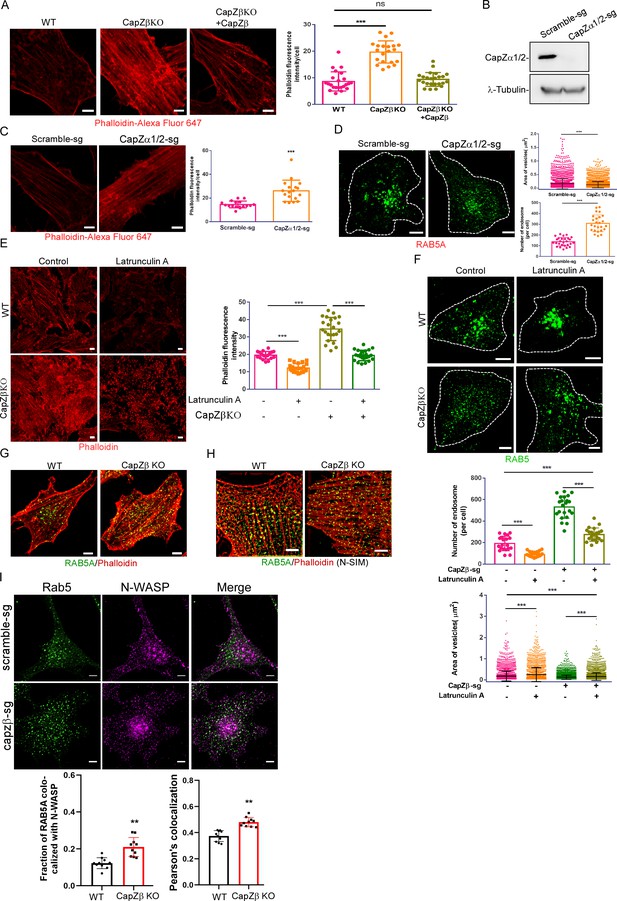
The role of actin density around endocytic vesicles in the maturation of early endosomes.
(A) F-actin levels in control, CapZβ-knockout, or CapZβ-reconstituted HeLa cells were visualized by phalloidin staining. (B) The expression of CapZα in control and CapZα1/2-knockout HeLa cells was determined by CapZα immunoblot analysis. (C) F-actin levels in control or CapZα1/2-knockout HeLa cells were visualized by phalloidin staining. (D) Control or CapZα1/2-knockout HeLa cells were immunostained with an anti-RAB5A antibody. The number and size of the early endosomes in these cells were quantified. (E) Control or CapZβ-knockout HeLa cells were treated with or without latrunculin A (1 μM) overnight, followed by phalloidin immunostaining. (F) Control or CapZβ-knockout HeLa cells were treated with or without latrunculin A (1 μM) overnight, and were then immunostained with an anti-RAB5A antibody. The number and size of the early endosomes in these cells were quantified. (G, H) Control or CapZβ-knockout HeLa cells were immunostained with Red-X Phalloidin and anti-RAB5A antibody, followed by confocal (G) or N-SIM S Super-resolution imaging (H). (I) The control or CapZβ-knockout HeLa cells were immunostained with antibodies against RAB5 and N-WASP, followed by confocal imaging. The colocalization coefficients (MCC or PCC) of RAB5A and N-WASP were quantified. The images and graphs represent data from at least three independent experiments. The data are presented as mean ± SD. The difference between the two groups was assessed using two-tailed Student’s t-test. Differences were considered statistically significant when p<0.05. * p<0.05, ** p<0.01, and *** p<0.001. Scale bar, 5 μm. MCC, Manders colocalization coefficient; PCC, Pearson’s correlation coefficient.
-
Figure 3—figure supplement 1—source data 1
Original immunoblots.
- https://cdn.elifesciences.org/articles/65910/elife-65910-fig3-figsupp1-data1-v2.xlsx
-
Figure 3—figure supplement 1—source data 2
Data for 1A, 1C–1F, 1I.
- https://cdn.elifesciences.org/articles/65910/elife-65910-fig3-figsupp1-data2-v2.xlsx
The reconstituted 3-D N-Sim images of endogenous Arp2/3 complex and endogenous RAB5 in wild-type HeLa cells.
The reconstituted 3-D N-Sim images of endogenous Arp2/3 complex and endogenous RAB5 in CapZβ-knockout cells.
RAB5-positive endosomes movements in wild-type HeLa cells.
RAB5A-positive endosomes movements in CapZβ-knockout HeLa cells.
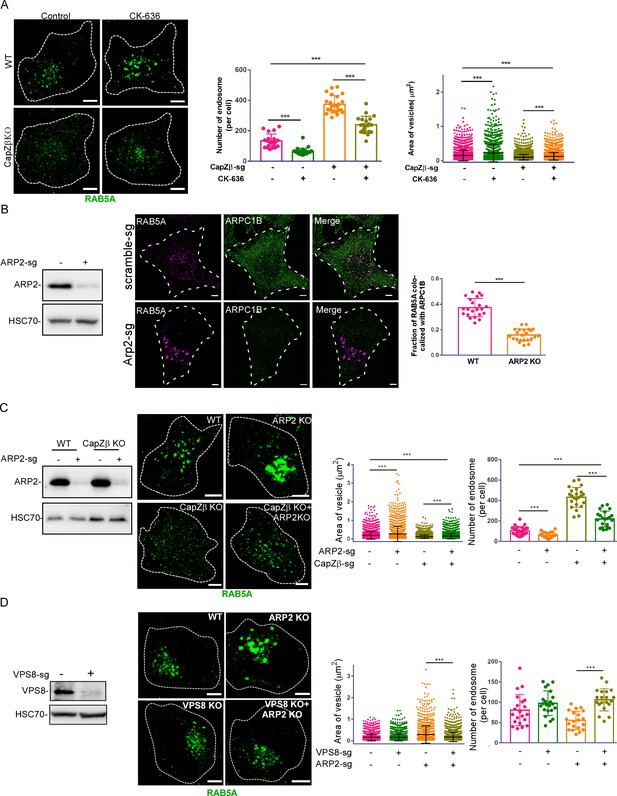
The actin density around early endosomes regulates its maturation.
(A) Control or CapZβ-knockout HeLa cells were treated with or without CK-636 (100 μM) overnight and were then immunostained with an anti-RAB5A antibody. The number and size of the early endosomes in these cells were quantified. (B) Control or ARP2-knockout HeLa cells were immunostained with antibodies against RAB5A and ARPC1B, followed by confocal imaging. The colocalization coefficients (MCC) of RAB5A and ARPC1B were quantified. The expression of ARP2 in these cells was analyzed by immunoblot analysis. (C) Control, CapZβ-knockout, ARP2-knockout, or CapZβ/ARP2-double knockout HeLa cells were immunostained with an anti-RAB5A antibody, and the number and size of the early endosomes in these cells were then quantified. The expression of ARP2 in these cells was analyzed by immunoblot analysis. (D) Control, VPS8-knockout, ARP2-knockout, or VPS8/ARP2-double knockout HeLa cells were immunostained with an anti-RAB5A antibody, and the number and size of the early endosomes in these cells were then quantified. The expression of VPS8 in control or VPS8-knockout cells was analyzed by immunoblot analysis. The images and graphs represent data from at least three independent experiments. The data are presented as mean ± SD. The difference between the two groups was analyzed using two-tailed Student’s t-test. Differences were considered statistically significant when p<0.05. * p<0.05, ** <0.01, and *** <0. 001. Scale bar, 5 μm. MCC, Manders colocalization coefficient.
-
Figure 4—source data 1
Original immunoblots.
- https://cdn.elifesciences.org/articles/65910/elife-65910-fig4-data1-v2.xlsx
-
Figure 4—source data 2
Data for 4A–4D.
- https://cdn.elifesciences.org/articles/65910/elife-65910-fig4-data2-v2.xlsx
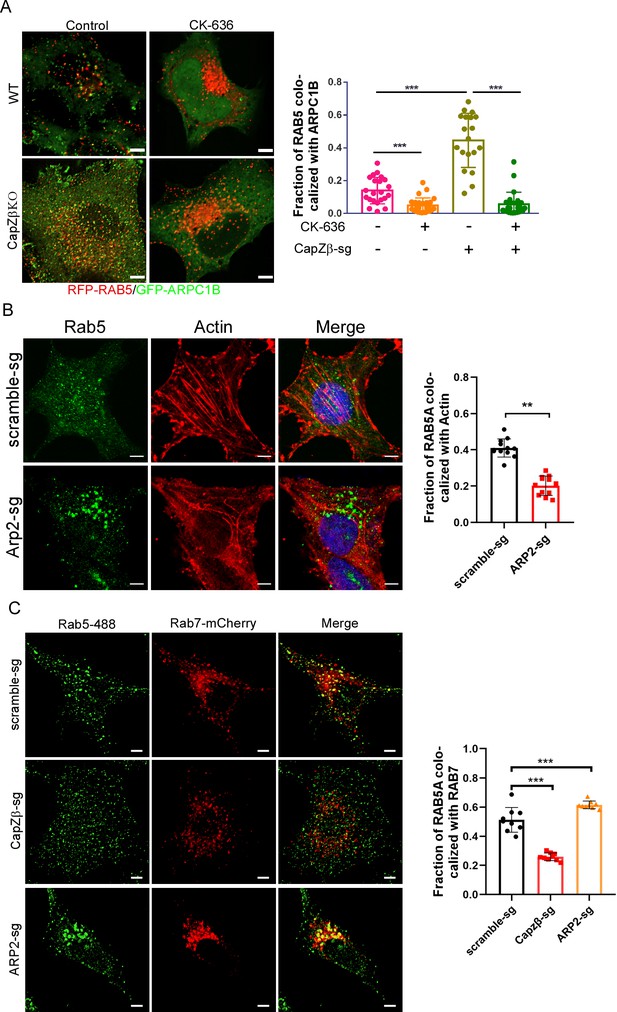
The role of actin density around endocytic vesicles in the maturation of early endosomes.
(A) The control or CapZβ-knockout HeLa cells were transiently transfected with RFP-RAB5A and ARPC1B-GFP, and were then treated with or without CK-636 (100 μM) overnight, followed by confocal imaging. The colocalization coefficients (MCC) of RFP-RAB5A and ARPC1B-GFP were quantified. (B) Control or ARP2-knockout HeLa cells were immunostained with anti-Actin and anti-RAB5A antibodies, followed by confocal imaging. The colocalization coefficients (MCC) of RAB5A and Actin were quantified. (C) Control, CapZβ-knockout, or ARP2-knockout HeLa cells were transfected with RAB7-mcherry, and immunostained with an anti-RAB5A antibody, followed by confocal imaging. The colocalization coefficients (MCC) of RAB5A and RAB7 were quantified. The images and graphs represent data from at least three independent experiments. The data are presented as mean ± SD. The difference between the two groups was assessed using two-tailed Student’s t-test. Differences were considered statistically significant when p<0.05. * p<0.05, ** p<0.01, and *** p<0.001. Scale bar, 5 μm. MCC, Manders colocalization coefficient.
-
Figure 4—figure supplement 1—source data 1
Data for 1A–1C.
- https://cdn.elifesciences.org/articles/65910/elife-65910-fig4-figsupp1-data1-v2.xlsx
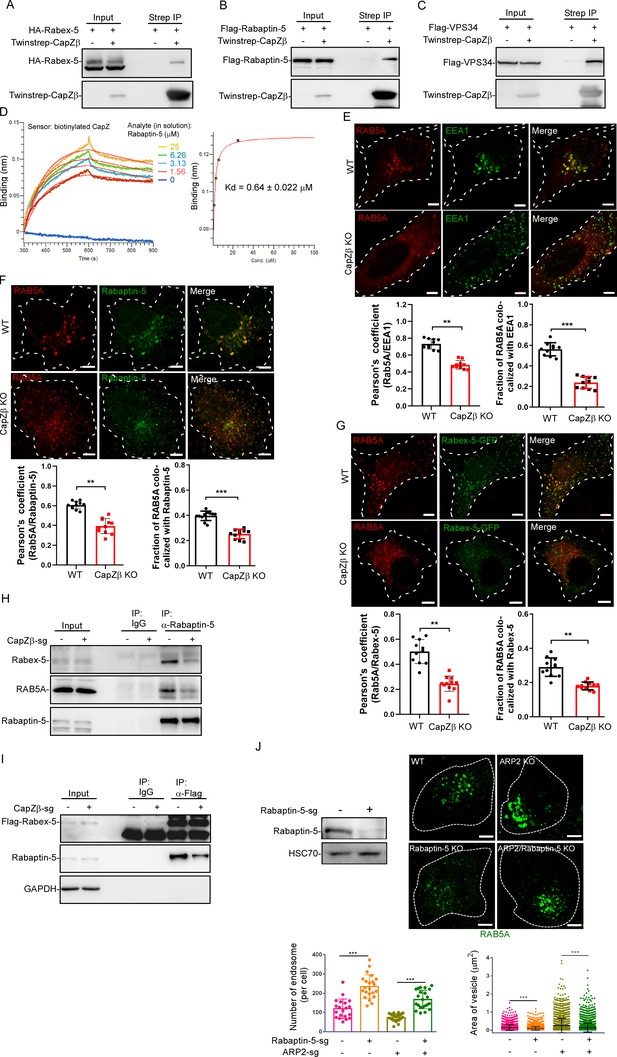
CapZ facilitates the recruitment of RAB5 effectors to early endosomes.
(A) HEK293 cells were transiently transfected with HA-Rabex-5, and/or Twinstrep-CapZβ, and the cell lysates were then incubated with Strep-Tactin Sepharose. The pulldowns were subjected to immunoblotting analysis against CapZβ and Rabex-5. (B, C) HEK293 cells were transiently transfected with Flag-Rabaptin-5 (B), Flag-VPS34 (C), and/or Twinstrep-CapZβ, and the cell lysates were then incubated with Strep-Tactin Sepharose. The pulldowns were subjected to immunoblot analysis against CapZβ, Rabaptin-5, and/or VPS34. (D) Wavelength shift (nm) generated by the addition of Rapaptin-5 at indicated concentrations to biotinylated CapZ (200 μg/ml) immobilized on a streptavidin biosensor. Association was monitored for 5 min, followed by a 5 min dissociation step in assay buffer. Binding affinity constant was generated by fitting both the association and dissociation curves to a 1:1 binding model. (E) Control or CapZβ-knockout HeLa cells were immunostained with an anti-RAB5A or anti-EEA1 antibody, after which the colocalization coefficients (PCC or MCC) of EEA1 and RAB5A were quantified. (F) Control or CapZβ-knockout HeLa cells were immunostained with an anti-RAB5A or anti-Rabaptin-5 antibody. The colocalization coefficients (PCC or MCC) of Rabaptin-5 and RAB5A were quantified. (G) Control or CapZβ-knockout HeLa cells were transiently transfected with Rabex-5-GFP, and subjected to immunostaining with RAB5A antibody. The colocalization coefficients (PCC or MCC) of Rabex-5 and RAB5A were quantified. (H) The lysates of control or CapZβ-knockout cells were immunoprecipitated with an anti-Rabaptin-5 antibody, and the immunoprecipitates were then subjected to immunoblot analysis against Rabex-5, Rabaptin-5, and RAB5A. (I) Control or CapZβ-knockout HeLa cells were stably transfected with Flag-Rabex-5, and the cell lysates were then immunoprecipitated with an anti-Flag antibody. The immunoprecipitates were subjected to immunoblot analysis against Rabex-5 and Rabaptin-5. (J) Control, ARP2-knockout, Rabaptin-5-knockout, or ARP2/Rabaptin-5-double knockout HeLa cells were immunostained with an anti-RAB5A antibody, and the number and size of the early endosomes in these cells were then quantified. The expression of Rapaptin-5 in control or Rapaptin-5-knockout HeLa cells was analyzed by immunoblot analysis. The BLI data (D) represent two independent experiments, and the rest of the blots, images, and graphs represent data from at least three independent experiments. The data are presented as mean ± SD. The difference between the two groups was assessed using two-tailed Student’s t-test. Differences were considered statistically significant when p<0.05. ** p<0.01, and *** p<0.001. Scale bar, 5 μm. BLI, biolayer interferometry; MCC, Manders colocalization coefficient; PCC, Pearson’s correlation coefficient.
-
Figure 5—source data 1
Original immunoblots.
- https://cdn.elifesciences.org/articles/65910/elife-65910-fig5-data1-v2.xlsx
-
Figure 5—source data 2
Data for 5D–5G, 5J.
- https://cdn.elifesciences.org/articles/65910/elife-65910-fig5-data2-v2.xlsx
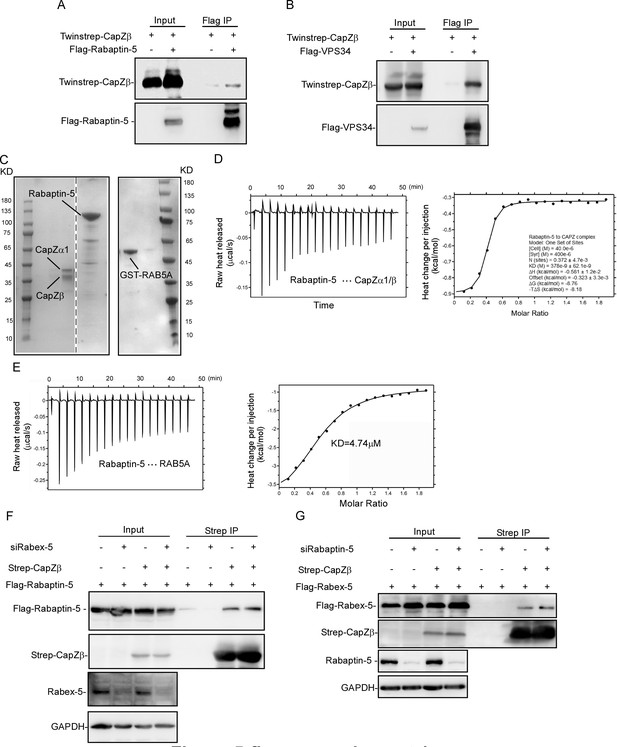
The role of CapZ in the recruitment of RAB5 effectors to early endosomes.
(A, B) Twinstrep-CapZβ with Flag-Rabaptin-5 (A) or Flag-VPS34 (B) were transfected into HEK293 cells. The cell lysates were immunoprecipitated with an anti-Flag antibody, and the pulldowns were subjected to immunoblot analysis against CapZβ, Rabaptin-5, or VPS34. (C) Coomassie staining of recombinant CapZα1-CapZβ, Rabaptin-5, and GST-RAB5 proteins. (D) The interaction between recombinant CapZ (CapZα1-CapZβ) and Rabaptin-5 proteins was assessed by isothermal titration calorimetry (ITC). (E) The interaction between RAB5A and Rabaptin-5 was assessed by ITC. (F) Flag-Rabaptin-5 and Twinstrep-CapZβ-expressing HEK293 cells were transfected with or without Rabex-5 siRNA. The cell lysates were incubated with Strep-Tactin Sepharose, and the pulldowns were subjected to immunoblot analysis against CapZβ and Rabaptin-5. (G) Flag-Rabex-5 and Twinstrep-CapZβ-expressing HEK293 cells were transfected with or without Rabaptin-5 siRNA. The cell lysates were incubated with Strep-Tactin Sepharose, and the pulldowns were subjected to immunoblot analysis against CapZβ and Rabex-5. The blots, images, and graphs represent data from two to three independent experiments.
-
Figure 5—figure supplement 1—source data 1
Original immunoblots.
- https://cdn.elifesciences.org/articles/65910/elife-65910-fig5-figsupp1-data1-v2.xlsx
-
Figure 5—figure supplement 1—source data 2
Data for 1D, 1E.
- https://cdn.elifesciences.org/articles/65910/elife-65910-fig5-figsupp1-data2-v2.xlsx
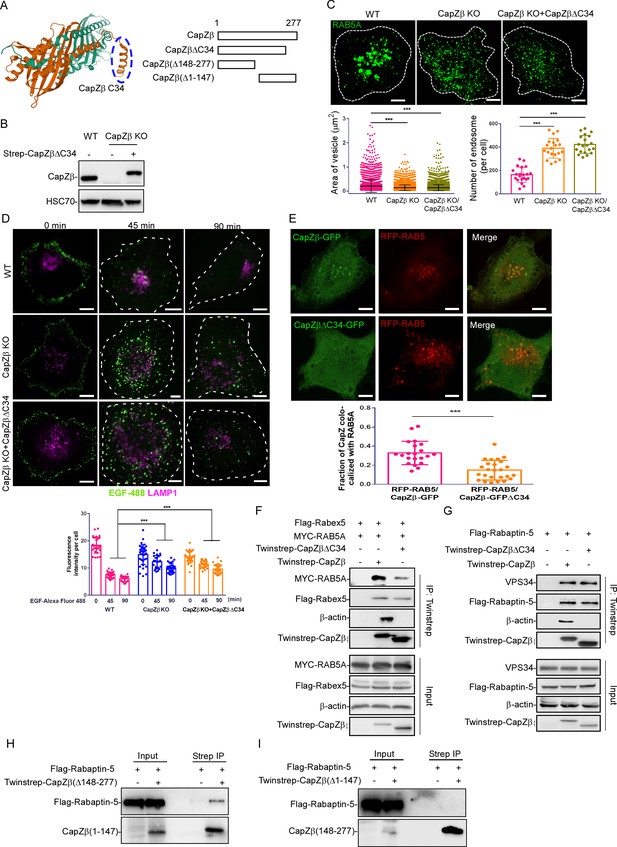
Recruitment of RAB5 effectors to the early endosomes by CapZ depends on its actin capping activity.
(A) The left panel shows the actin-binding motif in the CapZ structure generated from the protein data bank (PDB ID: 1IZN). Cyan color indicated CapZα structure, and brown color indicated CapZβ structure. The right panel shows various CapZβ deletion constructs. (B) The expression of CapZβ in control, CapZβ-knockout, or Twinstrep-CapZβ-ΔC34-reconstituted HeLa cells was determined by CapZβ immunoblotting. (C) Control, CapZβ-knockout, or Twinstrep-CapZβ-ΔC34-reconstituted HeLa cells were stained with an anti-RAB5A antibody. The number and size of the early endosomes in these cells were quantified. (D) Control, CapZβ-knockout, or Twinstrep-CapZβ-ΔC34-reconstituted HeLa cells were treated with EGF-488 for the indicated times, and processed for immunofluorescence analysis. (E) HeLa cells were transfected with RFP-RAB5A, and CapZβ-GFP or CapZβ-ΔC34-GFP, and subjected to confocal imaging 24 hr later. The colocalization coefficients (MCC) of RFP-RAB5A and CapZβ-GFP or RFP-RAB5A and CapZβΔC34-GFP were quantified. (F) HEK293 cells were transfected with Twinstrep-CapZβ or Twinstrep-CapZβ-ΔC34, MYC-RAB5A, and Flag-Rabex-5, and the cell lysates were then incubated with Strep-Tactin Sepharose. The pulldowns were subjected to immunoblotting analysis against the indicated antibodies. (G) HEK293 cells were transfected with Twinstrep-CapZβ or Twinstrep-CapZβ-ΔC34, and Flag-Rabaptin-5, and the cell lysates were then incubated with Strep-Tactin Sepharose. The pulldowns were subjected to immunoblotting analysis against the indicated antibodies. (H, I) HEK293 cells were transfected with Twinstrep-CapZβ(Δ148–277) (H) or Twinstrep-CapZβ(Δ1–147) (I), and Flag-Rabaptin-5, and the cell lysates were then incubated with Strep-Tactin Sepharose. The pulldowns were subjected to an immunoblotting analysis against the indicated antibodies. The blots, images, and graphs represent data from at least three independent experiments. The data are presented as mean ± SD. The difference between the two groups was calculated using two-tailed Student’s t-test. Differences were considered statistically significant when p<0.05. *** p<0.001. Scale bar, 5 μm. MCC, Manders colocalization coefficient.
-
Figure 6—source data 1
Original immunoblots.
- https://cdn.elifesciences.org/articles/65910/elife-65910-fig6-data1-v2.xlsx
-
Figure 6—source data 2
Data for 6C–6E.
- https://cdn.elifesciences.org/articles/65910/elife-65910-fig6-data2-v2.xlsx
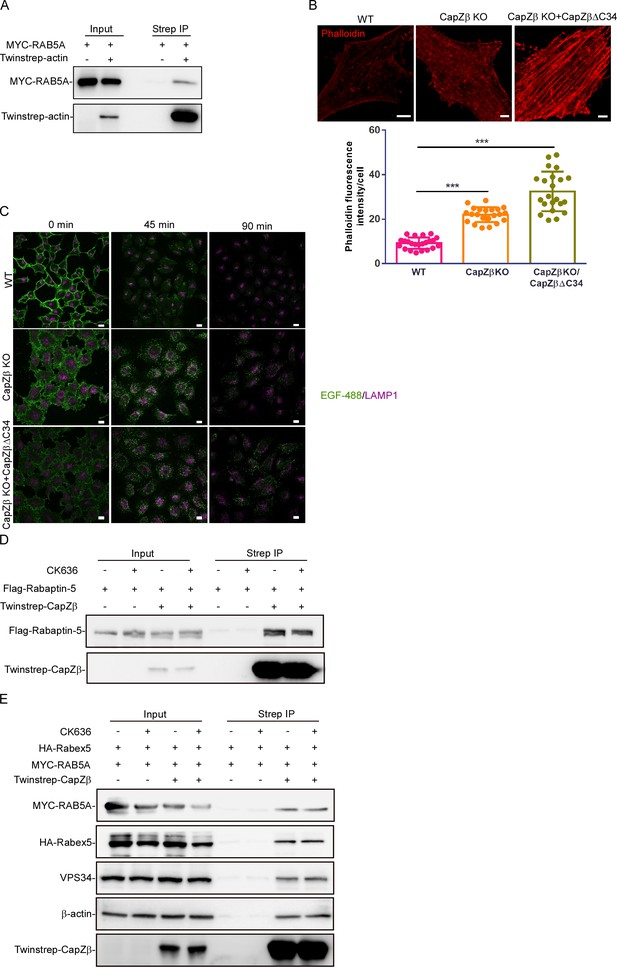
The role of the actin-binding motif of CapZ in endocytosis.
(A) HEK293 cells were transfected with Twinstrep-actin, and Myc-RAB5A, and the cell lysates were then incubated with Strep-Tactin Sepharose. The pulldowns were subjected to immunoblotting analysis against the indicated antibodies. (B) F-actin levels in control, CapZβ-knockout, or Twinstrep-CapZβΔC34-reconstituted HeLa cells were visualized by phalloidin staining. (C) Control, CapZβ-knockout, or Twinstrep-CapZβΔC34-reconstituted HeLa cells were treated with EGF-488 for the indicated times, and processed for immunofluorescence analysis. (D, E) HEK293 cells were transiently transfected with Flag-Rapaptin-5 (D), or MYC-RAB5A/HA-Rabex5 (E), and/or Twinstrep-CapZβ. The cells were then treated with or without CK-636 (200 μM) overnight, and the cell lysates were incubated with Strep-Tactin Sepharose. The pulldowns were subjected to immunoblotting analysis against CapZβ, Rapaptin-5, RAB5A, Rabex5, β-actin, and VPS34. The blots, images, and graphs represent data from at least three independent experiments. The difference between the two groups was calculated using two-tailed Student’s t-test. Differences were considered statistically significant when p<0.05, *** p<0.001. Scale bar, 5 μm.
-
Figure 6—figure supplement 1—source data 1
Original immunoblots.
- https://cdn.elifesciences.org/articles/65910/elife-65910-fig6-figsupp1-data1-v2.xlsx
-
Figure 6—figure supplement 1—source data 2
Data for 1B.
- https://cdn.elifesciences.org/articles/65910/elife-65910-fig6-figsupp1-data2-v2.xlsx
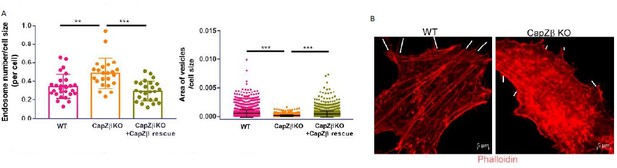
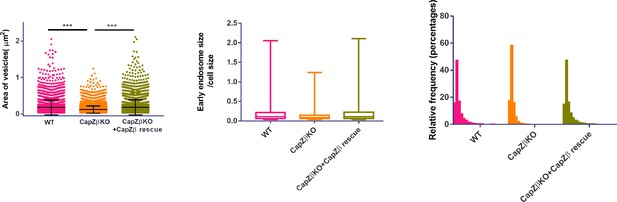
(A) Control or CapZβ-knockout HeLa cells were immunostained with an anti-RAB5A antibody.
The number and size of the early endosomes in these cells were quantified and weighted by the cell size. (B) Control or CapZβ-knockout HeLa cells were immunostained with Red-X Phalloidin; the white dashed lines indicate filopodia.
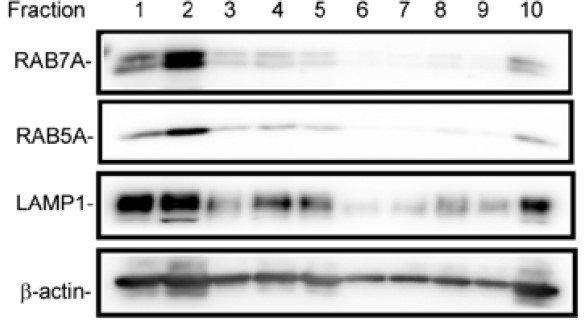
The lysates of control or CapZβ-knockout HeLa cells were subjected to sucrose gradient fractions, followed by respective immunoblot analysis.
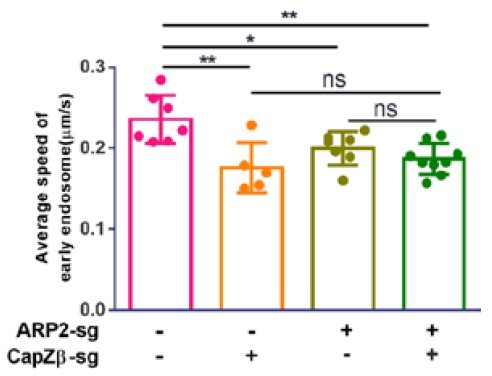
Control, CapZβ-knockout, ARP2-knockout, or CapZβ/ARP2 double-knockout HeLa cells were transiently transfected with RFP-RAB5A, and cells were then imaged without delay for 3 min by time-lapse Nikon high-speed confocal microscopy.
2D track analysis was performed using NIS-Elements AR software.
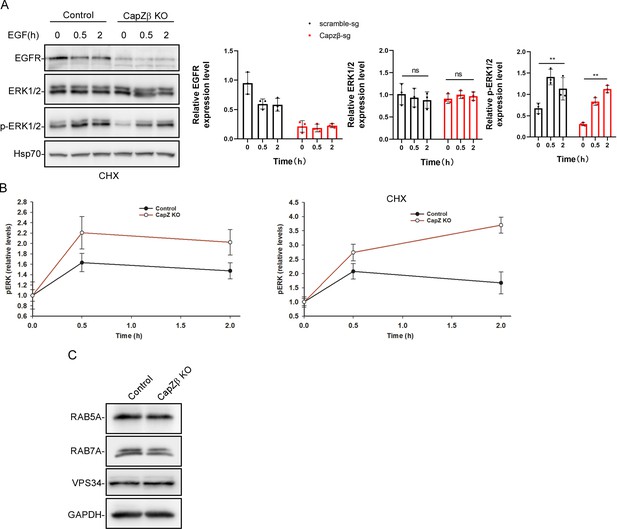
Control or CapZβ-knockout HeLa cells were treated with EGF (100 ng/ml) in the presence of cycloheximide (10 μg/ml) (A).
They were then collected at the indicated time points and subjected to western blot analysis against EGFR, ERK, pERK, and Hsp70. The p-ERKs in EGF-treated control or CapZβ-knockout cells were also normalized over non-treated cells, respectively (B). (C) The expression levels of RAB5A, RAB7A, and VPS34 in control or CapZβ-knockout HeLa cells were determined by western blot analysis.
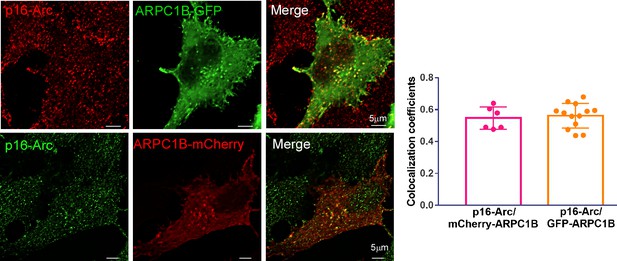
The ARPC1B-mCherry- or ARPC1B-GFP-expressing HeLa cells were stained with anti-p16-Arc antibody, followed by confocal imaging.
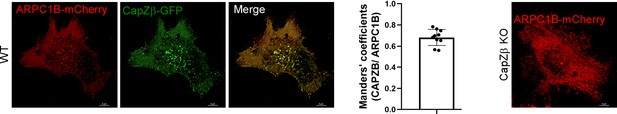
The control or CapZβ-knockout HeLa cells were transiently transfected with ARPC1B-mCherry or CapZβ-GFP, followed by confocal imaging.
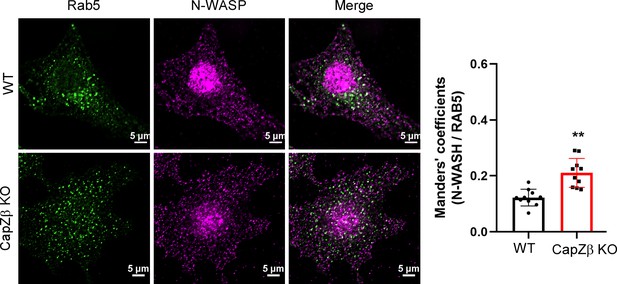
The control or CapZβ-knockout HeLa cells were immunostained with antibodies against RAB5 and N-WASP, followed by confocal imaging.
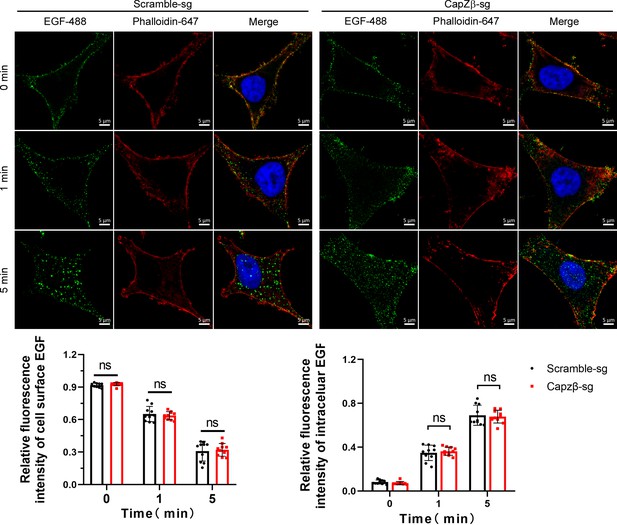
Control or CapZβ-knockout HeLa cells were treated with EGF-488 for the indicated times before fixation and staining with phalloidin-647 and DAPI (without permeabilization), and then processed for immunofluorescence analysis.
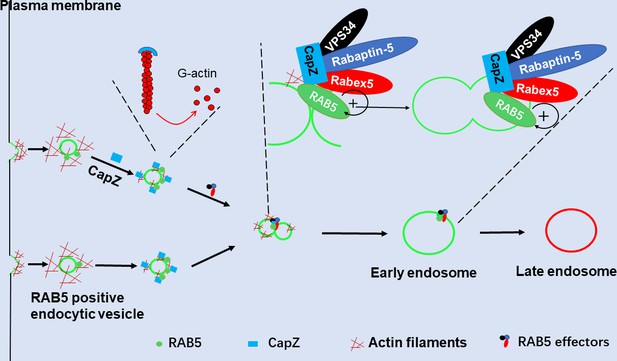
CapZ helps recruit Rabex5 and Rapaptin-5 to RAB5-positive early endosomes, thus establishing a positive feedback loop to further activate RAB5 for the early endosome fusion.
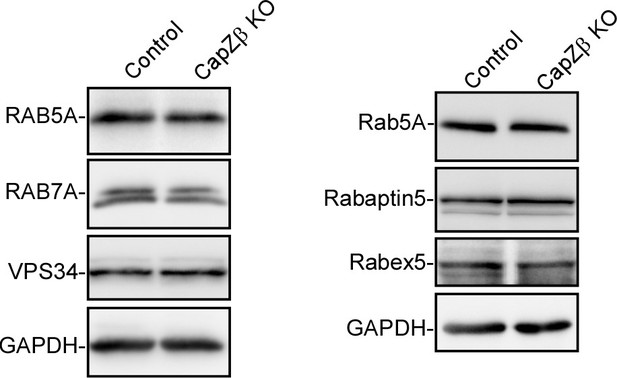
The expression levels of RAB5A, RAB7A, Rabex5, Rabaptin5, and VPS34 in control or CapZβ-knockout HeLa cells were determined by western blot analysis.
Videos
RAB5-positive endosomes movements in wild-type HeLa cells.
RAB5-positive endosomes movements in CapZb-knockout HeLa cells.
RAB5-positive endosomes movements in ARP2-knockout HeLa cells.
RAB5-positive endosomes movements in CapZb-ARP2 double knockout HeLa cells.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Antibody | APRC1B (Rabbit polyclonal) | Sigma-Aldrich | HPA004832 | IF (1:100) |
| Antibody | ARP2 (Rabbit polyclonal) | ProteinTech | 10922-1-AP | WB (1:2000) |
| Antibody | Pan Actin (Mouse monoclonal) | Cytoskeleton | AAN02-s | IF (1:500) |
| Antibody | CapZα (Mouse monoclonal) | DSHB | mAb 5B12.3 | WB (1:100) |
| Antibody | CapZβ (Rabbit polyclonal) | ProteinTech | 25043-1-AP | WB (1:2000) |
| Antibody | EGFR (Mouse monoclonal) | Santa Cruz | sc-373746 | WB (1:100) |
| Antibody | EEA1 (Rabbit monoclonal) | CST | 3,288 | IF (1:100) |
| Antibody | Flag-tag (Mouse monoclonal) | Sigma-Aldrich | F3165 | WB (1:1000) |
| Antibody | HA-tag (Rat monoclonal) | Sigma-Aldrich | 11867423001 | WB (1:3000) |
| Antibody | His-tag (Mouse monoclonal) | ProteinTech | 66005-1-Ig | WB (1:3000) |
| Antibody | HSC70 (Mouse monoclonal) | Santa Cruz | sc-7298 | WB (1:10,000) |
| Antibody | LAMP1 (Rabbit monoclonal) | CST | 9,091 | IF (1:200) |
| Antibody | MYC-tag (Mouse monoclonal) | ProteinTech | 67447-1-Ig | WB (1:3000) |
| Antibody | N-WASP (Rabbit polyclonal) | Novus Biologicals | NBP1-82512 | IF (0.25–2 µg/ml) |
| Antibody | p16-Arc (Rabbit monoclonal) | Abcam | ab51243 | IF (1:100) |
| Antibody | Rabaptin-5 (Rabbit polyclonal) | ProteinTech | 14350-1-AP | WB (1:1000) |
| Antibody | Rabex-5 (Mouse monoclonal) | Santa Cruz | sc-166049 | WB (1:500) |
| Antibody | StrepMAB-Classic HRP conjugate (Mouse monoclonal) | IBA | 2-1509-001 | WB (1:3000) |
| Antibody | VPS8 (Rabbit polyclonal) | ProteinTech | 15079-1-AP | WB (1:1000) |
| Antibody | RAB5A (Rabbit polyclonal) | ProteinTech | 11947-1-AP | WB (1:1000) |
| Antibody | RAB5A (Rabbit monoclonal) | CST | 3547 | IF (1:200) |
| Antibody | RAB5A (Mouse monoclonal) | CST | 46449 | IF (1:200)WB (1:1000) |
| Chemical compound, drug | CK-636 | Selleck | S7497 | |
| Chemical compound, drug | Latrunculin A | Santa Cruz | sc-202691 | |
| Chemical compound, drug | EGF | Bio-Rad | PHP030A | |
| Chemical compound, drug | GDP-β-S | Sigma-Aldrich | G7637 | |
| Chemical compound, drug | GTP-γ-S | Jena Bioscience | NU-412-2 | |
| Other | Transferrin-Alexa Fluor 594 | Invitrogen | T13343 | (25 μg/mL) |
| Other | EGF-Alexa Fluor 488 | Invitrogen | E13345 | (1 μg/ml) |
| Other | Phalloidin-iFluor 647 | Abcam | ab176759 | (1:1000) in 30 μl stock solution |
| Other | Texas Red-X Phalloidin | Invitrogen | T7471 | (1:40) in 1.5 ml stock solution |
| Cell line (Homo sapiens) | HeLa cells | ATCC | CRL-CCL-2 | |
| Cell line (H. sapiens) | HEK293 cells | ATCC | CRL-1573 |
Plasmids used in this study.
| pCDNA3.1-Flag-Rabaptin-5 | pRK5-mRFP-FYVE | pCDNA3.1-HA-Rabex-5 |
| pCDNA3.1-MYC-RAB5A | plenti-CapZβ-GFP | pCDNA3.1-CapZβ-His6 |
| pRP-CapZβ-TwinStrep | pGEX-4-R5BD | pCDNA3.1-Flag-VPS34 |
| pCDNA3.1-Twinstrep-RAB5A | iRFP-FRB-RAB5A | CFP-FKBP-CapZβ |
| mRFP-RAB5A | pAGW-GFP-RAB5A | plenti-CapZβ-mcherry |
| pGEX4T-1-RAB5A | pGEX4T-1 | plenti-Flag-Rabex-5 |
| Plenti-GFP-ML1N2* | plenti-CapZβ | pRSFDuet-1-CapZβ/CapZα1 |
| plenti-CapZβ-Twinstrep | psPAX2 | pMD2.G |
| pGEX4T-1-Rabaptin-5 | pCDNA3.1-β-actin-Twinstrep | plenti-CapZβ(1-147)-Twinstrep |
| plenti-CapZβ(148-277)-Twinstrep | mCherry-ARPC1B |