Dynamic dichotomy of accumbal population activity underlies cocaine sensitization
Figures
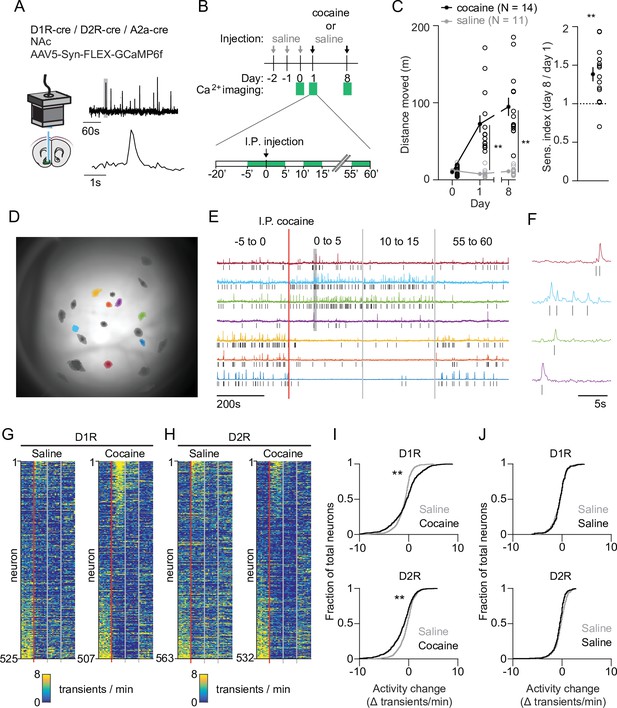
Acute changes in accumbal neuronal activity in response to cocaine.
(A) Schematic of calcium imaging setup. Inset graph shows calcium recording trace, gray line indicates zoomed area in lower graph. Top trace horizontal bar is 60 s, bottom trace horizontal bar is 1 s. (B) Schematic of behavioral setup. Green squares indicate recording days (top) and 5-min recording windows (bottom). (C) Left panel: distance moved 1 hr after I.P. injection in mice that either receive cocaine (N = 14) or saline control (N = 11) injections, for two sessions separated by 1 week. Average responses (mean ± S.E.M.): cocaine group: day 1: 72.5 ± 11.2 m, day 8: 94.8 ± 11.8 m. Saline group: day 1: 7.8 ± 1.6 m, day 8: 11.0 ± 1.5 m. Repeated measures ANOVA: Days x Treatment: F (2,46) = 30.74, Bonferroni-corrected post hoc T-test: ** = p < 0.01. Right panel: sensitization index in cocaine treated animals. Average response (mean ± S.E.M.): sensitization index 1.38 ± 0.09. Paired T-test: p = 1.2 x 10–3. ** = p < 0.01 (D) Field of view of a single animal (D1R-cre), overlaid with a subset of neurons recorded during a single cocaine-treated session. (E) Temporal trace (CNMF-E label ‘C_raw’) of highlighted neurons in panel (D), showing diverse activity responses to first cocaine injection within the same D1R-cre mouse. Raw traces are rescaled to their own maximum. Tick marks indicate detected calcium transients. (F) Zoom of highlighted area (gray box) in panel (F), showing calcium signals over time. Tick marks indicate detected calcium transients. (G) Calcium transient frequency heatmap of all neurons recorded in D1R-cre cocaine treated animals during either saline (left) or cocaine (right) treatment. Data is sorted by amplitude of change in activity. N = 7 animals. (H) Same as panel (G) but for D2R-cre cocaine treated animals. N = 7 animals. (I) Top panel: Cumulative distribution of activity change (−5 to 0 vs 0 to 5) minutes after I.P. injection in D1R neurons of the cocaine-treated groups around either saline (grey) or cocaine (black) treatment. Saline: n = 525 neurons, cocaine n = 507 neurons. Two-sample Kolmogorov–Smirnov test: p = 1.3 x 10–10. Bottom panel: same but for D2R neurons. Saline: n = 563 neurons, cocaine n = 532 neurons. Kolmogorov-Smirnov test: p = 1.3 x 10–07. ** = p < 0.01 (J) Top panel: cumulative distribution of activity change (−5 to 0 vs. 0 to 5 min around I.P. injection) in D1R neurons of control animals treated only with saline on 2 separate days. First saline: n = 273 neurons, second saline: n = 235 neurons. Kolmogorov-Smirnov test: p = 1. Bottom panel: same but for D2R neurons. First saline: n = 231 neurons, second saline: n = 256 neurons. Kolmogorov-Smirnov test: p = 0.054. Kolmogorov-Smirnov tests are corrected for multiple comparisons.
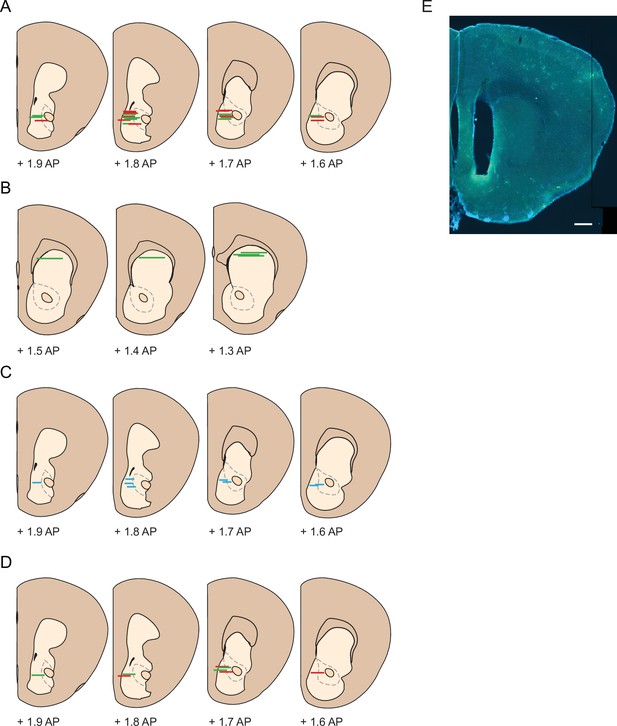
Schematic overview of fiber / GRIN placement for different experimental groups.
(A) GRIN lens placement for animals used in cocaine sensitization experiment (Figures 1—4) N = 13 D1R-cre animals, N = 12 D2R/A2a-cre animals. Green lines indicate D1R-cre, red lines indicate D2R/A2a-cre. (B) GRIN lens placement for dStr animals (Figure 4). N = 5 D1R-cre animals. (C) Optic fiber placement for animals used in dLight experiment (Figure 5). N = 9 wildtype animals. (D) GRIN lens placement for animals used in SL327 manipulation experiment (Figure 6). N = 4 D1R-cre animals, N = 4 D2R-cre animals. Green lines indicate D1R-cre, red lines indicate D2R-cre. Schematics are modified from Paxinos and Franklin (Franklin and Paxinos, 1997). (E) Representative photograph with the fiber track lesion. Scale bar 500 µm.
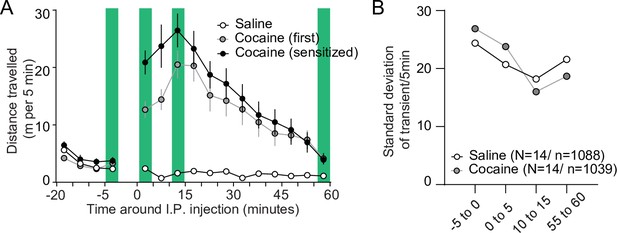
Diverse movement and calcium activity responses after cocaine.
(A) Average distance moved over 5-min bins around saline/cocaine injections in the cocaine-treated group. Green bars indicate moments of calcium imaging. N = 14 animals. Spread shows S.E.M. (B) Standard deviation over the number of transients in each 5-min recording window over all SPNs recorded in cocaine-treated animals during either saline or first cocaine treatment. N = number of animals, n = number of cells.
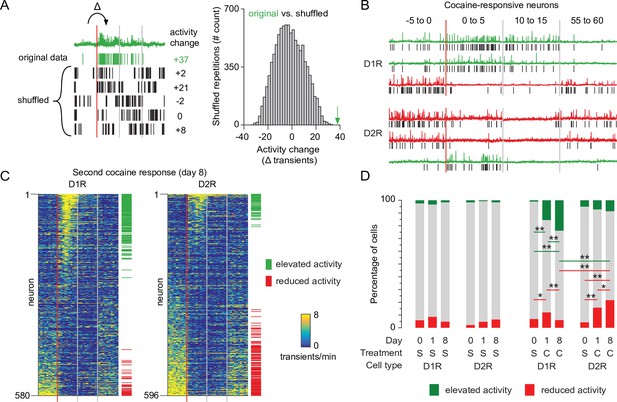
Recruitment of cocaine-responsive neurons during cocaine sensitization.
(A) Schematic overview of methodology to identify responsive neurons. Calcium transients are detected from traces (green trace shows raw trace, green ticks show calcium transients), and activity change around cocaine injection is compared to shuffled sequences of the same inter-transient intervals (bottom left and histogram). Vertical lines indicate edges of five-minute recording windows, red line indicates cocaine injection. Green arrow indicates non-shuffled result. (B) Calcium traces of example D1R (top three) or D2R (bottom three) neurons that either show significant increased (green) or decreased (red) activity to cocaine. Vertical red line indicates cocaine injection moment, red and gray lines indicate edges of recording periods. Black ticks indicate detected calcium transients. (C) Calcium transient frequency heatmap of all neurons recorded around second cocaine injection in either D1R-cre mice (left, n = 580 neurons from seven animals) or D2R-cre mice (right, n = 596 neurons from seven animals). Neurons are sorted by their response to cocaine, green and red ticks indicate significantly responsive neurons. (D) Fraction of responsive neurons among D1R-cre and D2R-cre neurons in saline control or cocaine-treated animals. S = saline. C = cocaine. D1R control n = 273/235/229 neurons (day 0 /day 1 /day 8) from 6 mice. D2R control n = 231/256/231 neurons from 5 mice. D1R cocaine n = 525/507/580 neurons from 7 mice. D2R cocaine n = 563/532/596 neurons from 7 mice. Fishers Exact test: D1R cocaine group, elevated activity: day 0 vs day 1: p = 5.9 x 10–19, day 1 vs day 8: p = 1.9 x 10–3, day 0 vs day 8: 1.5 × 10–34. Reduced activity: day 0 vs day 1: p = 1.6 x 10–2, day 1 vs day 8: p = 1.7 x 10–3. D2R cocaine group reduced activity: day 0 vs day 1: p = 3.1 x 10–10. day 1 vs day 8: p = 4.4 x 10–2. Day 0 vs day 8: p = 3.4 x 10–19. All other intra-group comparisons p > 0.05. Day 8 cocaine, D1R vs D2R: elevated activity, p = 9.0 x 10-13, reduced activity, p = 4.1 x 10-15. * = p < 0.05, ** = p < 0.01.

Analysis of the cocaine response, accounting for individual animals.
(A) Standard deviation of the activity change (i.e. Δ transients/min = (number of transients within 5 min after injection – number of transients within 5 min before injection)/5 min), for all animals across days. N = 6/5/7/7. S = saline, C = cocaine. (B) Analysis of the cocaine response with a linear mixed model, taking into account all cells and individual animals. Box plots (minimum, maximum, median, 25th and 75th percentile) represent the ratios of activity (number of transients within 5 min after injection +10) / (number of transients within 5 min before injection +10) for all neurons, with a log2 transformation to approach normality. Linear mixed model of the log2(ratio of activity) as a function of time or cell type, and individuals as random effect. Post-hoc Tukey test: D1R cocaine day 0 vs day 1, p < 10–4, day 0 vs day 8, p = 3 × 10–4, day 1 vs day 8, p < 10–4; D2 R cocaine day 0 vs day 1, p < 10–4, day 0 vs day 8 p < 10–4, day 1 vs day 8 p = 6 × 10–4; D1R cocaine vs D2R cocaine day 8, p = 3.7 × 10–2. All saline intra-group comparison p > 0.05. D1R saline (N = 6 animals, n = 19–66 neurons per animal per day, ntotal(days 0/1/8) = 273/235/229), D2R saline (N = 5 animals, n = 24–76 neurons per animal per day, ntotal(days 0/1/8) = 231/256/231), D1R cocaine (N = 7 animals, n = 50–134 neurons per animal per day, ntotal(days 0/1/8) = 525/507/580), D2R cocaine (N = 7 animals, n = 51–147 neurons per animal per day, ntotal(days 0/1/8) = 563/532/596). * = p < 0.05, ** = p < 0.01, S = saline, C = cocaine.
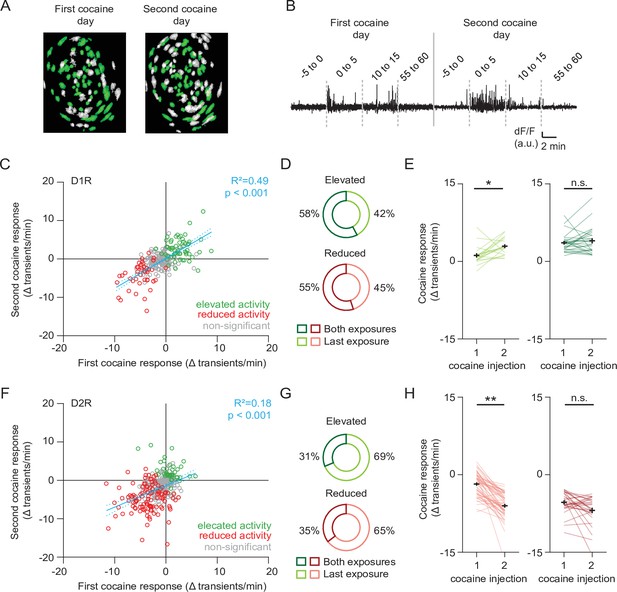
Profiling cocaine responsive neurons over cocaine treatments.
(A) Example of identified neurons on two different sessions in the same animal. Green indicates neurons present on both session (see Methods). (B) Example trace of a single neuron that was present during both cocaine-treatment days. (C) Scatter plot showing activity change of D1R neurons during the first and second cocaine injections. Neurons that are cocaine responsive on at least one session are highlighted (green – increased activity, red decreased activity). Blue line shows linear correlation across all neurons (dotted line is 95% C.I.) Pearson’s r = 0.77, p = 2.8 × 10–38, n = 251 neurons from 7 mice. (D) Pie chart showing fraction of D1R neurons with increased activity on second cocaine exposure day, that were either responsive during both sessions (dark colors), or non-significantly responding during the first cocaine exposure (light colors). Neurons per group: elevated activity: both exposures n = 26/45, last exposure n = 19/45. Reduced activity: both exposures n = 11/20, last exposure n = 9/20. (E) Activity change between cocaine-treated days among D1R neurons that increase their activity following cocaine: those that are non-responsive during the first cocaine exposure and are responsive during the second exposure (left), and neurons that are responsive to cocaine on both days (right). Wilcoxon signed-rank test: left panel: n = 19, p = 0.014, right panel: n = 26, p = 0.80. * = p < 0.05. (F) Same as (C) but for D2R neurons that decrease their activity following cocaine. Pearson’s r = 0.56, p = 1.4 x 10–15, n = 330 neurons from 7 mice. (G) Same as (D) but for D2R neurons that reduce their activity following cocaine. Neurons per group: elevated activity: both exposures n = 10/32, last exposure n = 22/32. Reduced activity: both exposures n = 28/79, last exposure n = 51/79. (H) Same as (E) but for D2R neurons. Wilcoxon signed-rank test: left panel: n = 51, p = 2.3 x 10–9, right panel: n = 28, p = 0.08. ** = p < 0.01.
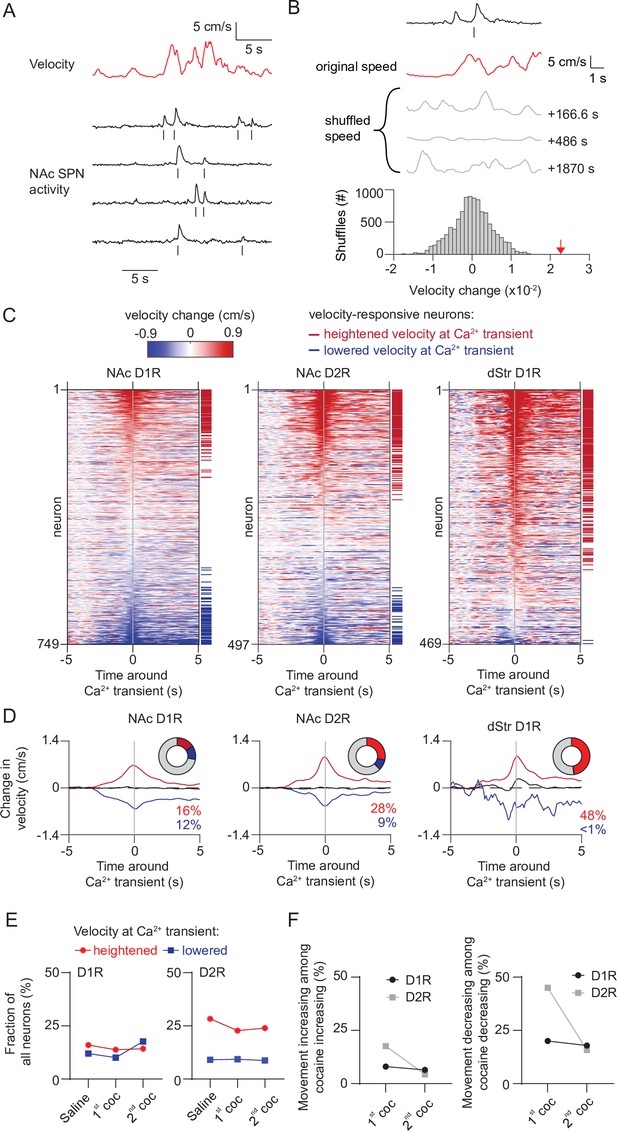
Movement-related activity of NAc neurons does not change during cocaine sensitization.
(A) Example trace showing velocity over time and the activity of D1R neurons recorded during a saline-treated session. Ticks below each trace indicate detected calcium transients. (B) Schematic overview of methodology to identify neurons correlated to velocity. Calcium transients are detected from traces and velocity change around each transient is computed, and then averaged per neuron. Alignment is then compared to temporally shifted shuffled data (where the velocity is offset by a random time period) to identify movement-correlating neurons (see Materials and methods). Gray lines indicate examples of temporally shifted data, and histogram shows distribution of this data. Red arrow indicates non-shuffled result. (C) Heatplot showing average velocity change aligned to calcium transients in either NAc D1R neurons (left), NAc D2R neurons (middle) or dStr D1R neurons (right) under saline injection conditions. Neurons are sorted by their movement response amplitude. Horizontal red and blue lines indicate significantly velocity-responsive neurons. (D) Average activity over time of neurons correlated with either heightened (red), non-significant (black) or lowered (blue) movement at the time of the calcium transient under saline conditions. Pie chart insets shows fraction of total neurons with significant response. (E) Fraction of neurons correlated to movement over days. (F) Left: fraction of neurons correlated to increases of movement among cocaine-responsive neurons with elevated activity. Right: fraction of neurons correlated to decreases of movement among cocaine-responsive neurons with reduced activity.
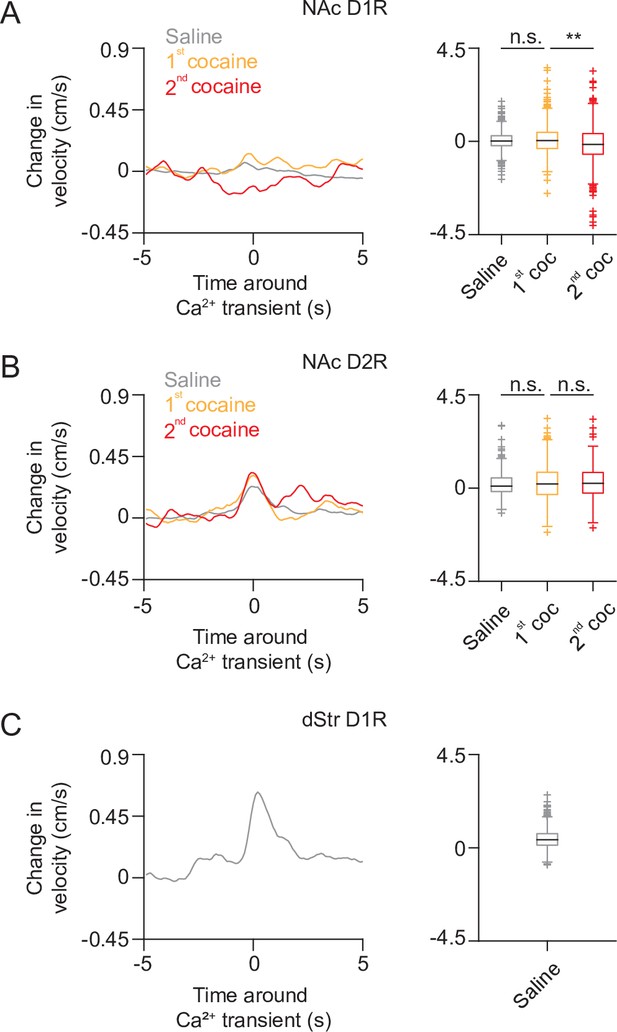
Movement around calcium transients in all recorded neurons over days.
(A) Left Panel: average movement velocity change over time around calcium transient for all NAc D1R SPNs, during saline, or cocaine-treated sessions. Right panel: box plot of the average movement velocity around calcium transient for NAc D1R SPNs. Saline: n = 724 neurons from 12 mice, first cocaine: n = 456 from 6 mice, second cocaine: n = 504 from 6 mice. (B) Same as (A) but for NAc D2R. Saline: n = 487 neurons from 8 mice, first cocaine: n = 268 neurons from 4 mice, second cocaine: 309 neurons from 4 mice. (C) Same as (A) but for D1R neurons recorded in the dStr of saline treated mice. Saline: n = 469 neurons from 5 mice. Box plots center line shows median, box indicates interquartile range (IQR), whiskers extend to extreme values not considered outliers. Outliers (plus signs) are defined as values more than 1.5 time the IQR away from the box.
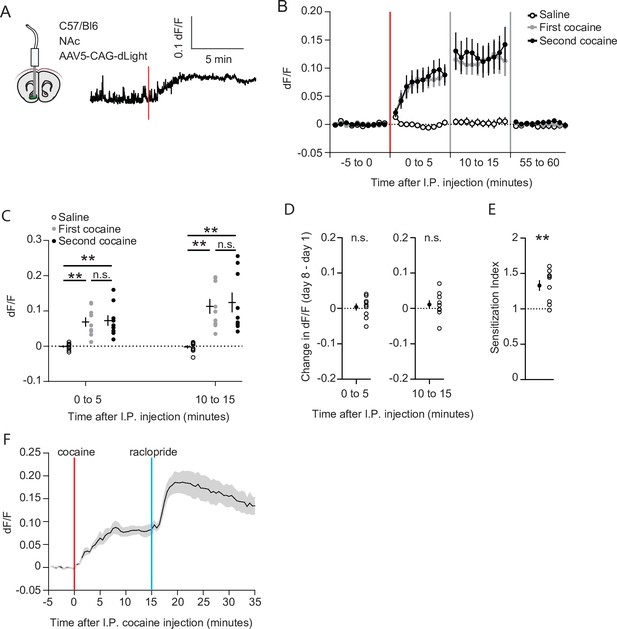
Cocaine-evoked NAc dopamine levels remain stable during cocaine sensitization.
(A) Experimental setup. Wild-type mice were injected with an AAV9 CAG-dLight construct in the NAc, and an optic fiber was placed above the area. Trace represents dLight fluorescence over time. Vertical red line indicates moment of cocaine injection. (B) Average dF/F over time around I.P. injection of either saline or cocaine. Red line indicates cocaine injection. N = 9 mice. (C) Average dF/F over 5-min periods. Greenhouse-Geisser corrected Repeated measures ANOVA F (1.100, 8.802) = 8.816, p = 0.015. Bonferroni-corrected two-tailed paired students T-test: 0–5 min: saline vs first cocaine: p = 1.6 x 10–3, saline vs. second cocaine: p = 3.5 x 10–3, first cocaine vs second cocaine p > 0.99. 10–15 minutes: saline vs first cocaine: p = 1.7 x 10–3, saline vs second cocaine: p = 6.2 x 10–3, first cocaine vs second cocaine: p > 0.99. ** = p < 0.01. (D) Within animal change of five-minute average dF/F between cocaine exposures. Paired students T-test: 0–5 minutes: p = 0.71, 10–15 min: p = 0.39. (E) Sensitization index of the same animals shown in panels B-D. Paired students T-test: p = 2.5 x 10–3. ** p < 0.01. (F) Average dF/F over time around I.P. injection of sequential cocaine (20 mg/kg, I.P.) and D2 antagonist raclopride (0.1 mg/kg, I.P.). N = 5 mice.
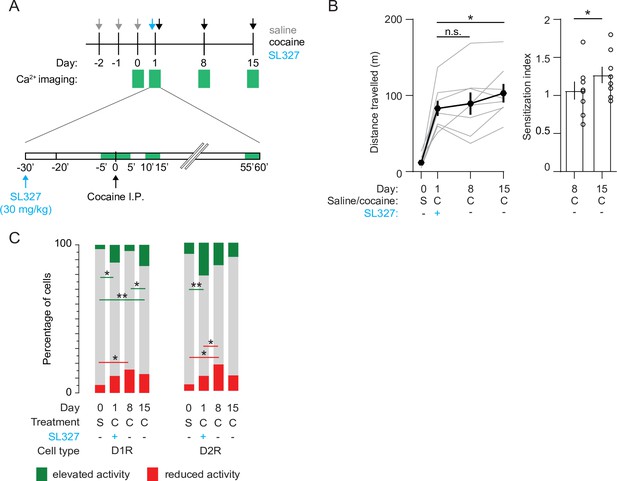
Blocking ERK signaling pathway decreases the fraction of sensitized D1R SPN responses, leaves sensitized D2R SPN responses unaffected, and abolishes LS.
(A) Schematic overview of experimental setup. Animals are pre-treated with SL327 (30 mg/kg) 30 min before the first cocaine injection. Green squares indicate recording days (top) and 5-min recording windows (bottom). (B) Left panel: average distance travelled among recorded animals over different days. Paired student T-tests: day 8 vs day 1, p = 0.6, day 15 vs day 1, p = 0.02. Right panel: sensitization index on day 8 versus day 15. Spread shows S.E.M. Students T-test, p = 0.04, N = 8 mice. * = p < 0.05, n.s. = non significant. (C) Fraction of significantly responsive D1R and D2R neurons over days. D1R n = 205/192/185/181 neurons (day 0 /day 1 /day 8 /day 15) from 4 mice. Fishers Exact test: elevated activity: day 0 vs day 1: p = 1.6 x 10–2, day 8 vs day 15: p = 2.7 x 10–2, day 0 vs day 15: p = 5.2 x 10–4. Reduced activity: day 0 vs day 8: p = 1.4 x 10–2. All other comparisons p> 0.05. D2R n = 141/153/160/176 neurons (day 0 /day 1 /day 8 /day 15) from 4 mice. Fishers exact test, elevated activity: day 0 vs day 1: p = 3.4 x 10–3, reduced activity: day 0 vs day 8: p = 4.1 x 10–2, day 1 vs day 8: p = 4.5 x 10–2. All other intra-group comparisons p > 0.05. * = p < 0.05, ** = p < 0.01. S = saline, C = cocaine.
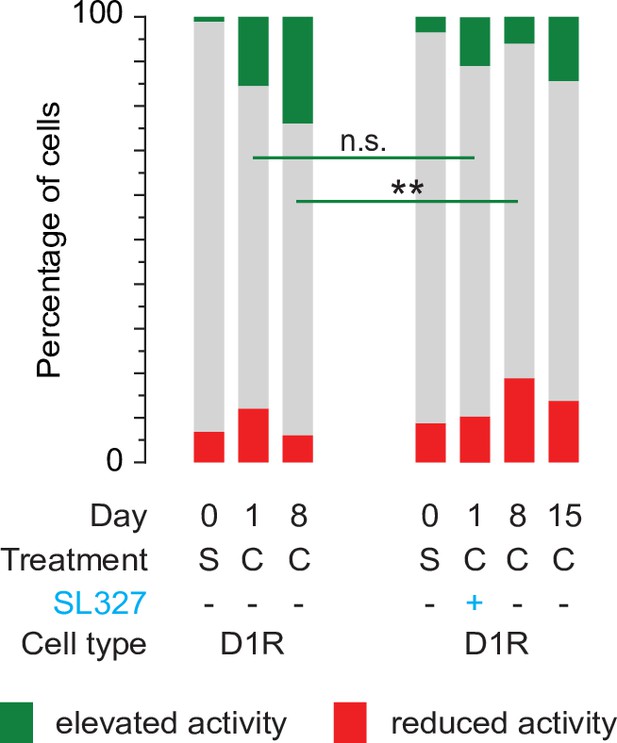
Blocking ERK signaling pathway prevents D1R sensitization on day 8.
Fraction of significantly responsive D1R neurons over days, with and without SL327 treatment (comparison of data presented in Figures 2D and 6C). D1R cocaine without SL327 n = 525/507/580 neurons from 7 mice. D1R cocaine with SL327 n = 205/192/185/185 neurons from 4 mice. Fishers Exact test: day 1, p = 0.15; day 8, p = 1.4 x 10–7. ** = p < 0.01. S = saline, C = cocaine, n.s. = non significant.





