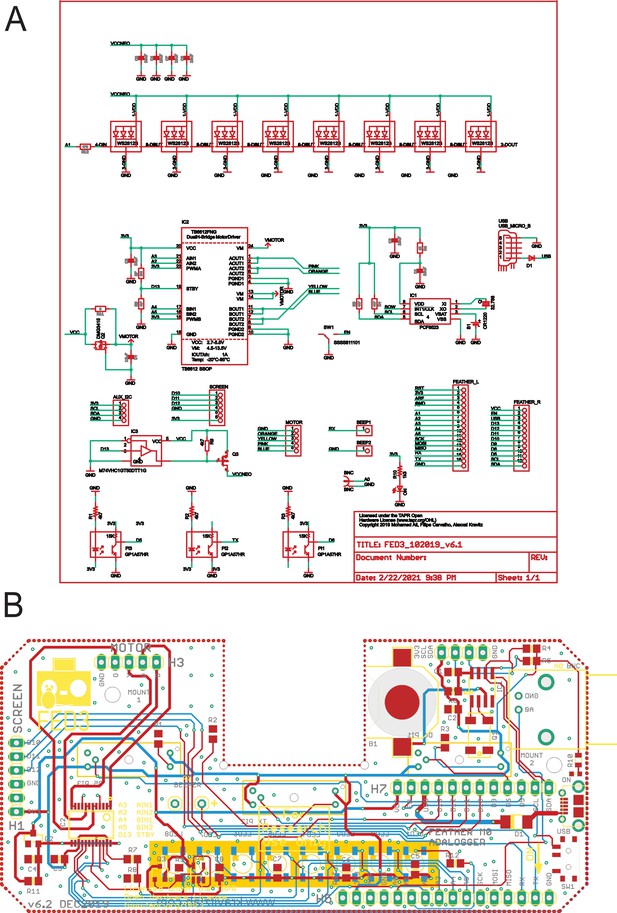
An open-source device for measuring food intake and operant behavior in rodent home-cages
Figures
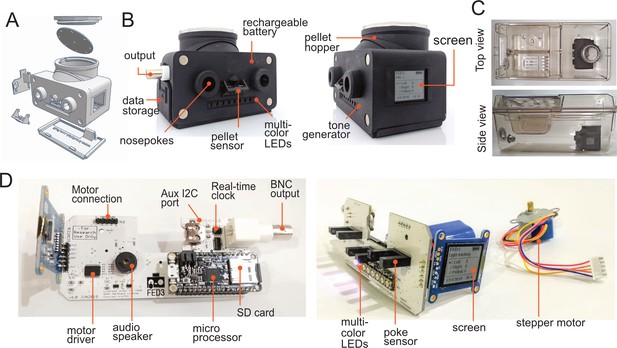
Assembly of FED3.
(A) Exploded view schematic and (B) photos of FED3 with main components highlighted. (C) Assembled FED in an Allentown NextGen home-cage, top view, and side view. (D) Back view (left) of assembled and populated FED3 PCB, and side view (right) of assembled FED3 electronics.
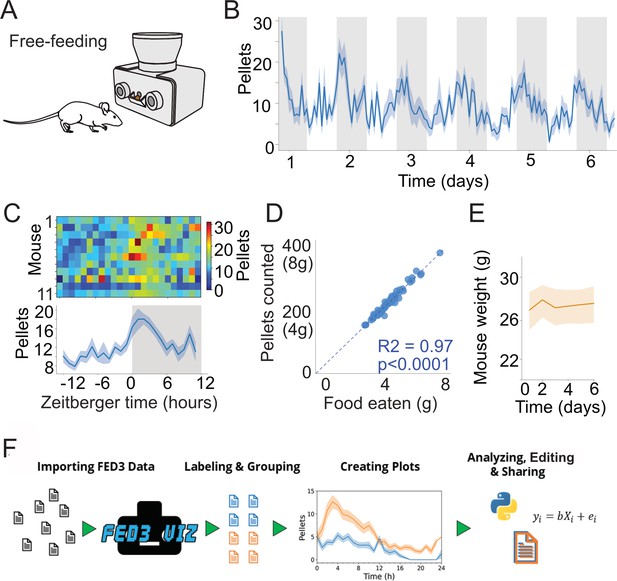
FED3 tracks food intake.
(A) Schematic of FED3 in free-feeding mode. (B) Pellets per hour across six consecutive days. Shaded areas indicate dark cycle. (C) Chronograms of pellets eaten in heatmap (top) and line plot. (D) Regression of the calculated weight of the pellets recorded by FED vs. the measured difference in weight of the FED between days. (E) Mice weight across time during free feeding. (F) Schematic of FED3VIZ workflow. Data is shown as means ± SEM in (B, C, E).
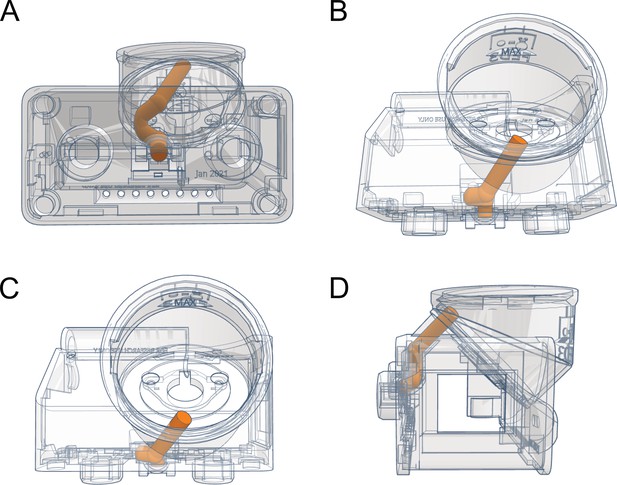
FED3 pellet delivery cute.
A specially designed release chute is curved to prevent pellets from ricocheting at the end of the chute, and to prevent jamming. 3D renderings with pellet chute highlighted in orange from front (A), top angled (B), top (C), and side (D) view.
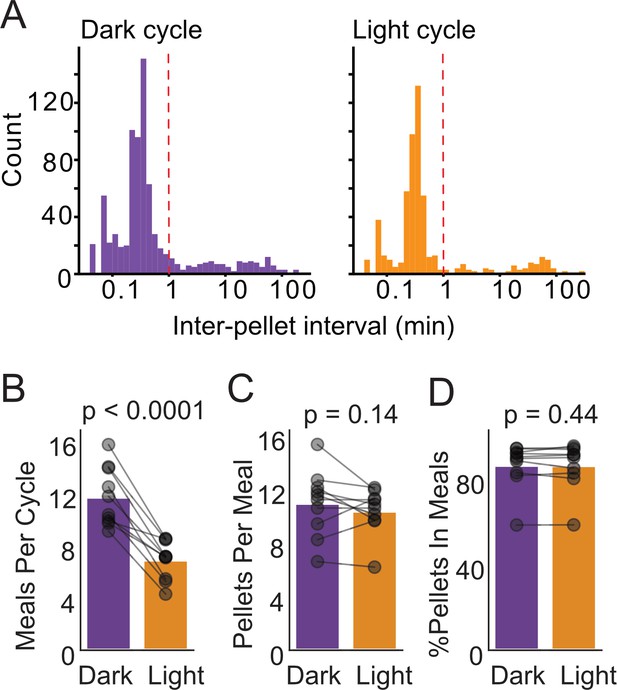
Meal analysis with FED3.
(A) Inter-pellet interval histograms for dark and light cycle feeding. (B) Meals per day, (C) pellets per meal, and (D) % of pellets within meals for dark and light cycle feeding. N = 10 mice, paired t-tests.
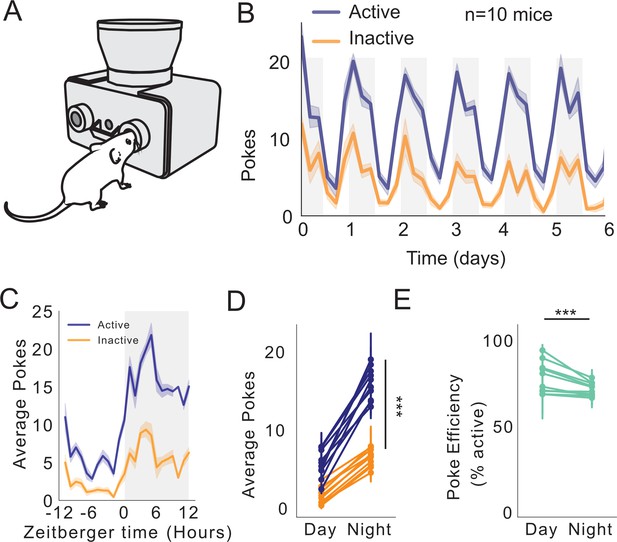
FED3 reveals circadian feeding patterns.
(A) Schematic of FED3 in FR1 mode. (B) Active (blue) and inactive (orange) pokes over 6 days. n = 10 mice. (C) Average pokes (active, blue; inactive, orange) over 24 hr cycle. (D) Average pokes during light and dark cycles. Significant interaction between day/night and average pokes (F(1,792)=85.225, p<0.0001); significant effect of day/night (F(1,792)=668.700, p<0.0001); significant effect of active or inactive pokes (F(1,792)=610.824, p<0.0001). Fitted linear model for two-way ANOVA, (F(3,792)=454.917, p<0.0001.) (E) Average poke efficiency (p=0.0001, student’s t-test).
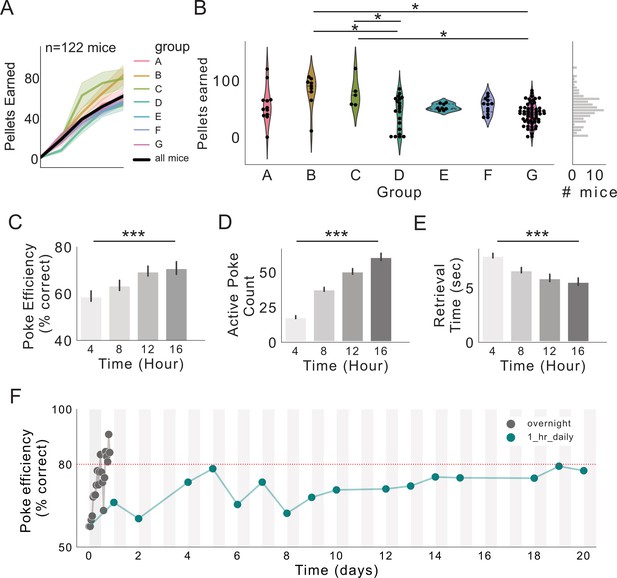
FR1 acquisition across seven research sites.
(A) Pellets earned over first 16 hr of exposure to FED3 at seven research sites. (B) Scatterplots and kernel density estimation plots showing pellets earned after 16 hr with FED3, effect of group (F(6,115)=6.4223, p<0.0001), significant post hoc differences between groups B and D (p=0.001), B and G (p=0.001), C and D (p=0.030), and C and G (p=0.010). (C) Poke efficiency across the session. Significant effect of time, p=0.0007, F(3,386)=5.79. (D) Active pokes across the session. Significant effect of time, p=0.0001, F(3, 393)=115.2. (E) Retrieval time across the session. Significant effect of time, p=0.0001, F(3,351)=14.02. (E) Linear models with Tukey post-tests were conducted in (C-E). (F) Poke efficiency across continuous 16 hr sessions (gray line, n = 122 mice) and across multiple days with 1 hr sessions each day (teal line, n = 11 mice). Two-way ANOVA revealed significant effect of time (p<0.0001), no significant effect of group or interaction. F(7,500)=3.974.
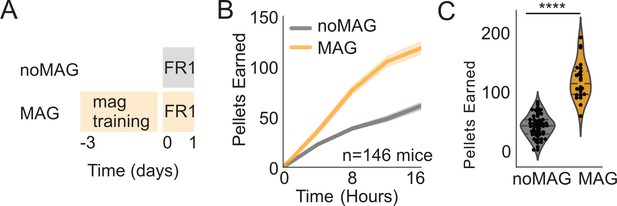
Effect of magazine training.
(A) Schematic showing paradigm for magazine trained group (MAG) vs. no magazine training (noMAG). (B) Active pokes over time during first exposure to FED3 FR1 between noMAG and MAG groups. (C) Scatterplots and kernel density estimation plots showing distribution of active poke counts at 16 hr (p=0.0001). Student’s t-test. N = 146 mice.
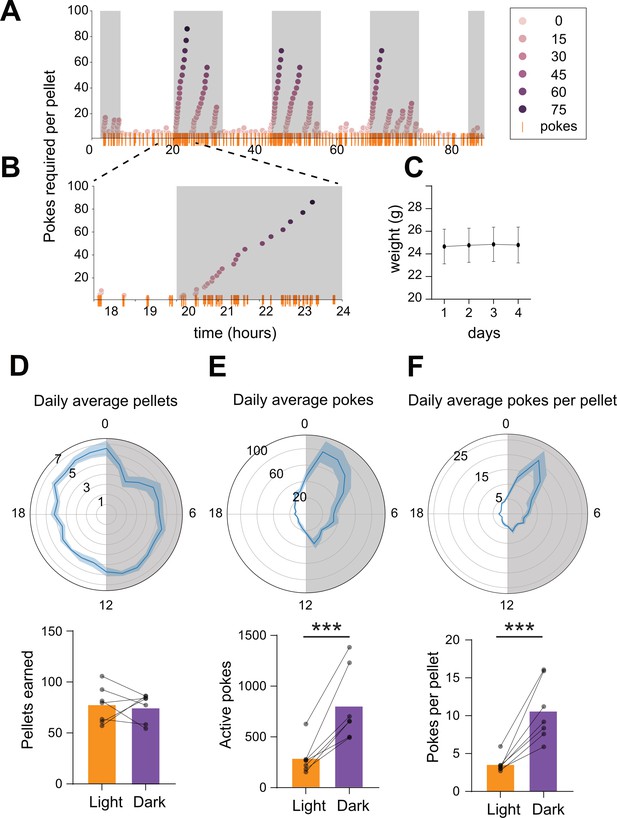
Longitudinal closed-economy feeding with FED3.
(A) Active pokes required per pellet in a 4 day closed-economy progressive ratio task with 30 min resets. (B) Inset illustrating increasing number of pokes (y-axis, and orange rasters) per pellet earned during dark cycle. (C) Mouse weights over time (n = 7 mice). (D–F) Chronograms (top) and bar/scatter plots (bottom) showing daily average pellets (D), pokes (E), and pokes per pellet (F) over time, binned by 1 hr. Shaded region in chronograms indicates dark cycle. Significant increase in active pokes, p=0.0044 (E, bottom), and pokes per pellet, p=0.0007 (F, bottom) during dark cycle. No significant difference in pellets earned during dark cycle, p=0.8622 (D, bottom).
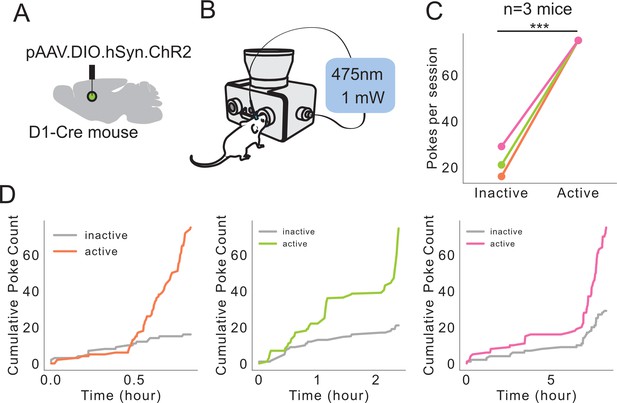
Self-stimulation of dMSNs using FED3.
(A) Schematic showing Cre-dependent ChR2 injected into dorsal medial striatum with fiber optic implanted. (B) Schematic showing FED3 operant self-stim setup. (C) Mice poked significantly more on the active port (p=0.0002, Student’s t-test, n = 3 mice). (D) Cumulative inactive and active pokes plotted over time for each mouse.
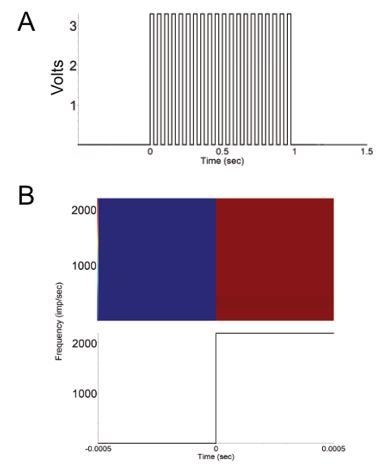
FED3 as a function generator.
(A) Recorded 20 Hz Pulse train generated by FED3. (B) Peripulse rasters (top) and histogram showing change in voltage in 1ms around the onset of each pulse in a 20Hz train. Note that the observable lag in this test was zero. Y-axis is mV, x-axis in sec.
Videos
Example movie of mouse using FED3 in a home-cage, and with FED3 mounted on a plastic box.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Mus musculus) | Mus musculus with name C57BL/6J from IMSR. | https://www.jax.org/strain/000664 | RRID:IMSR_JAX:000664 | |
| Software, algorithm | Python 3.7 (Anaconda Distribution) | https://www.anaconda.com/ | ||
| Software, algorithm | Spyder 4.1 | https://github.com/spyder-ide/spyder/releases | RRID:SCR_017585, version 4.1 | |
| Software, algorithm | Arduino IDE 1.8.1 | https://www.arduino.cc/ | ||
| Software, algorithm | FED3_VIZ | https://github.com/earnestt1234/FED3_Viz | Analysis package for FED3 data files | |
| Software, algorithm | TinkerCAD | https://www.tinkercad.com/ | ||
| Other | FED3 – commercially assembled | https://open-ephys.org/fed3/fed3 | ||
| Other | FED3 – open-source | https://github.com/KravitzLabDevices/FED3 | ||
| Other | 5TUM grain-based rodent enrichment pellets | https://www.testdiet.com/Diet-Enrichment-Products/Lab-Treat-Tablets-and-Pellets/index.html |