Differential conditioning produces merged long-term memory in Drosophila
Figures
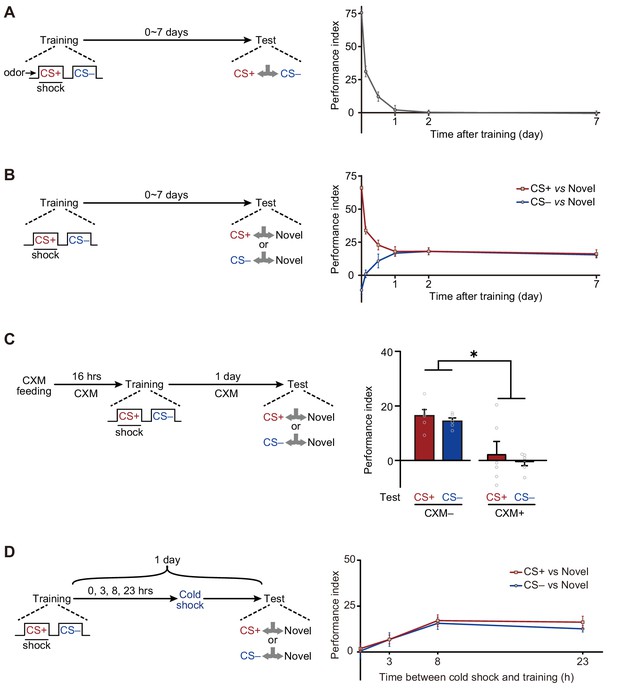
Long-term avoidances of both conditioned stimulus (CS+) and non-conditioned stimulus (CS–) induced by aversive differential conditioning.
(A) Single-trial differential conditioning induced discriminative memory that was forgotten within 1 day (n = 6–8). (B) Conditioned flies exhibited long-lasting avoidances of both CS+ and CS– (n = 8–10). (C) Cycloheximide (CXM) treatment abolished 1 day avoidances of CS+ and CS– (n = 6). (D) The 1 day avoidances were tested after cold-shock anesthesia at different time points following training. Aversive memory to CS+ and CS– requires a multi-hour consolidation process (n = 6–8). All data shown are presented as mean ± SEM. *p < 0.05.
-
Figure 1—source data 1
Behavioral data of each group in Figure 1.
- https://cdn.elifesciences.org/articles/66499/elife-66499-fig1-data1-v2.xlsx
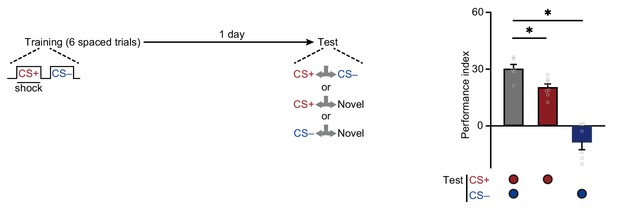
Repetitive spaced training forms complementary long-term memories (LTMs).
Multi-trial spaced training (six trials of training with 15 min interval) induced 24 hr aversive memory to conditioned stimulus (CS+) and approach memory to non-conditioned stimulus (CS–) (n = 6–8). All data shown are presented as mean ± SEM. *p < 0.05.
-
Figure 1—figure supplement 1—source data 1
Behavioral data of each group in Figure 1—figure supplement 1.
- https://cdn.elifesciences.org/articles/66499/elife-66499-fig1-figsupp1-data1-v2.xlsx
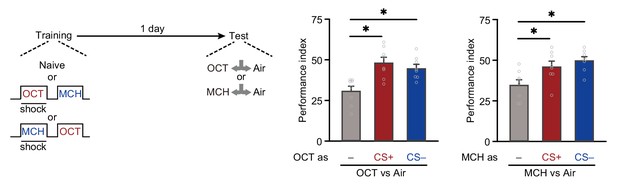
Single-trial differential conditioning increases conditioned stimulus (CS+) and non-conditioned stimulus (CS–) odor avoidances.
Odor avoidances of 3-octanol (OCT) and 4-methylcyclohexanol (MCH) were significantly increased when they were used as CS+ or CS– during training (n = 8). All data shown are presented as mean ± SEM. *p < 0.05.
-
Figure 1—figure supplement 2—source data 1
Behavioral data of each group in Figure 1—figure supplement 2.
- https://cdn.elifesciences.org/articles/66499/elife-66499-fig1-figsupp2-data1-v2.xlsx
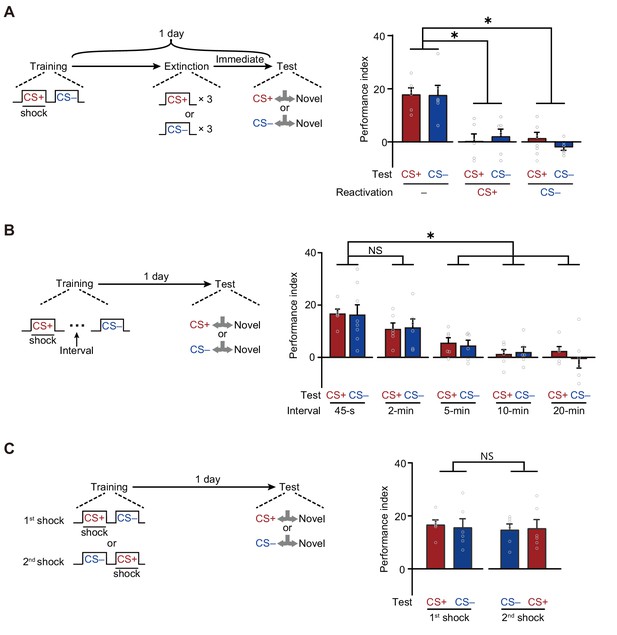
A merged long-term memory (mLTM) underlies the long-term avoidances of conditioned stimulus (CS+) and non-conditioned stimulus (CS–).
(A) Three trials of re-exposure to either CS+ or CS– alone can impair both CS+ avoidance and CS– avoidance 1 day after training (n = 6). (B) Prolonging the inter-trial interval (ITI) between CS+ and CS– to more than 5 min significantly impaired 1 day avoidances (n = 6–8). (C) Changing the sequence of CS+ and CS– during training did not affect 1 day avoidances (n = 6). All data shown are presented as mean ± SEM. *p < 0.05. NS, non-significant.
-
Figure 2—source data 1
Behavioral data of each group in Figure 2.
- https://cdn.elifesciences.org/articles/66499/elife-66499-fig2-data1-v2.xlsx
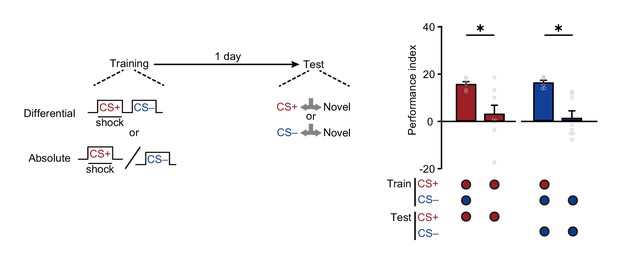
Absolute trainings fail to induce 24 hr avoidances.
Training without conditioned stimulus (CS+) or non-conditioned stimulus (CS–) abolished the 24 hr avoidance to CS– or CS+ (n = 6–8). All data shown are presented as mean ± SEM. *p < 0.05.
-
Figure 2—figure supplement 1—source data 1
Behavioral data of each group in Figure 2—figure supplement 1.
- https://cdn.elifesciences.org/articles/66499/elife-66499-fig2-figsupp1-data1-v2.xlsx
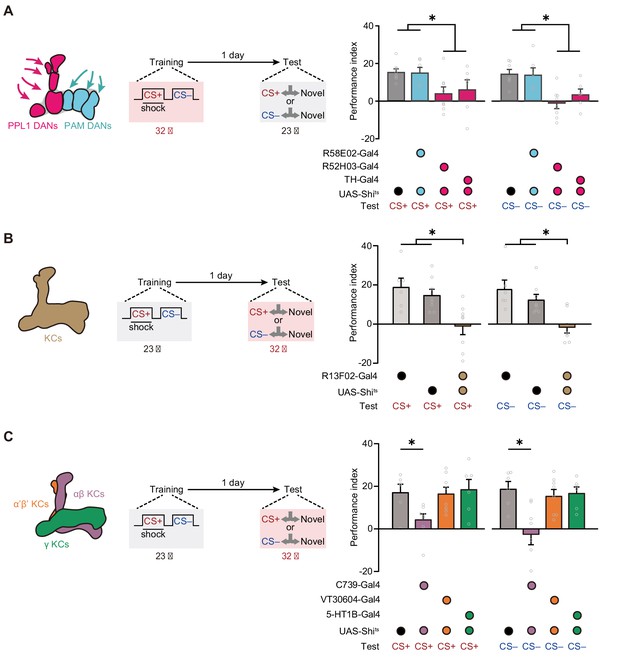
Expression of the merged long-term memory (mLTM) requires the αβ Kenyon cells (KCs).
(A) Blocking paired posterior lateral 1 (PPL1) dopaminergic neurons (DANs) (R52H03-Gal4 or TH-Gal4) but not protocerebral anterior medial (PAM) DANs (R58E02-Gal4) during training significantly impaired mLTM (n = 6–8). (B) Blocking mushroom body (MB) during testing using R13F02-Gal4>UAS-Shits impaired the mLTM (n = 6–8). (C) The mLTM was impaired by blocking αβ KCs (C739-Gal4), but not that of α’β’ KCs (VT30604-Gal4) or γ KCs (5-HT1B-Gal4) (n = 6–8). All data shown are presented as mean ± SEM. *p < 0.05.
-
Figure 3—source data 1
Behavioral data of each group in Figure 3.
- https://cdn.elifesciences.org/articles/66499/elife-66499-fig3-data1-v2.xlsx
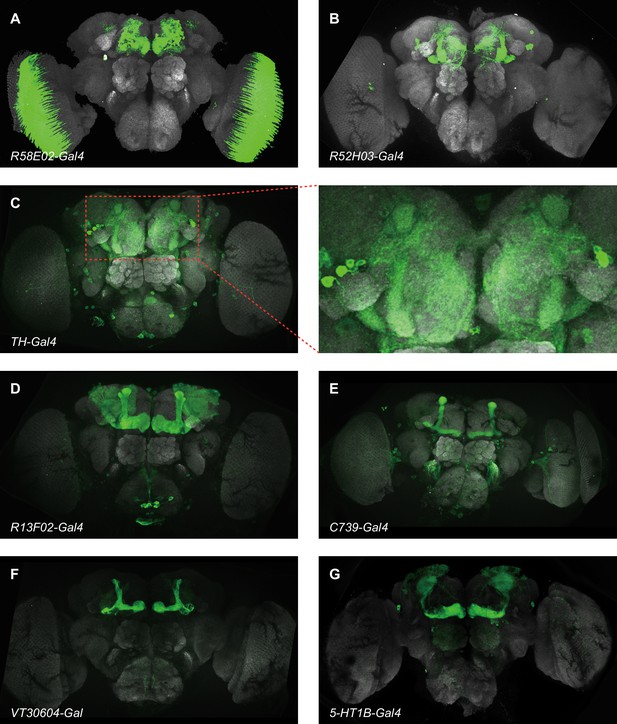
The expression patterns of GAL4 lines used in Figure 3.
Panels show GFP expression driven by the relevant GAL4 (green) and general neuropil stained with an antibody to the presynaptic marker nc82 (gray). (A) R58E02-Gal4 labels all protocerebral anterior medial (PAM) dopaminergic neurons (DANs). (B) R52H03-Gal4 labels all paired posterior lateral 1 (PPL1) DANs. (C) TH-Gal4 broadly labels all PPL1 DANs. (D) R13F02-Gal4 labels all Kenyon cells (KCs) in mushroom body (MB). (E) C739-Gal4 labels αβ KCs. (F) VT20604-Gal4 labels α’β’ KCs. (G) 5-HT1B-Gal4 labels γ KCs.
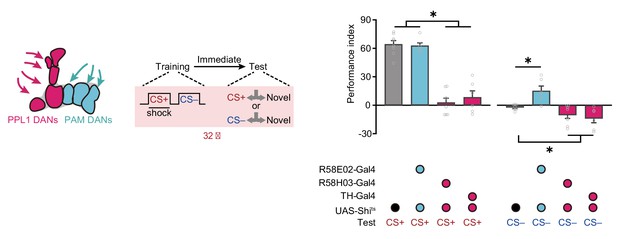
Paired posterior lateral 1 (PPL1) and protocerebral anterior medial (PAM) dopaminergic neurons (DANs) play different roles in conditioned stimulus (CS+) and non-conditioned stimulus (CS–) memory encoding.
Blocking PAM DANs did not affect the 3 min avoidance to CS+ but induced a significant avoidance to CS–, whereas blocking PPL1 DANs abolished the 3 min avoidance to CS+ but increases the 3 min approach to CS– (n = 6–8). All data shown are presented as mean ± SEM. *p < 0.05.
-
Figure 3—figure supplement 2—source data 1
Behavioral data of each group in Figure 3—figure supplement 2.
- https://cdn.elifesciences.org/articles/66499/elife-66499-fig3-figsupp2-data1-v2.xlsx
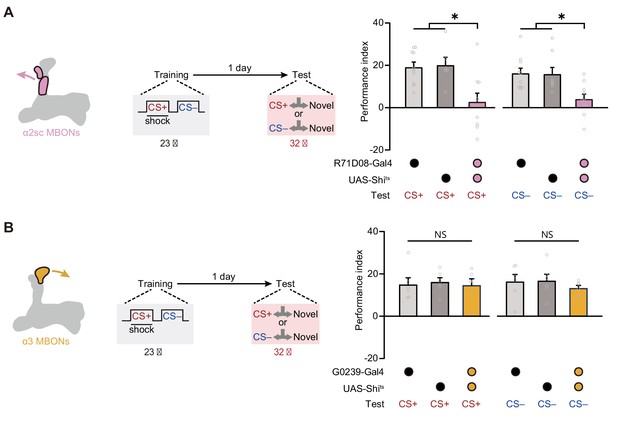
Expression of the merged long-term memory (mLTM) requires α2sc mushroom body output neurons (MBONs).
(A) Blocking α2sc MBONs using R71D08-Gal4>UAS-Shits impaired mLTM expression (n = 6–10). (B) Blocking α3 MBONs using G0239-Gal4>UAS-Shits did not affect mLTM expression (n = 6). All data shown are presented as mean ± SEM. *p < 0.05. NS, non-significant.
-
Figure 4—source data 1
Behavioral data of each group in Figure 4.
- https://cdn.elifesciences.org/articles/66499/elife-66499-fig4-data1-v2.xlsx
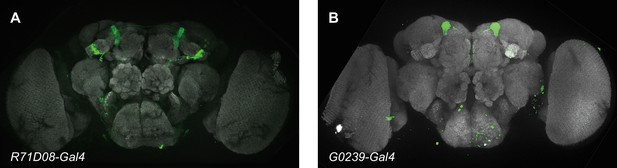
The expression patterns of GAL4 lines used in Figure 4.
Panels show GFP expression driven by the relevant GAL4 (green) and general neuropil stained with an antibody to the presynaptic marker nc82 (gray). (A) R71E08-Gal4 labels α2sc mushroom body output neurons (MBONs). (B) G0239-Gal4 labels α3 MBONs.
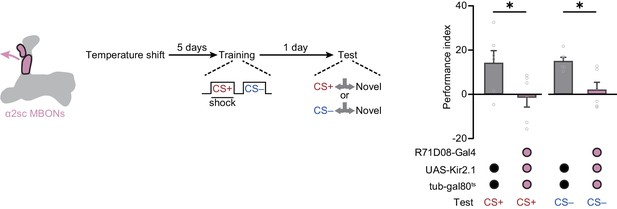
Retrieving the merged long-term memory (mLTM) relies on the neural activity of α2sc mushroom body output neurons (MBONs).
Blocking the neural activity of α2sc MBONs using R71D08-Gal4>UAS-Kir2.1;tub-gal80ts abolished the mLTM expression (n = 6). Flies were moved to high temperature (32°C) 5 days before training for the induction of Kir2.1. All data shown are presented as mean ± SEM. *p < 0.05.
-
Figure 4—figure supplement 2—source data 1
Behavioral data of each group in Figure 4—figure supplement 2.
- https://cdn.elifesciences.org/articles/66499/elife-66499-fig4-figsupp2-data1-v2.xlsx
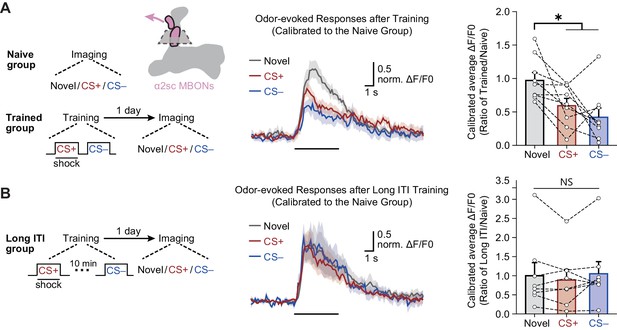
The merged long-term memory (mLTM) can be recorded as depressed odor-evoked responses in α2sc mushroom body output neurons (MBONs).
(A) Left: training and imaging protocols. The imaging plane for α2sc MBONs is shown. The novel odor-evoked response, conditioned stimulus (CS+) odor-evoked response, and non-conditioned stimulus (CS–) odor-evoked response were calibrated to the average responses of corresponding odors in naïve flies. Right: the mLTM can be recorded as depressed odor-specific responses in α2sc MBONs (n = 9). (B) Prolonging the inter-trial interval (ITI) between CS+ and CS– during training abolished the depressed odor-specific responses (n = 8). All data shown are presented as mean ± SEM. *p < 0.05. NS, non-significant.
-
Figure 5—source data 1
Calcium imaging data of each group in Figure 5.
- https://cdn.elifesciences.org/articles/66499/elife-66499-fig5-data1-v2.xlsx
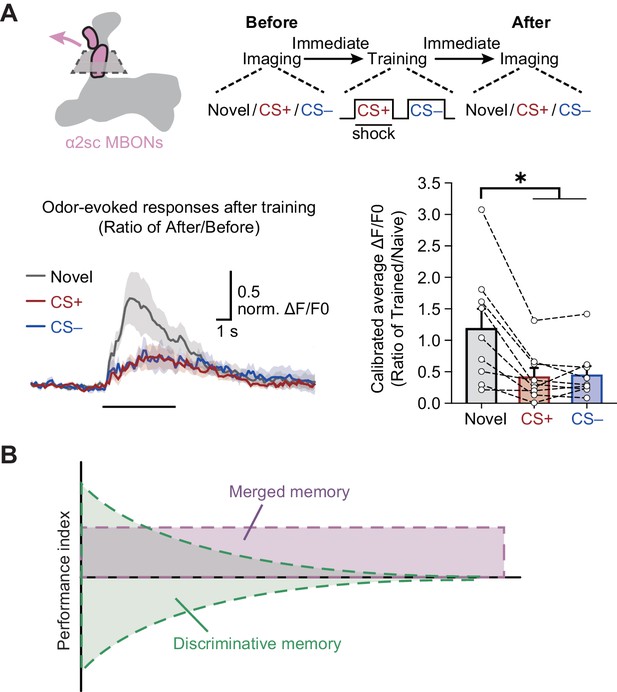
The depression of odor-evoked responses in α2sc mushroom body output neurons (MBONs) can be observed immediately after training.
(A) Top: training and imaging protocols. The odor-evoked responses of novel odor, conditioned stimulus (CS+), and non-conditioned stimulus (CS–) were sequentially recorded before and after training. Down: the depressed CS+ and CS– odor-evoked responses were recorded immediately after training (n = 9). All post-training responses were calibrated to the average responses of corresponding in same flies before training. (B) Model of memory components induced by single-trial differential conditioning. After training, two categories of memories are formed: the short-lasting discriminative memory guiding avoidance to CS+ and approach to CS–; and the long-lasting merged memory guiding avoidance to both CS+ and CS–. All data shown are presented as mean ± SEM. *p < 0.05.
-
Figure 5—figure supplement 1—source data 1
Calcium imaging data of each group in Figure 5—figure supplement 1.
- https://cdn.elifesciences.org/articles/66499/elife-66499-fig5-figsupp1-data1-v2.xlsx





