Sensing the world and its dangers: An evolutionary perspective in neuroimmunology
Figures
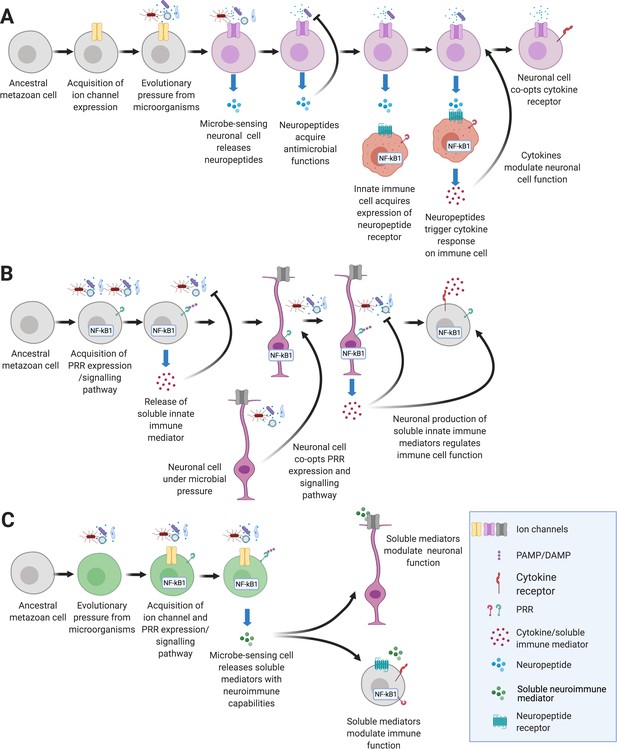
Hypothetical pathways that lead to the emergence of NICUs in early metazoans.
(A) An ancient metazoan cell under microbial pressure acquires ion channels gaining the ability to detect danger (Elkhatib et al., 2019; Senatore et al., 2016). Eventually neuropeptides that have antimicrobial functions are released in response to microbes (Augustin et al., 2017). Innate immune cells gain neuropeptide receptors that trigger cytokine release (Pinho-Ribeiro et al., 2017). Finally, neuronal cells gain the ability to detect and respond to immune signaling cytokines (Chatterjea and Martinov, 2015; Chu et al., 2020). (B) An ancient metazoan cell acquires pattern recognition receptors and gains the ability to detect microbes (Boller and Felix, 2009; Rosenstiel et al., 2009). Eventually soluble immune mediators are released in response that trigger antimicrobial functions (Hanson et al., 2019). Neurons gain immune ligand receptors that trigger neuropeptide release (Lezi et al., 2018). Finally, immune cells gain the ability to detect and respond to neuronally derived neuropeptides (Chu et al., 2020; Foster et al., 2017). (C) An ancient metazoan cell under microbial pressure acquires pathogen pattern recognition receptor pathways concurrently with acquisition of ion channels that, under selective pressure gain the ability to detect danger (Tian et al., 2019). Eventually soluble neuroimmune mediators are secreted in response to pathogens modulate functions of neuronal and immune systems.
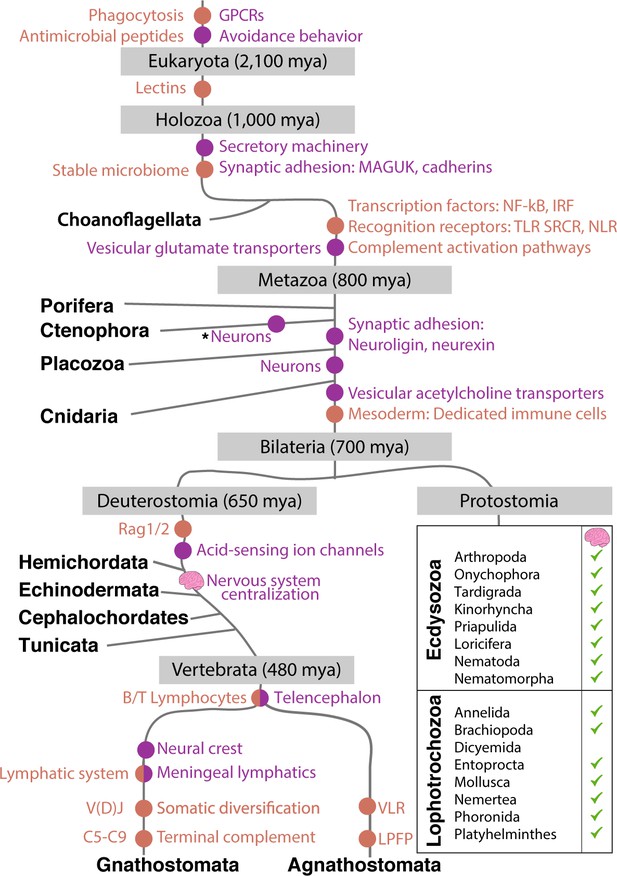
Emergence of the immune and nervous systems predates the emergence of Metazoa.
Analysis of genome sequences indicates that much of the cellular machinery involved in extant nervous and immune systems was present in basal metazoan lineages. This includes ion channels, pattern recognition receptors, and antimicrobial peptides (Franzenburg et al., 2012; Rosenstiel et al., 2009; Senatore et al., 2016). The evolutionary origin of significant innovations is indicated by dots (immune system in orange; nervous system in purple). Centralization of nervous systems within bilaterian phyla is indicated by small brain icons. Note CNS structures may be very diverse among invertebrate groups. Asterisk indicates possible independent, convergent evolution of Ctenophore neurons, a current point of debate (Moroz et al., 2014).
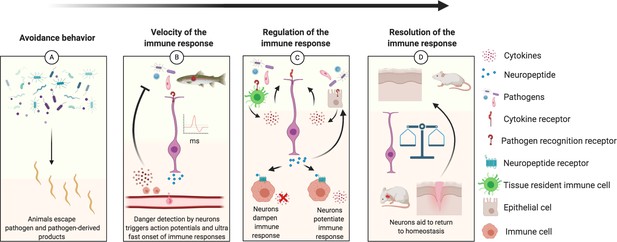
Schematic diagram illustrating how neurons play a role in the regulation of each step of the immune response, from inception to resolution.
(A) Sensory neurons detect danger cues that are then transduced to move the animals away from potential deleterious infection. For example, in C elegans npr-1 expressing neurons detect oxygen levels to avoid harmful pathogens (Hoffman and Aballay, 2019; Singh and Aballay, 2019; Styer et al., 2008). Furthermore, bacterially infected female Drosophila will lay fewer eggs (Kurz et al., 2017; Masuzzo et al., 2019). (B) Sensory neurons directly detect pathogen presence resulting in rapid action potentials and release of neurotransmitters that increase the velocity of an immune response. Neurons ultra-rapid communication to immune cell reservoirs deploy immune cells quicker than chemokine signals; such as recruiting CD8+ cells to the olfactory organ after detection of neurotrophic virus by sensory neurons (Chavan and Tracey, 2017; Sepahi et al., 2019). The immune responses triggered by neurons can be biased to be neuroprotective while dealing with infection such as in the myenteric plexus of the gut where b2 adrenergic receptor signaling polarizes muscularis macrophages to express Arg1, associated with an M2 phenotype (Gabanyi et al., 2016; Lai et al., 2020). (C) Pathogens detected by immune and epithelial cells provoke release of cytokines and other immune factors that are pro-inflammatory and potentially deleterious to the tissue if allowed to propagate inflammation without regulation. Local neurons are rapidly activated by the inflammatory environment and PAMPs, releasing neurotransmitters that tune the immune response (immune cells and epithelial cells) toward a less pro-inflammatory phenotype (Baral et al., 2018; Basbaum et al., 2009; Cardoso et al., 2017; Chu et al., 2020; Labed et al., 2018; Ramirez et al., 2020). A specific example is neuromedin U that binds to its cognate receptor on ILC2s enhancing their type 2/repair phenotype mediating protection against worm infections (Cardoso et al., 2017). Another example includes the reduction of systemic inflammation when the vagus nerve is stimulated (Borovikova et al., 2000). However, suppressive neuronal responses can be detrimental and favor infection as when TRPV1 neurons release CGRP and suppress neutrophil recruitment, leading to S. pyogenes lesion progression compared to animals lacking CGRP signaling (Pinho-Ribeiro et al., 2017; Chu et al., 2020). (D) The immune system and nervous system must balance their responses over time to return to homeostasis (Aurora and Olson, 2014; Veiga-Fernandes and Artis, 2018). An initial immune response is necessary for clearance of pathogen and debris and to initiate growth factor responses in neurons, but prolonged inflammation can be detrimental to neuronal regeneration as seen in the olfactory system and spinal cords with NF-κb/chemokine and TNFα signaling, respectively, leading to loss of regenerative abilities (Chen et al., 2019a; Godwin et al., 2013; Tsai et al., 2019; Tsarouchas et al., 2018). Another example includes the resolution of inflammation controlled by the sympathetic nervous system in mice (Körner et al., 2019).
Tables
Molecules with dual roles in the immune and nervous systems.
| Factors classically associated with immune functions | ||||
|---|---|---|---|---|
| Protein | Immune system properties | Nervous system properties | References | |
| Antimicrobial peptides (AMPs) |
|
| Hanson et al., 2019; Lezi et al., 2018; Su et al., 2010; Zasloff, 2002 | |
| Cytokines | TGF-β |
|
| Arnold et al., 2014; Arrieta-Bolaños et al., 2012; Eisenstein and Williams, 2009; Hirota et al., 2015; Makhijani et al., 2017; Morishima et al., 2009; Singh and Aballay, 2019; Yi et al., 2010; You et al., 2008; Zheng et al., 2006 |
| IL-4, IL-13 |
|
| Fallon et al., 2002; Gadani et al., 2012; Kolosowska et al., 2019; McKenzie et al., 1998; Yang et al., 2016; Zhang et al., 2019a | |
| TNF-α |
|
| Borsini et al., 2015; Lambertsen et al., 2009; Liu et al., 1994; Takei and Laskey, 2008; Vanderheyden et al., 2018 | |
| Complement System Proteins | Complement factors |
|
| Hammad et al., 2018; Nonaka, 2001; Rupprecht et al., 2007; Shinjyo et al., 2009; Stevens et al., 2007 |
| Perforin-like factors |
|
| Ni and Gilbert, 2017 | |
| Pattern Recognition Receptors | Toll-like receptors (TLRs) |
|
| Chen et al., 2019c; Donnelly et al., 2020; Foldi et al., 2017; Franzenburg et al., 2012; Lemaitre et al., 1996 |
| Nod-like receptors (NLRs) |
|
| Gharagozloo et al., 2017 | |
| Peptidoglycan recognition protein LC (PGRP-LC) |
|
| Harris et al., 2015 | |
| Formyl peptide receptors (FPRs) |
|
| Dietschi et al., 2017 | |
| Histamine |
|
| Yuan and Silberstein, 2018 | |
| Factors classically associated with neuronal functions | ||||
| Transient receptor potential (TRPs) |
|
| Alpizar et al., 2017; López-Requena et al., 2017; Parenti et al., 2016 | |
| Nerve growth factor (NGF) |
|
| Minnone et al., 2017; Pinho-Ribeiro et al., 2017; Takei and Laskey, 2008 | |
| Brain-derived neurotrophic factor (BDNF) |
|
| Fauchais et al., 2008; Lee et al., 2012; Linker et al., 2015; Schuhmann et al., 2005 | |
| Olfactory receptors |
|
| Heimroth et al., 2020; Li et al., 2013 | |
| Calcitonin gene related peptide (CGRP) |
|
| Chung, 2017; Kerage et al., 2019; Pinho-Ribeiro et al., 2017; Xu et al., 2019 | |
| Substance P |
|
| Mashaghi et al., 2016 | |
| Dopamine |
|
| Kerage et al., 2019; Matt and Gaskill, 2020 | |
| DSCAMs |
|
| Goyal et al., 2019; Hattori et al., 2009; Ng and Kurtz, 2020 | |
| NCAM/CD56 |
|
| Van Acker et al., 2017; Vukojevic et al., 2020 | |
| SNARE |
|
| Ramakrishnan et al., 2012; Tang, 2015 | |





