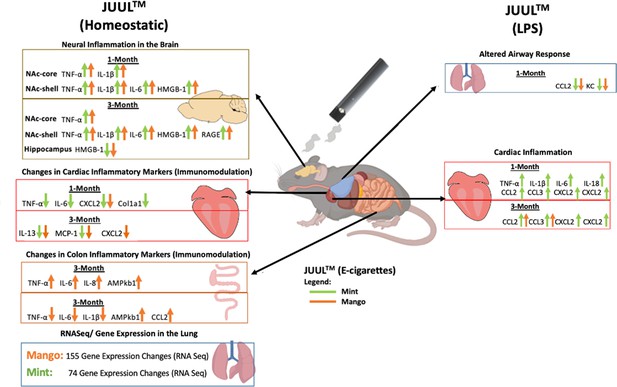
Effects of mango and mint pod-based e-cigarette aerosol inhalation on inflammatory states of the brain, lung, heart, and colon in mice
Figures
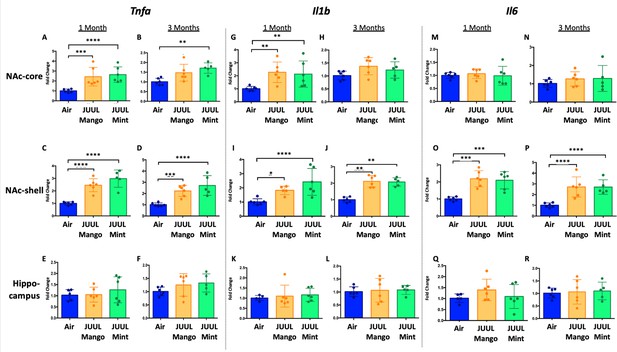
Three months of JUUL aerosol inhalation leads to an increase of pro-inflammatory cytokines in different regions of the brain.
Brains were harvested at the end point and the regions for NAc-core, NAc-shell and Hippocampus were harvested and frozen. RNA was extracted and qPCR was performed to quantify the expression of Tnfa, Il1b, Il6. Tnfa expression is shown from NAc-core at (A) 1 month and (B) 3 months, from NAc-shell at (C) 1 month and (D) 3 months, and from Hippocampus at (E) 1 month and (F) 3 months. Il1b expression is shown from NAc-core at (G) 1 month and (H) 3 months, from NAc-shell at (I) 1 month and (J) 3 months, and from Hippocampus at (K) 1 month and (L) 3 months. Il6 expression is shown from NAc-core at (M) 1 month and (N) 3 months, from NAc-shell at (O) 1 month and (P) 3 months, and from Hippocampus at (Q) 1 month and (R) 3 months. Data were analyzed with two-way ANOVA with Dunnett’s multiple comparisons for each brain region and timepoint. Data are presented as individual data points ± SEM with n = 5–6 mice per group. *p < 0.05, **p < 0.01, *** p < 0.001 and **** p < 0.0001.
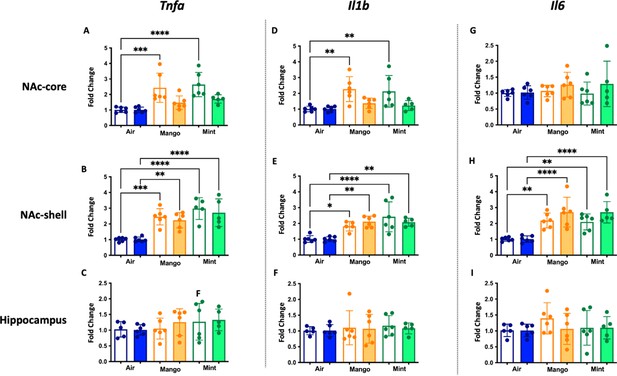
Inflammatory gene expression changes in the central nervous system in mice exposed to JUUL Mango and JUUL Mint.
These data are graphed with each exposure type (air – blue, mango – orange, and mint – green) grouped for ease of comparison of gene expression at 1 month (white columns) and 3 months (solid columns) of exposure. Data were analyzed with one-way ANOVA with Holm-Sidak multiple comparisons for each brain region and gene of interest. Data are presented as individual data points ± SD with n = 5–6 mice per group. *p < 0.05, **p < 0.01, *** p < 0.001 and **** p < 0.0001.
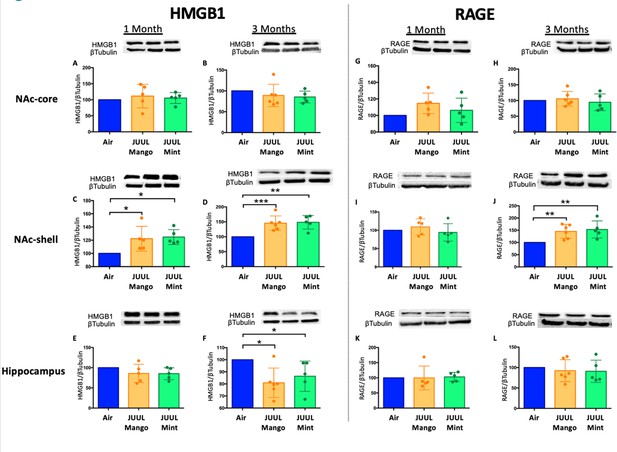
Three months of JUUL aerosol inhalation leads to an increase of inflammatory mediators HMGB1 and RAGE.
Brains were harvested at the end point and the regions for NAc-core, NAc-shell and Hippocampus were sectioned. Later, protein was extracted and Western Blot was performed to quantify the expression of HMGB1-1 and RAGE. HMGB1-1 relative protein level are shown from NAc-core at (A) 1 month and (B) 3 months, from NAc-shell at (C) 1 month and (D) 3 months, and from Hippocampus at (E) 1 month and (F) 3 months. RAGE protein levels are shown from NAc-core at (G) 1 month and (H) 3 months, from NAc-shell at (I) 1 month and (J) 3 months, and from Hippocampus at (K) 1 month and (L) 3 months. Changes in proteins levels are relative to Air controls. Data are presented as individual data points ± SEM with n = 5–6 mice per group. *p < 0.05, **p < 0.01 and *** p < 0.001.
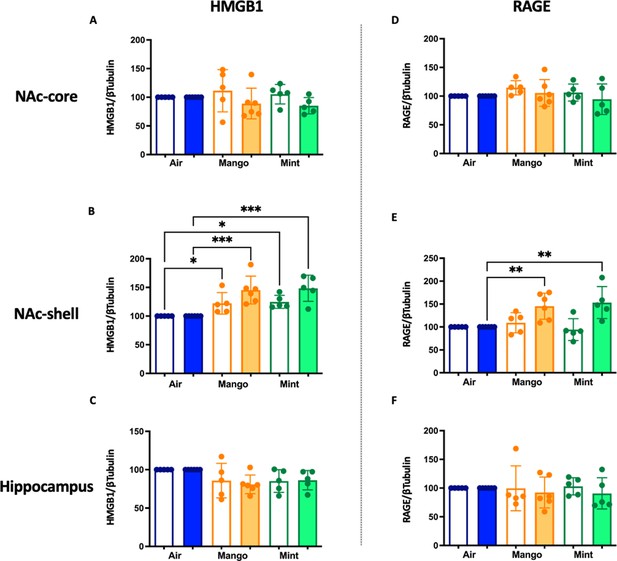
Inflammatory protein level changes in the central nervous system in mice exposed to JUUL Mango and JUUL Mint.
These data are graphed with each exposure type (air – blue, mango – orange, and mint – green) grouped for ease of comparison of protein levels at 1 month (white columns) and 3 months (solid columns) of exposure. Data were analyzed with one-way ANOVA with Holm-Sidak multiple comparisons for each brain region and protein of interest. Data are presented as individual data points ± SD with n = 5–6 mice per group. *p < 0.05, **p < 0.01, and *** p < 0.001.
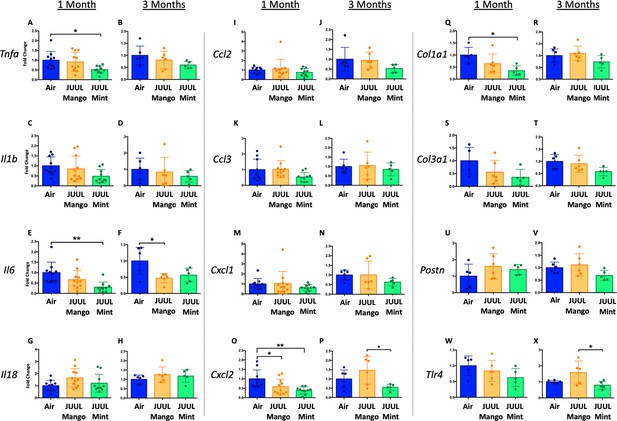
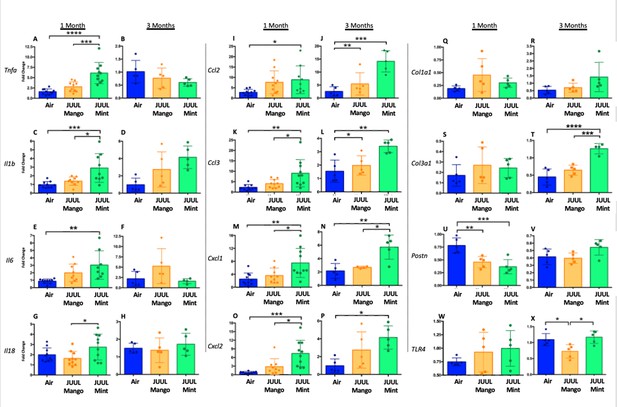
Three months of inhalation of JUUL aerosols alters inflammatory and fibrosis associated gene expression in cardiac tissue.
Hearts were harvested, and RNA was extracted from the left ventricle and qPCR was performed to quantify the gene expression of different cytokines, chemokines and fibrosis-associated genes. Cytokines include Tnfa at (A) 1 month and (B) 3 months, Il1b at (C) 1 month and (D) 3 months, Il6 at (E) 1 month and (F) 3 months, and Il18 at (G) 1 month and (H) 3 months. Chemokines include Ccl2 at (I) 1 month and (J) 3 months, Ccl3 at (K) 1 month and (L) 3 months, Cxcl1 at (M) 1 month and (N) 3 months, and Cxcl2 at (O) 1 month and (P) 3 months. Fibrosis-associated genes include Col1a1 at (Q) 1 month and (R) 3 months, Col3a1 at (S) 1 month and (T) 3 months, Postn at (U) 1 month and (V) 3 months, and Tlr4 at (W) 1 month and (X) 3 months. Changes in expression levels are relative to Air controls. Data are presented as individual data points ± SEM with n = 5–11 mice per group. *p < 0.05 and **p < 0.01.

Inflammatory gene expression changes in the hearts of mice exposed to JUUL Mango and JUUL Mint.
Mice exposed to JUUL Mint aerosols three times daily (60 min total) for 1 month had diminished expression of Tnfa, Il6, Col1a1, and Col3a1 relative to air controls. Data were analyzed with one-way ANOVA with Holm-Sidak multiple comparisons for each gene of interest. Data are presented as individual data points ± SD with n = 5–6 mice per group. *p < 0.05, **p < 0.01, and *** p < 0.001.
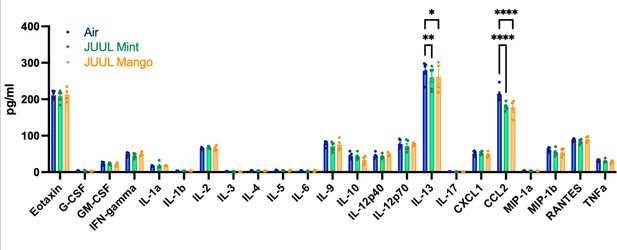
Chronic inhalation of JUUL aerosols alters Ccl2 and Il13 levels in cardiac tissue.
Cardiac apex tissue was lysed, total protein isolated, and inflammatory proteins quantified by Bio-Plex Pro Mouse Cytokine 23-plex Assay. Both Il13 and Ccl2 levels were diminished in cardiac tissue from mice exposed for 3 months to JUUL Mint (green) and JUUL Mango (orange) aerosols, relative to Air controls (blue). Data was analyzed by two-way ANOVA with Dunnett’s corrections for multiple comparisons, and are presented as individual data points ± SEM, with n = 4–6 mice per group. *p = 0.017, **p < 0.01, and ****p < 0.0001.
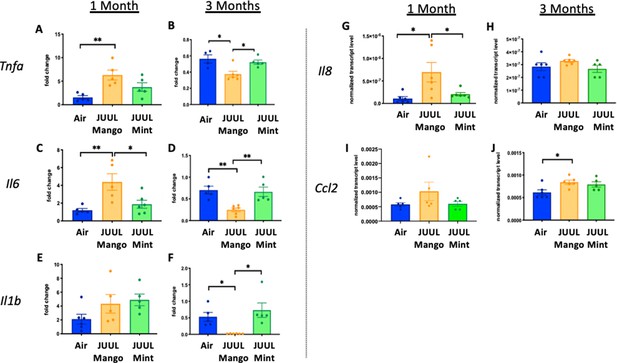
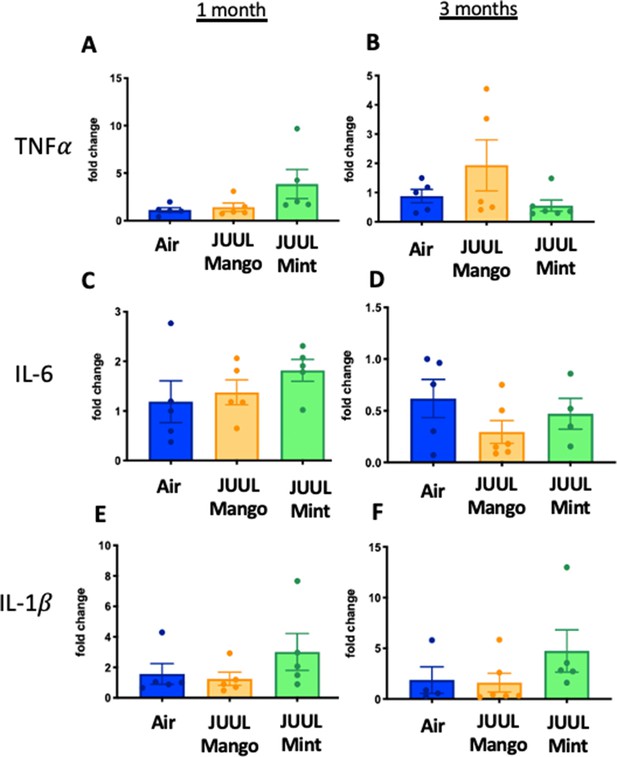
Three months of JUUL aerosol inhalation alters pro-inflammatory markers in colon.
Inflammation was assessed in the colon at 1 and 3 months. Panels show inflammation markers in the colon in Tnfa (A) 1 month and (B) 3 months, Il6 at (C) 1 month and (D) 3 months, Il1b at (E) 1 month and (F) 3 months,Il8 (G) and (H), and Ccl2I 1 month and (J) 3 months. Data for inflammation markers is presented as individual data points ± SEM. * p < 0.01 and ** p < 0.001.

Inflammatory gene expression changes in the colon of mice exposed to JUUL Mango and JUUL Mint.
These data are graphed with each exposure type (air – blue, mango – orange, and mint – green) grouped for ease of comparison of gene expression at 1 month (white columns) and 3 months (solid columns) of exposure. Notably, inhalation of JUUL Mango aerosols three times daily for 1 month led to increased expression of Tnfa, Il6, and Il8 in the colon. Data were analyzed with one-way ANOVA with Holm-Sidak multiple comparisons for each gene of interest. Data are presented as individual data points ± SD with n = 5–6 mice per group. *p < 0.05, **p < 0.01, *** p < 0.001 and **** p < 0.0001.
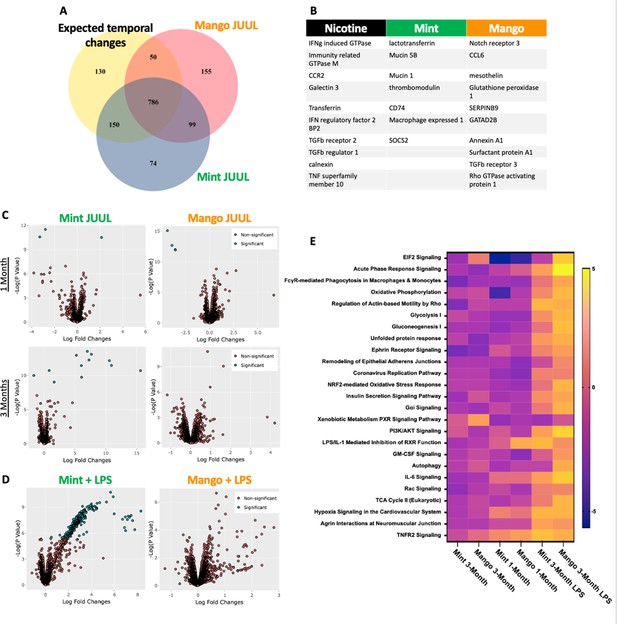
Unique RNAseq signatures in the lungs exposed to different flavors of JUUL aerosols.
(A) Venn diagram of gene expression unique to JUUL Mango (155) and JUUL Mint (Alhaddad et al., 2014b). Gene expression changes common to both aerosols (99) suggest that they are due to chemicals found in both of the flavored e-liquids (nicotinic salts, propylene glycol, glycerin, benzoic acid, etc). (B) Greatest gene expression changes associated with nicotine and flavorant chemicals within aerosols. (C) Volcano plots demonstrating gene expression changes specific for each flavor after sub-acute exposure (1 month; top row) and 3 months of exposure (bottom row). (D) Gene expression changes associated with inhalation of JUUL aerosols with different flavors in the setting of inflammatory challenge with inhaled LPS.(E) Heat map of the pathways most notably impacted by 1- and 3-month daily exposures to JUUL Mint and Mango, as well as in the setting of LPS challenge at 3 months. IFNg: interferon gamma; CCR: C-C motif chemokine related; IFN: interferon; BP: binding protein; TGFb: transforming growth factor beta; TNF: tumor necrosis factor; SOCS: suppressor of cytokine signaling; CCL: C-C motif ligand.
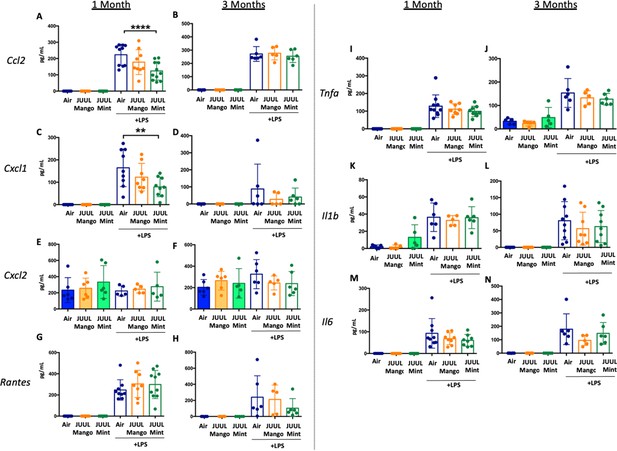
JUUL exposure alters airway inflammatory responses in the setting of inhaled LPS challenge.
BAL was harvested at the endpoints, and cytokines and chemokines were quantified by ELISA. Ccl2 at (A) 1 month, and (B) 3 months, Cxcl1 at (C) 1 month and (D) 3 months, Cxcl2 at (E) 1 month and (F) 3 month, RANTES at (G) 1 month and (H) 3 months, Tnfa at (I) 1 month and (J) 3 months, Il1b at (K) 1 month and (L) 3 months, Il6 at (M) 1 month and (N) 3 months. Data are presented as individual data points ± SEM with n = 5–11 mice per group. **p < 0.01 and **** p < 0.0001.

Inflammatory cytokine levels in the BAL of mice exposed to JUUL Mango and JUUL Mint prior to inhaled LPS challenge.
These data are graphed with each exposure type (air – blue, mango – orange, and mint – green) grouped for ease of comparison of protein levels at 1 month (white columns) and 3 months (solid columns) of exposure. Data were analyzed with one-way ANOVA with Holm-Sidak multiple comparisons for each brain region and gene of interest. Data are presented as individual data points ± SD with n = 5–6 mice per group.
Cardiac inflammation induced by inhaled LPS challenge is increased in the setting of 3 months of JUUL aerosol inhalation.
Hearts were harvested, and RNA was extracted from the left ventricle and qPCR was performed to quantify the gene expression of different cytokines, chemokines and fibrosis-associated genes. Cytokines include Tnfa at (A) 1 month and (B) 3 months, Il1b at (C) 1 month and (D) 3 months, Il6 at (E) 1 month and (F) 3 months, and Il18 at (G) 1 month and (H) 3 months. Chemokines include Ccl2 at (I) 1 month and (J) 3 months, Ccl3 at (K) 1 month and (L) 3 months, Cxcl1 at (M) 1 month and (N) 3 months, and Cxcl2 at (O) 1 month and (P) 3 months. Fibrosis-associated genes include Col1a1 at (Q) 1 month and (R) 3 months, Col3a1 at (S) 1 month and (T) 3 months, Postn at (U) 1 month and (V) 3 months, and Tlr4 at (W) 1 month and (X) 3 months. Changes in expression levels are relative to Air controls. Data are presented as individual data points ± SEM with n = 5–11 mice per group. *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001.
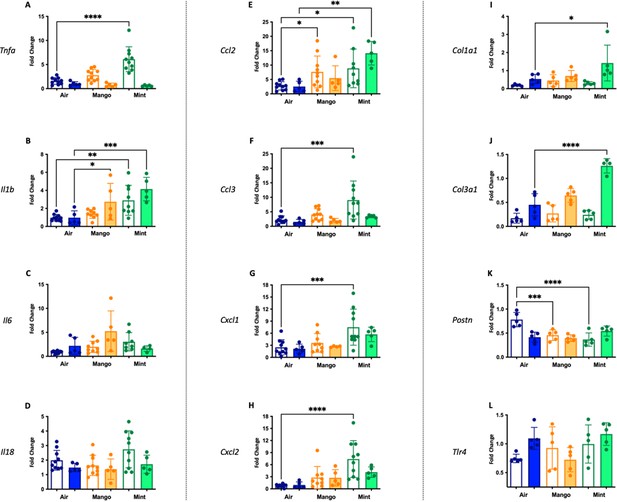
Inflammatory gene expression changes in the hearts of mice exposed to JUUL Mango and JUUL Mint and challenged with inhaled LPS.
Mice exposed to JUUL Mint aerosols three times daily (60 min total) for 1 month prior to LPS challenge had increased expression of Tnfa, Il1b, Ccl2, Ccl3, Cxcl1, and Cxcl2, and decreased Postn, relative to air controls, while mice exposed to JUUL Mango aerosols for 1 month had increased Ccl2 and decreased Postn. After 3 months of JUUL Mint exposure, cardiac tissue had increased Il1b, Ccl2, Il1b, Ccl2, and Col3a1 in the setting of LPS challenge, while JUUL Mango had increased Il1b. Data were analyzed with one-way ANOVA with Holm-Sidak multiple comparisons for each gene of interest. Data are presented as individual data points ± SD with n = 5–6 mice per group. *p < 0.05, **p < 0.01, *** p < 0.001, and ****p < 0.0001.
Three months of JUUL aerosol inhalation does not alter inflammatory markers in the setting of by inhaled LPS challengein the gastrointestinal tract.
Inflammation was assessed in the colon at 1 and 3 months. Panels show inflammation markers in the colon in Tnf (A) 1 month and (B) 3 months, Il6 at (C) 1 month and (D) 3 months, Il1b at (E) 1 month and (F) 3 months. Data for inflammation markers are presented as individual data points ± SEM.

Inflammatory gene expression in the colon of mice exposed to JUUL Mango and JUUL Mint and challenged with inhaled LPS.
These data are graphed with each exposure type (air – blue, mango – orange, and mint – green) grouped for ease of comparison of gene expression at 1 month (white columns) and 3 months (solid columns) of exposure. Data were analyzed with one-way ANOVA with Holm-Sidak multiple comparisons for each gene of interest. Data are presented as individual data points ± SD with n = 5–6 mice per group.
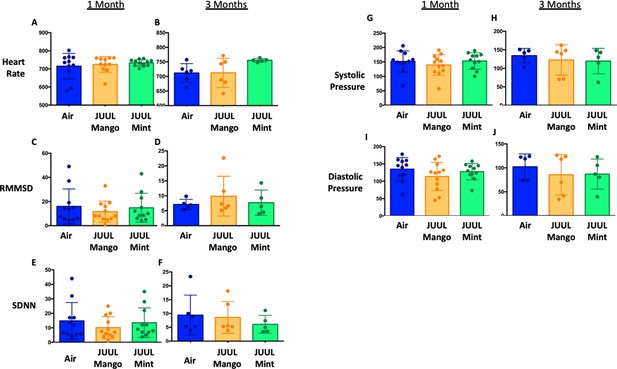
JUUL aerosol inhalation does not alter heart rate, heart rate variability or blood pressure.
Before assessment of lung function, mice underwent heart rate and blood pressure measurements using Emka non-invasive ECG Tunnels and the CODA non-invasive blood pressure system at 1 and 3 months. Heart rate (A–B), heart rate variability as measured by RMMSD and SDNN (C–F), systolic blood pressure (G–H) and diastolic blood pressure (I–J) were unaltered by chronic e-cigarette aerosol inhalation. Data are presented as individual data points ± SEM with n = 5–11 mice per group.
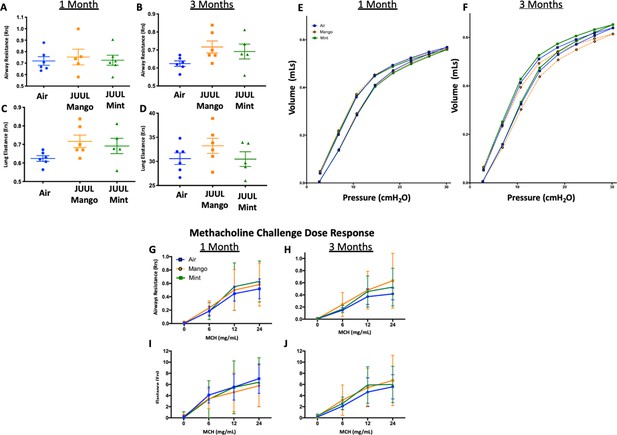
Chronic exposure of JUUL does not increase airways resistance or induce airways hyperreactivity.
At end points prior to harvest, mice underwent tracheostomy and attached to the FlexiVent mouse ventilator (SciReq). Airways resistance, lung elastance and pressure-volume (PV) loops were measured by mechanics scans using the FlexiVent mouse ventilator (SciReq) (A–F), followed by the assessment of responses to methacholine (MCH) challenge at 0, 6, 12 and 24 mg/mL (G–J). These parameters were assessed after 1 and 3 months of exposure of JUUL. Panels show airways resistance (Rrs) at (A) 1 month and (B) 3 months, Elastance at (C) 1 month and (D) 3 months, and PV loops at (E) 1 month and (F) 3 months. There were no differences in responses to methacholine challenge by Rrs at (G) 1 month and (H) 3 months, or Ers at (I) 1 month and (J) 3 months. Data for airways resistance and lung elastance are presented as individual data points ± SEM with n = 5–11 mice per group, and PV loops as means with n = 6 mice per group.
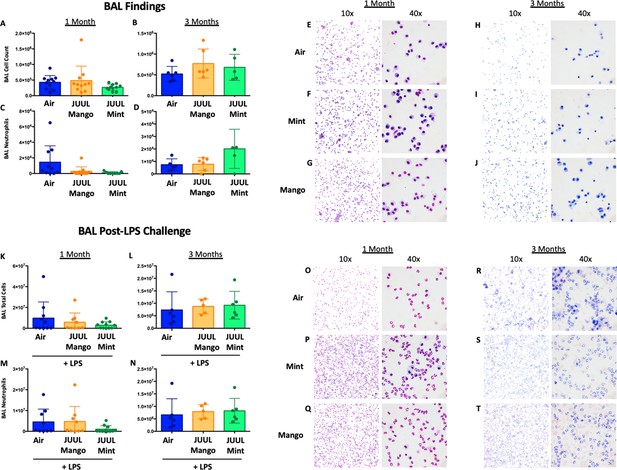
Chronic JUUL exposure does not affect leukocyte levels in the airways or influx into the airways and parenchyma in the setting of inhaled LPS challenge.
BAL was obtained, leukocyte counts were performed. BAL total cell counts in air versus JUUL exposed mice were no different at (A) 1 month and (B) 3 months, nor were neutrophils counts at (C) 1 month and (D) 3 months. Representative pictures from Giemsa Wright stained BAL cells are shown in (E,F,G) for 1 month and (H,I,J) for 3 months. In the setting of acute lung injury induced by inhaled LPS challenge, cell counts in the BAL were no different across groups (K–N), with representative images from BAL cells demonstrating the same (O–T). Data for cell counts are presented as individual data points ± SEM with n = 5–11 mice per group.
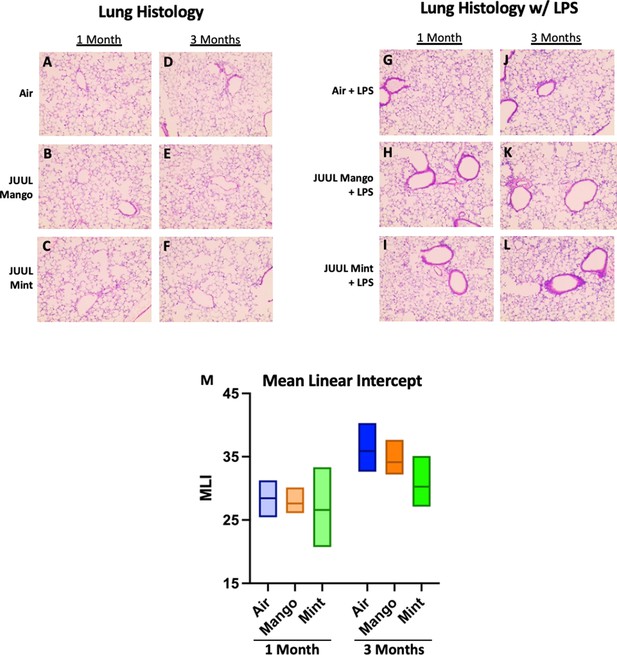
Chronic JUUL exposure does not affect lung parenchyma at baseline or in the setting of inhaled LPS challenge.
The left lung lobe was fixed with formalin at 25 cm3 water pressure and stained with H&E. Representative pictures from H&E staining of lung tissue are shown in A,B,C for 1 month and (D,E,F) for 3 months. In the setting of acute lung injury induced by inhaled LPS challenge, lung inflammation was no different by histologic evaluation (G–L). Mean linear intercept (MLI) was calculated for all lungs and were no different across groups at 1 and 3 months (M). n = 5–11 mice per group.
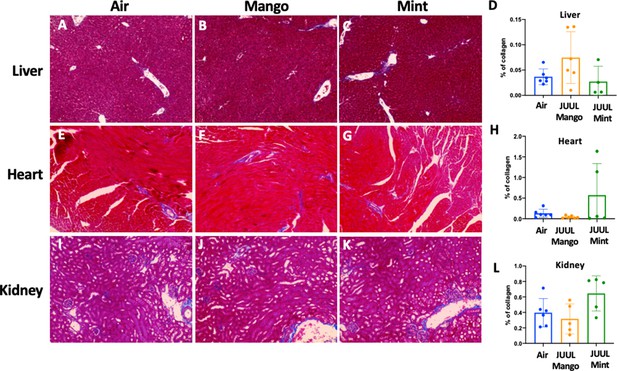
Chronic exposure of JUUL for 3 months does not induce fibrosis in the liver, heart, and kidney.
Collagen deposition was quantified by image analysis (using ImageJ) of lung histological slides stained with Masson’s trichrome. Representative pictures are shown for liver tissue in (A) Air control, (B) JUUL Mango, (C) JUUL Mint and (D) Quantification of collagen percentage in liver tissue. Representative pictures for heart tissue are shown in (E) Air control, (F) JUUL Mango, (G) JUUL Mint and (H) Quantification of collagen percentage in heart tissue. Representative pictures for heart tissue are shown in (I) Air control, (J) JUUL Mango, (K) JUUL Mint and (L) Quantification of collagen percentage in kidney tissue. Data for quantification is presented as individual data points ± SEM with n = 4–6 mice per group.
Tables
Primer sequences for qRT-PCR on colonic tissues.
| qPCR primers (Mouse) | Forward primer (3’- 5’) | Reverse primer (3’- 5’) |
|---|---|---|
| Mouse 18 s | GTAACCCGTTGAACCCCATT | CCATCCAATCGGTAGTAGCG |
| Mouse Il6 | CCCCAATTTCCAATGCTCTC C | CGCACTAGGTTTGCCGAGTA |
| Mouse Il1b | GAAATGCCACCTTTTGACAG T | CTGGATGCTCTCATCAGGAC A |
| Mouse Tnfa | CCACCACGCTCTTCTGTCTA | AGGGTCTGGGCCATAGAAC T |
| Mouse Il8 | CCTGCTCTGTCACCGATG | CAGGGCAAAGAACAGGTCA G |
| Mouse Ccl2 | AAGTGCAGAGAGCCAGACG | TCAGTGAGAGTTGGCTGGTG |
Primer sequences for qRT-PCR on brain tissues.
| Targets | Primers | Sequences | References |
|---|---|---|---|
| Gapdh | Forward (Sense) | 5′-ATGACATCAAGAAGGTGGTG-3′ | Sandhir et al., 2008 |
| Reverse (Antisense) | 5′-CATACCAGGAAATGAGSCTTG-3′ | ||
| Il1b | Forward (Sense) | CCAGCTTCAAATCTCACAGCAG | Kawane et al., 2010 |
| Reverse (Antisense) | CTTCTTTGGGTATTGCTTGGGATC | ||
| Tnfa | Forward (Sense) | CACAGAAAGCATGATCCGCGACGT | Kawane et al., 2010 |
| Reverse (Antisense) | CGGCAGAGAGGAGGTTGACTTTCT | ||
| Il6 | Forward (Sense) | TCCAGTTGCCTTCTTGGGAC | Kawane et al., 2010 |
| Reverse (Antisense) | GTACTCCAGAAGACCAGAGG |