The cryptic gonadotropin-releasing hormone neuronal system of human basal ganglia
Figures
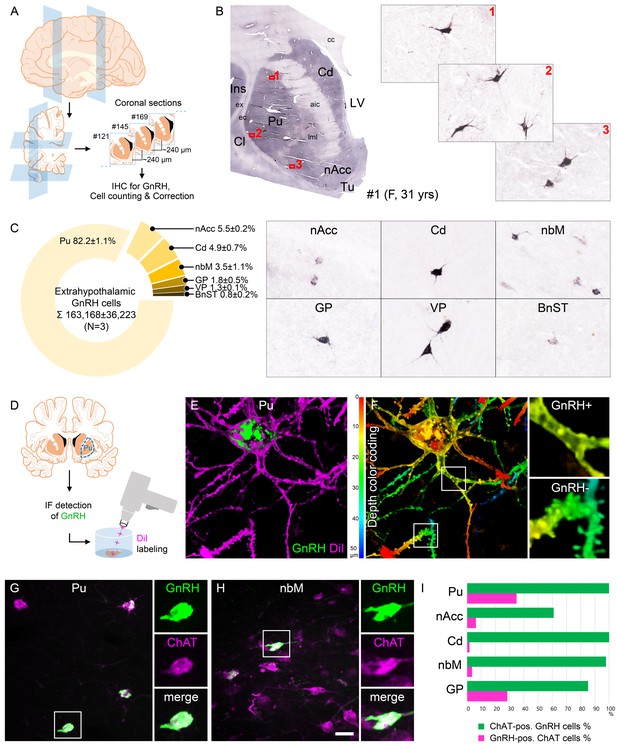
Anatomical approaches unveil the distribution, number, fine structure, and cholinergic phenotype of extrahypothalamic gonadotropin-releasing hormone (GnRH) neurons in the adult human brain.
(A) Extrahypothalamic GnRH-immunoreactive (GnRH-IR) neurons were mapped with immunohistochemistry (IHC) and quantified in the brain of three adult human individuals (#1–3). (B) Representative coronal section of a 31-year-old female subject (#1) illustrates the caudate nucleus (Cd), putamen (Pu), claustrum (Cl), insular cortex (Ins), anterior limb of the internal capsule (aic), external capsule (ec), extreme capsule (ex), corpus callosum (cc), lateral medullary lamina (lml), lateral ventricle (LV), nucleus accumbens (nAcc), and olfactory tubercle (Tu). High-power insets (1–3) reveal extrahypothalamic GnRH neurons many of which can be found in the Pu. (C) The majority (82.2%) of the 163,168 ± 36,223 extrahypothalamic GnRH neurons occurred in the Pu, followed by the nAcc, Cd, nucleus basalis magnocellularis (nbM), globus pallidus (GP), ventral pallidum (VP), and bed nucleus of the stria terminalis (BnST). (D) To visualize the fine structure of dendrites, the immunofluorescent (IF) detection of GnRH was combined with cell membrane labeling using DiI delivered with a Gene Gun. (E) 3-D reconstruction of the DiI-labeled (magenta) GnRH-IR (green) neurons revealed large multipolar cells, which exhibited only few dendritic spines. (F) Depth color coding, where colors represent distance from the section surface, allowed better distinction between DiI-labeled processes of the GnRH neuron (upper inset; GnRH+) from other DiI-labeled neuronal elements many of which belonged to medium spiny GABAergic projection neurons (lower inset; GnRH-). (G) Double-IF experiments addressed the presence of the cholinergic marker enzyme choline acetyltransferase (ChAT) in GnRH neurons. Nearly all GnRH neurons in the Pu contained ChAT signal. (H) The GnRH neuron population also overlapped with cholinergic projection neurons of the nbM. (I) With few exceptions, GnRH neurons were ChAT-immunoreactive (green columns), whereas they represented relatively small subsets of cholinergic cells (magenta columns) being the highest in the Pu (~35%). Scale bar (shown in H): 3.5 mm in low-power photomicrograph (B), 50 μm in (B, C, G and H) (insets in G and H: 25 μm), 12.5 μm in (E) and (F) (insets: 3.7 μm).
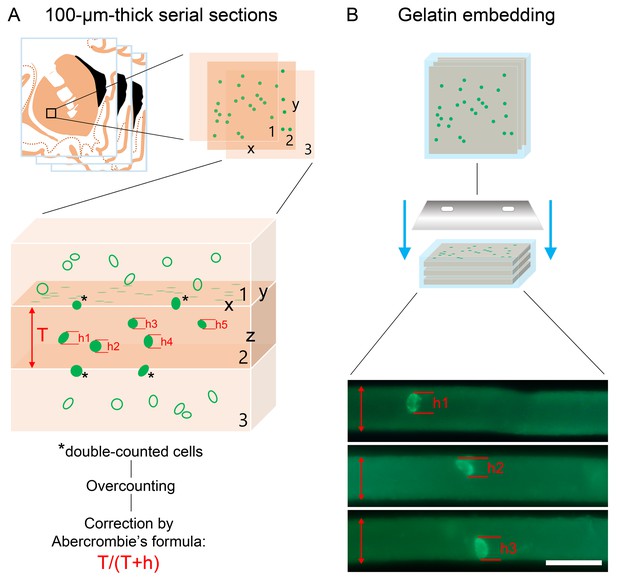
Cell numbers determined with light microscopic analysis require compensation for overcounting using Abercrombie’s correction factor.
Total extrahypothalamic gonadotropin-releasing hormone (GnRH) cell numbers were determined in three brains (#1–3) by counting GnRH-immunoreactive neurons in every 24th 100-µm-thick coronal section of a single hemisphere. This count was multiplied by 24 and then doubled with the assumption that neurons are distributed evenly between right and left hemispheres. (A) When section thickness (T) and cell diameter (h) along the Z axis are close to each other, a relatively high proportion of cells visualized in section 2 will be transected (asterisks). Note that taking into account the transected neurons at both surfaces of section 2 causes considerable overcounting within the tissue volume. This systematic error can be corrected by using Abercrombie’s correction factor calculated from the actual section thickness (T) and the mean diameter of uncut GnRH neurons (h) along the Z axis (schemas based on Guillery, 2002). (B) To determine T and h, GnRH neurons were detected in floating section of the putamen. Then, the sections were embedded into 2% agarose and recut perpendicular to the original section plane with a Vibratome, mounted on glass slides, coverslipped, and analyzed with confocal microscopy. Abercrombie’s correction factor obtained with this approach for the putamen was 0.712. Scale bar: 100 µm.
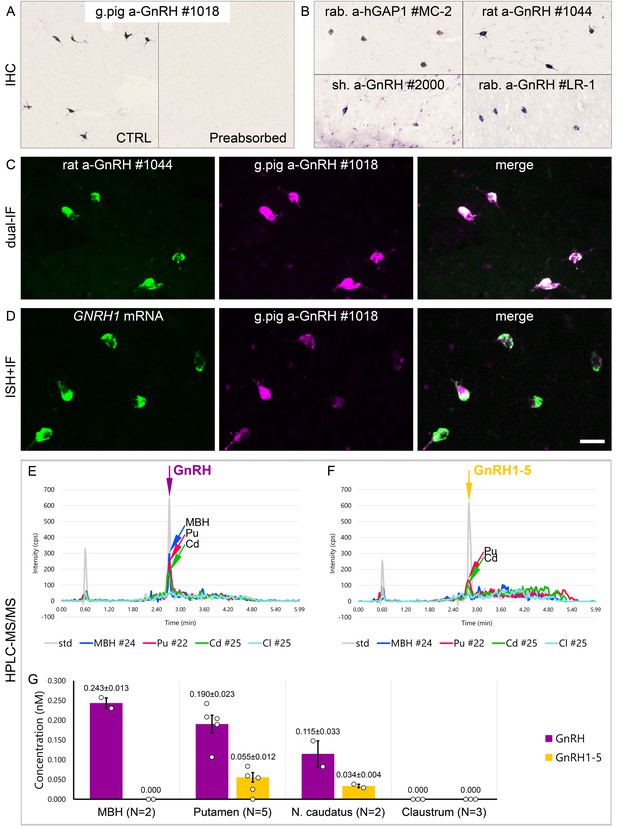
Combined evidence from immunohistochemistry (IHC), in situ hybridization (ISH), and high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) indicates that extrahypothalamic gonadotropin-releasing hormone (GnRH) neurons synthesize bona fide GnRH decapeptide derived from the GNRH1 transcript.
(A) IHC labeling of the human putamen with the guinea pig polyclonal GnRH antiserum #1018 reveals a large number of immunoreactive neurons in control sections (Ctrl) of a 64-year-old male subject (#19). All labeling is eliminated if the working solution of #1018 is preabsorbed overnight with 0.1 µg/ml GnRH. (B) A series of additional primary antisera against the human GnRH-associated peptide (hGAP1) or GnRH also recognize immunoreactive neurons in the human putamen (#6). Such antibodies include the LR-1 rabbit primary antiserum, which was reported previously not to label extrahypothalamic GnRH neurons in the developing monkey brain. (C) Positive control with the combined use of two GnRH antibodies from different host species for dual-immunofluorescence (IF) experiments on sample #5 provides evidence that the antibodies detect the same neuronal elements. (D) Non-isotopic ISH/IF dual-labeling studies reveal that GnRH-immunoreactive neurons express GNRH1 mRNA, indicating that extrahypothalamic GnRH is a GNRH1 gene product (sample #15). (E) As illustrated in representative chromatograms, HPLC-MS/MS ( #21–28) detects bona fide GnRH decapeptide in tissue extracts from the mediobasal hypothalamus (MBH), putamen (Pu), and nucleus caudatus (Cd), but not the claustrum (Cl). (F) The GnRH1-5 degradation product is present in the Pu and Cd and undetectable in the MBH and Cl. (G) Quantitative analysis reveals the highest tissue concentrations of GnRH in the MBH, somewhat lower levels in the Pu and the Cd, and no detectable GnRH decapeptide signal in the Cl. Note that tissue concentrations of GnRH in the Pu and the Cd are 3–4 times higher than those of GnRH1-5. Scale bar (shown in D): 120 μm in (A, B), 50 μm in (C, D).
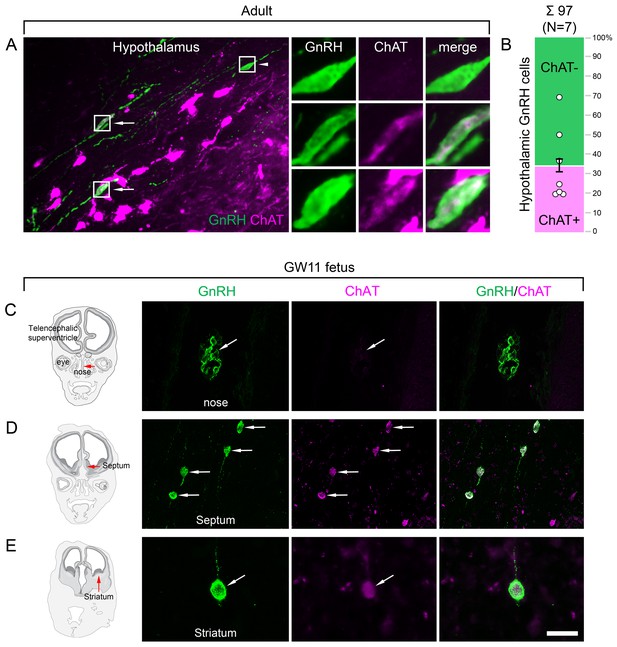
Both hypothalamic and extrahypothalamic gonadotropin-releasing hormone (GnRH) neurons exhibit cholinergic phenotype gained during early fetal development.
(A) The cholinergic phenotype is not a hallmark of human extrahypothalamic GnRH neurons because large subsets of GnRH neurons in the adult human hypothalamus (green immunofluorescent signal; subject #3) also exhibit choline acetyltransferase (ChAT; magenta) immunoreactivity. High-power insets show single- (arrowhead) and dual-labeled (arrows) GnRH neurons from framed regions. (B) Quantitative analysis of 97 GnRH neurons from seven subjects (#3; 6–11) reveals the ChAT phenotype in 34.6 ± 7.1% of hypothalamic GnRH neurons. (C–E) The cholinergic phenotype of GnRH neurons is gained during early fetal development. Left panels illustrate coronal views of the fetal head at GW11 (#29). Representative photomicrographs taken from sites indicated by the red arrows show results of dual-immunofluorescence experiments. (C) At this stage of development, a large subset of GnRH neurons (green immunofluorescent signal) migrate in the nasal region toward the brain and do not exhibit ChAT signal. (D, E) In contrast, GnRH neurons migrating through the septal area (D, arrows) or located in the striatum (E, arrow) express ChAT (magenta). Scale bar (shown in E): 50 µm in (A, C, D) (insets in A: 12.5 µm), 20 µm in (E).
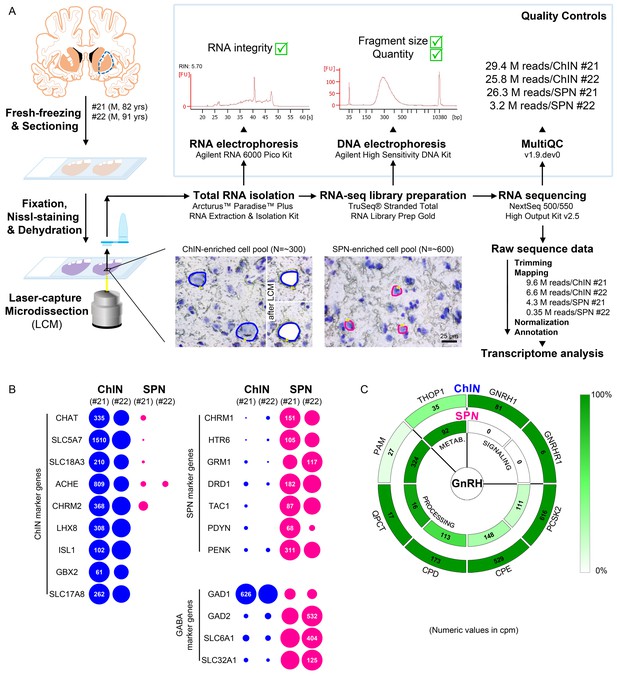
Deep transcriptome profiling of cholinergic interneurons (ChINs) and spiny projection neurons (SPNs) provides new insight into extrahypothalamic gonadotropin-releasing hormone (GnRH) signaling mechanisms and the molecular connectome of the human putamen.
(A) 20-µm-thick coronal sections were collected on PEN membrane slides from frozen putamen samples of two male human subjects (#21 and 22) and fixed with an ethanol/paraformaldehyde mixture. Neurons were visualized using Nissl-staining and isolated with laser-capture microdissection (LCM). 300 neurons included in each ChIN-enriched cell pool were recognized based on their large perikaryon size. The vast majority of the 600 medium-sized microdissected and pooled neurons corresponded to SPNs, the major putamen cell type. Total RNA was isolated and RNA-seq library prepared from both cell types and sequenced with the Illumina NextSeq 500/550 High Output (v2.5) kit. (B) Bioinformatic analysis verified high enrichment of known cholinergic markers in the two ChIN pools and of SPN markers in the two SPN pools. Expression levels in dots reflect counts per million reads (cpm), and in each case, dot areas reflect transcript abundances relative to the highest cpm (100%). (C) Key elements of proGnRH processing, GnRH signaling, and GnRH metabolism are illustrated in two concentric circles. The GNRH1 and GNRHR1 transcripts are present in ChINs only (outer circle). ChINs express all enzymes required for proGnRH processing. The promiscuous THOP1 enzyme, which may account for GnRH cleavage, occurs in both cell types, at higher levels in SPNs than in ChINs. Color coding reflects relative transcript abundances, whereas numbers indicate cpms (mean cpms of subjects #21 and 22).
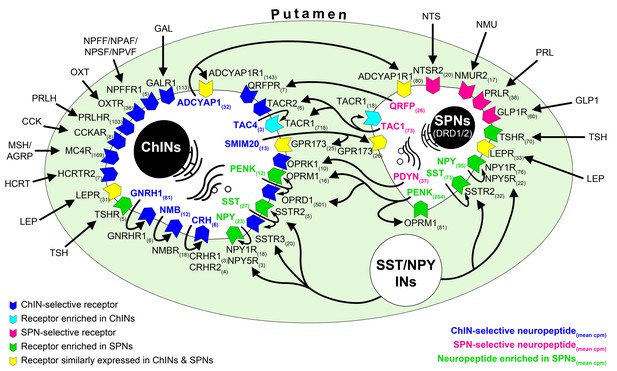
RNA-seq studies reveal the neuropeptide and neuropeptide receptor expression profiles of cholinergic interneurons (ChINs) and spiny projection neurons (SPNs) and provide insight into the molecular connectome of putamen cell types.
Proposed signaling mechanisms are based on neuropeptide and peptide receptor expression profiles of the two cell types. ChINs appear to use GNRHR1, CRHR1/2, and NMBR autoreceptor signaling. SSTR2, NPY1R/5R, OPRM1, and TACR1 may serve, at least partly, as autoreceptors in SPNs. Proposed peptidergic communication between the two cell types is also indicated by arrows. Other receptors receive ligands from different neuronal sources within (e.g., QRFPR, NPY1R/5R, TACR1, SSTR2/3) or outside (e.g., OXTR, MC4R, GLP1R, PRLR) the putamen. Numbers in receptor symbols reflect transcript abundances expressed as mean counts per million (cpms) from subjects #21 and 22. The figure illustrates receptors that were consistently observed in the given cell type of both human samples. INs: interneurons.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Biological sample (Homo sapiens) | Striatum, hypothalamus, putamen, n. caudatus, claustrum (adult) | 1st Department of Pathology and Experimental Cancer Research, Semmelweis University, Budapest, Hungary | See Supplementary file 1 | |
| Biological sample (Homo sapiens) | Head (fetus) | Agence de la Biomédecine, Saint-Denis la Plaine, France, protocol n°: PFS16–002 | See Supplementary file 1 | |
| Antibody | Anti-GAP (rabbit polyclonal) | Dr. M. D. Culler Culler and Negro-Vilar, 1986 | MC-2 | IHC (1:5000) |
| Antibody | Anti-GnRH (rabbit polyclonal) | Dr. R. A. Benoit Silverman et al., 1990 | LR-1 | IHC (1:10,000) |
| Antibody | Anti-GnRH (guinea pig polyclonal) | Made in-house Hrabovszky et al., 2011 | #1018 | IHC, IF-TSA (1:30,000) IF (1:10,000) |
| Antibody | Anti-GnRH (rat polyclonal) | Made in-house Skrapits et al., 2015 | #1014 | IHC, IF-TSA (1:20,000) |
| Antibody | Anti-GnRH (sheep polyclonal) | Made in-house Skrapits et al., 2015 | #2000 | IHC (1:1000) |
| Antibody | Anti-ChAT (goat polyclonal) | Merck | Cat#AB144P; RRID:AB_2079751 | IF (1:150), IF-TSA (1:2000) |
| Antibody | Anti-guinea pig IgG (H + L) Alexa Fluor 488 (donkey polyclonal) | Jackson ImmunoResearch | Cat#706-545-148; RRID:AB_2340472 | IF (1:400) |
| Antibody | Anti-goat IgG (H + L) Alexa Fluor 568 (donkey polyclonal) | Invitrogen | Cat#A-11057; RRID:AB_2534104 | IF (1:400) |
| Antibody | Biotin-SP-AffiniPure anti-guinea pig IgG (H + L) (donkey polyclonal) | Jackson ImmunoResearch | Cat#706-065-148; RRID:AB_2340451 | IHC (1:500) |
| Antibody | Biotin-SP-AffiniPure anti-rabbit IgG (H + L) (donkey polyclonal) | Jackson ImmunoResearch | Cat#711-065-152; RRID:AB_2340593 | IHC (1:500) |
| Antibody | Biotin-SP-AffiniPure anti-rat IgG (H + L) (donkey polyclonal) | Jackson ImmunoResearch | Cat#712-065-153; RRID:AB_2315779 | IHC (1:500), IF-TSA (1:500) |
| Antibody | Biotin-SP-AffiniPure anti-sheep IgG (H + L) (donkey polyclonal) | Jackson ImmunoResearch | Cat#713-065-147; RRID:AB_2340716 | IHC (1:500) |
| Antibody | Biotin-SP-AffiniPure anti-goat IgG (H + L) (donkey polyclonal) | Jackson ImmunoResearch | Cat#705-065-147; RRID:AB_2340397 | IF-TSA (1:500) |
| Antibody | Peroxidase-AffiniPure anti-guinea pig IgG (H + L) (donkey polyclonal) | Jackson ImmunoResearch | Cat#706-035-148; RRID:AB_2340447 | IF-TSA (1:250) |
| Antibody | Peroxidase-conjugated anti-digoxigenin, Fab fragments (sheep polyclonal) | Roche | 11207733910; RRID:AB_514500 | ISH (1:100) |
| Other | FITC-tyramide | Synthesized in-house Hopman et al., 1998 | IF-TSA (1:1000) | |
| Other | Cy3-tyramide | Synthesized in-house Hopman et al., 1998 | IF-TSA, ISH (1:1000) | |
| Other | ABC elite reagent | Vector Laboratories | PK-6100; RRID:AB_2336819 | IHC, IF-TSA (1:1000) |
| Sequence-based reagent | Antisense proGnRH | This paper | ISH probe | CATTCACAACACAGCA CTTTATTATGGAATATG TGCAACTTGGTGTAAGG ATTTCTGAAATTCATACC ATTTACAGGTATTTAATG GGTTATAAATTTTCAATG TCAGAATTATACTTAAGT CATGTTAGTAATGGTCAT TCCTTCTGGCCCAATGG ATTTAAATCTTCTTCTGC CCAGTTTCCTCTTCAATC AGACTTTCCAGAGCTCC TTTCAGGTCTCGGAGGG GAGAACGTGGCTGGTGC GTGGTGCATTCGAAGCG TTGGGTTTCTGCCAGTT GACCAACCTCTTTGACT ATCTCTTGGAAAGAATCA ATCAAATTTTCGGCATCT CTCTTTCCTCCAGGGCG CAGTCCATAGGACCAGT GCTGGCTGGAGCAGCCT TCCACGCACCAAGTCAG TAGAATAAGGCCAGCTA GGAGTTTTTGAATTGGC TTCATTCTGTTTAGAGG CAGAGAGCCAAAAAGATCC |
| Peptide, recombinant protein | GnRH decapeptide | Merck | Cat#L8008 | |
| Commercial assay or kit | Arcturus Paradise Plus RNA Extraction and Isolation Kit | ThermoFisher | Cat#KIT0312I | |
| Commercial assay or kit | RNA 6000 Pico Kit | Agilent | 5067-1513 | |
| Commercial assay or kit | TruSeq Stranded Total RNA Library Preparation Gold Kit | Illumina | Cat#20020598 | |
| Commercial assay or kit | High Sensitivity DNA Kit | Agilent | 5067-4626 | |
| Commercial assay or kit | NextSeq 500/550 High Output (v2.5) Kit | Illumina | Cat#20024906 | |
| Software, algorithm | AxioVision Imaging System 4.6 | Carl Zeiss | RRID:SCR_002677 | |
| Software, algorithm | Zen Black v.14.0.12.201 | Carl Zeiss | RRID:SCR_018163 | |
| Software, algorithm | PALM MicroBeam | Carl Zeiss | RRID:SCR_020929 | |
| Software, algorithm | Agilent Bioanalyzer 2100 Expert | Agilent Technologies | RRID:SCR_018043 |
Additional files
-
Supplementary file 1
Demographic information about the 30 donors and use of tissue specimens in different experiments.
ChAT: choline acetyltransferase; ChINs: cholinergic interneurons; GW11: gestational week 11; IF: immunofluorescence; IHC: immunohistochemistry; ISH: in situ hybridization; HPLC-MS/MS: high-performance liquid chromatography-tandem mass spectrometry; PMI: postmortem interval; RIN: RNA integrity number; RNA-seq: RNA sequencing; SPNs: medium spiny projection neurons.
- https://cdn.elifesciences.org/articles/67714/elife-67714-supp1-v2.docx
-
Supplementary file 2
Basic data on antibody specification, concentration, previous characterization, and on immunohistochemical reagents and applied detection methods.
ChAT: choline acetyltransferase; GAP1: GnRH-associated peptide-1; GnRH: gonadotropin-releasing hormone; IF: immunofluorescence; IHC: immunohistochemistry; TSA: tyramide signal amplification.
- https://cdn.elifesciences.org/articles/67714/elife-67714-supp2-v2.docx
-
Supplementary file 3
Detailed receptor expression profile and full list of expressed genes in cholinergic interneurons (ChINs) and spiny projection neurons (SPNs) of the human putamen.
Numeric values in counts per million reads.
- https://cdn.elifesciences.org/articles/67714/elife-67714-supp3-v2.xlsx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/67714/elife-67714-transrepform-v2.docx





