Characterization of the neurogenic niche in the aging dentate gyrus using iterative immunofluorescence imaging
Figures
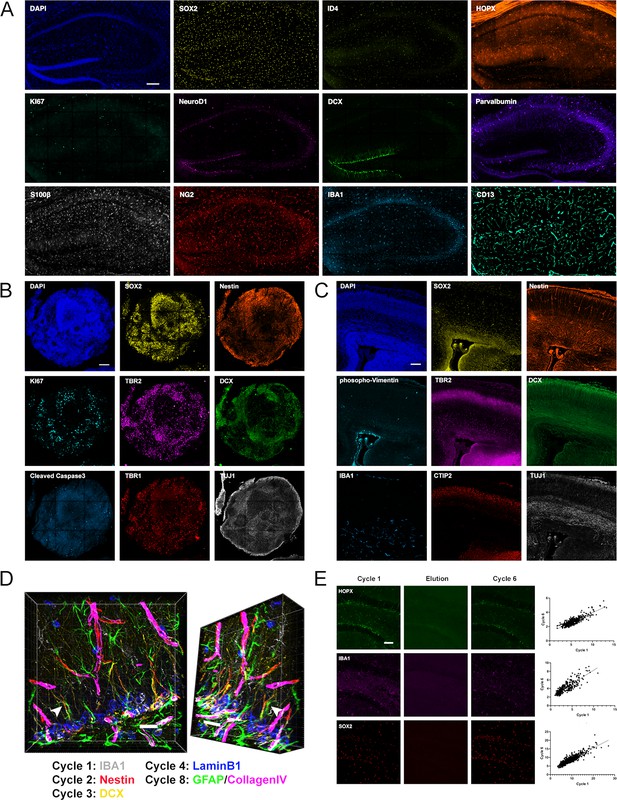
Iterative immunostaining in mouse and human tissue sections.
(A) Shown is an adult mouse dorsal hippocampal section labeled with 11 antibodies, as indicated in the individual panels, acquired over five rounds of 4i. SOX2/ID4/HOPX astrocytes/NSCs; KI67, proliferation; NeuroD1/DCX, immature neurons; Parvalbumin, interneurons; S100β, astrocytes; NG2, oligodendroglia; IBA1, microglia; and CD13, pericytes. Nuclei were counterstained with DAPI. (B) An hESC-derived, forebrain organoid section fixed at day 40 in vitro and labeled with eight antibodies, as indicated in the individual panels, over four rounds of 4i. SOX2/Nestin, apical progenitors; KI67, proliferation; TBR2, basal progenitors; DCX, immature neurons; cCaspase3, apoptotic cells; and TBR1/TUJ1, neurons. Nuclei were counterstained with DAPI. (C) Shown is a cortical section from an E14.5 mouse embryo labeled with eight antibodies, as indicated in the individual panels, over four rounds of 4i. Phopho-Vimentin, intermediate filaments; IBA1, microglia; SOX2/Nestin, astrocytes/NSCs; TBR2, basal progenitors; CTIP2, neurons; and DCX/TUJ1, immature neurons. Nuclei were counterstained with DAPI. (D) 3D reconstruction of a region in the dorsal DG analyzed with antibodies as indicated, with an arrow highlighting a Nestin+/GFAP+ R cell radial process indicating the spatial fidelity of 4i across cycles. (E) Examples of HOPX, IBA1, and SOX2 labeling in adult mouse brain sections, with proof of elution efficacy and restaining quality. The normalized fluorescent intensities for each stain were correlated between rounds. For details of statistics please refer to Supplementary file 1. Scale bars represent 200 µm (A), 100 µm (B, C), 25 µm (D), and 50 μm (E). 4i, immunofluorescence imaging; DG, dentate gyrus; hESC, human embryonic stem cell.
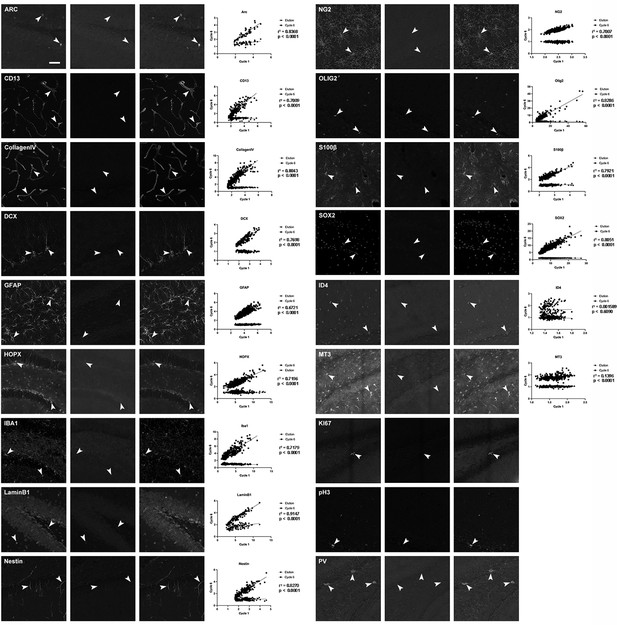
Examples of used antibody labeling in adult mouse brain sections, with proof of elution efficacy and restaining quality.
The normalized fluorescent intensities for each stain were correlated between rounds. For details of statistics please refer to Supplementary file 1. Scale bars represent 50 μm.
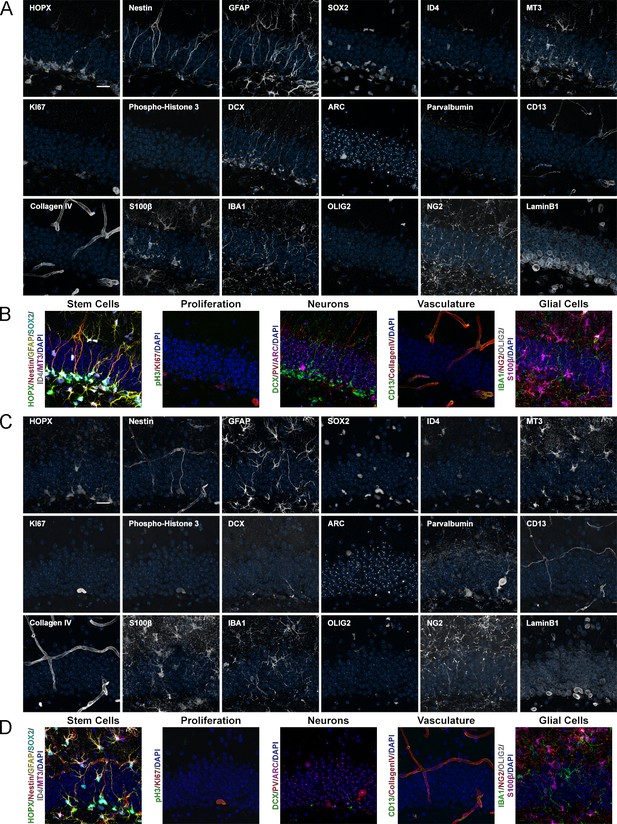
Expression of 18 proteins in the DG of mice with different ages.
(A) Images of 18 proteins labeled in the same area of the DG from a 2-month-old mouse. HOPX/Nestin/GFAP/SOX2/ID4/MT3 astrocytes/NSCs; KI67/phospho-Histone 3, proliferation; DCX, immature neurons; ARC, mature neurons; Parvalbumin, interneurons; CD13, pericytes; CollagenIV, vasculature; S100β, astrocytes; IBA1, microglia; OLIG2/NG2, oligodendroglia; and LaminB1, nuclear lamina. (B) shows merged channels for stem cells, proliferation, neurons, vasculature, and glial cells. Nuclei were counterstained with DAPI. (C) Images of 18 proteins labeled in the same area of the DG from a 12-month-old mouse. (D) shows composites grouping markers based on cell type. Note that the same sections were used for Figure 4, highlighting the power of iterative immunostaining using 4i. Scale bars represent 25 μm. DG, dentate gyrus; NSC, neural stem cell.
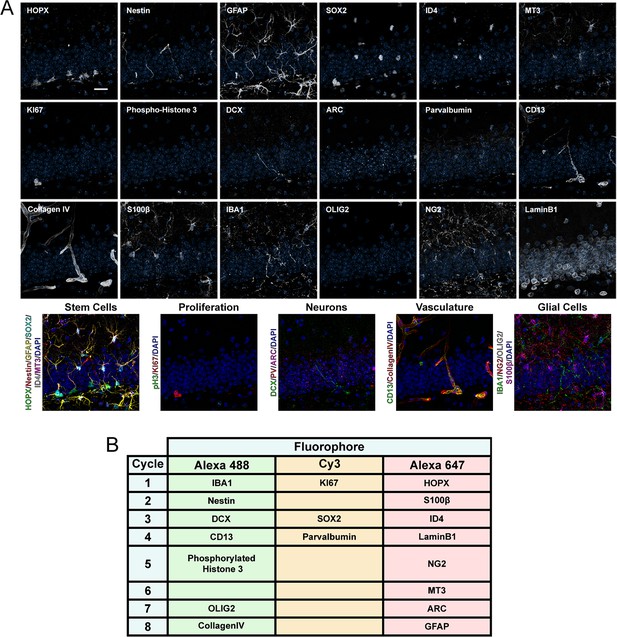
Expression of 18 proteins in the DG of 6-month-old mice.
(A) Close-up images of the 18 proteins labeled in the same area of the DG from a 6-month-old mouse with composites grouping markers based on cell type. (B) Combinations used for iterative immunostaining. Scale bar represents 25 μm. DG, dentate gyrus.
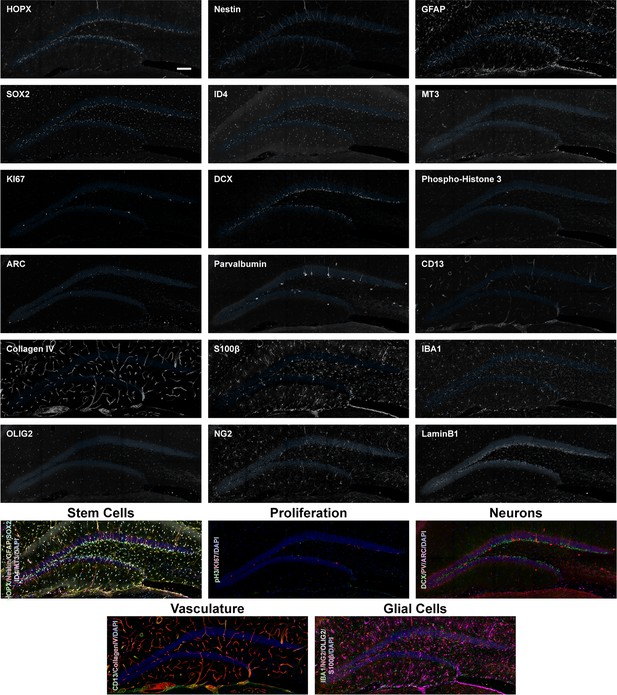
Overview expression of 18 proteins in the DG of 2-month-old mice.
Overview images of the 18 proteins labeled in the same area of the DG from a 2-month-old mouse with composites grouping markers based on cell type. Scale bar represents 100 µm. DG, dentate gyrus.

Overview expression of 18 proteins in the DG of 6-month-old mice.
Overview images of the 18 proteins labeled in the same area of the DG from a 6-month-old mouse with composites grouping markers based on cell type. Scale bar represents 100 µm. DG, dentate gyrus.
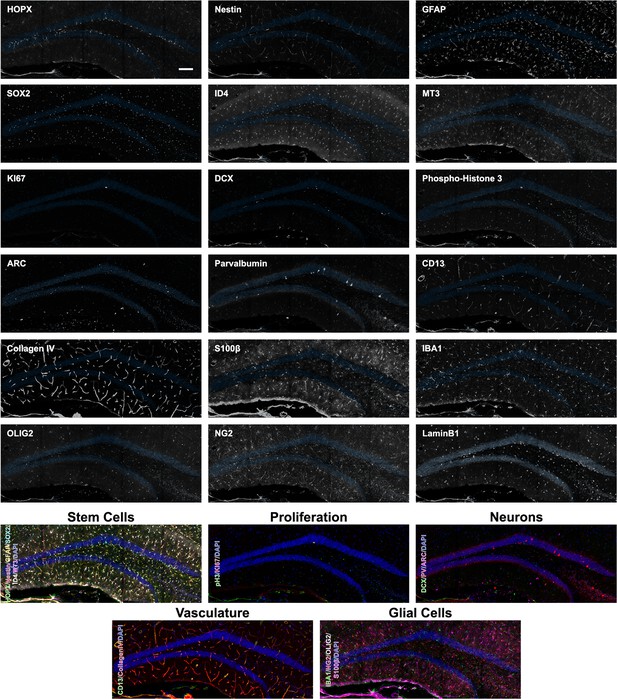
Overview expression of 18 proteins in the DG of 12-month-old mice.
Overview images of the 18 proteins labeled in the same area of the DG from a 12-month-old mouse with composites grouping markers based on cell type. Scale bar represents 100 µm. DG, dentate gyrus.
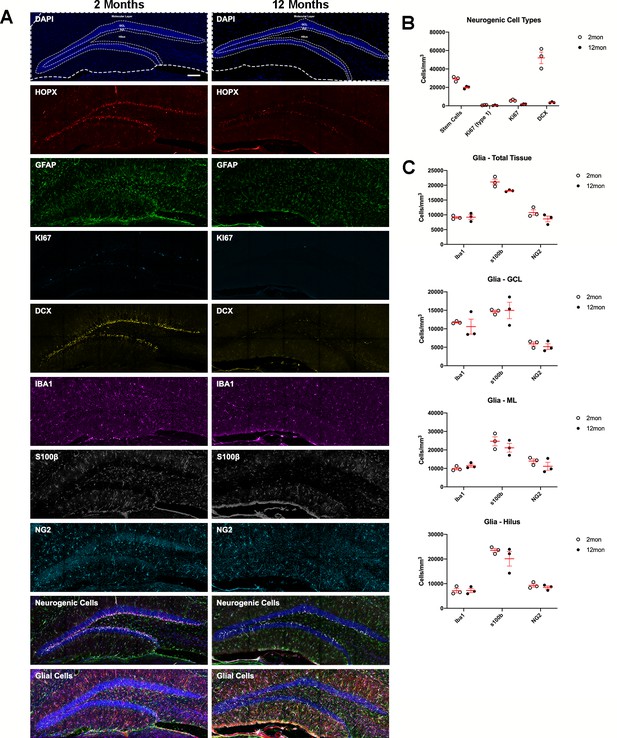
Age-related dynamics of cell populations in the mouse DG.
(A) Example overviews of the same DGs from 2- and 12-month-old mice showing the neurogenic and glial markers used to phenotype cells in population density measures. Nuclei were counterstained with DAPI. (B) Density analyses of neurogenic cells in the SGZ, defined as the number of R cells. For details of statistics please refer to Supplementary file 1. (C) Density analyses of microglia, OPCs, and astrocytes in the total sampled area of the DG, in the granule cell layer, the molecular layer, and hilus. For details of statistics please refer to Supplementary file 1. Scale bar represents 100 μm. *p<0.05, **p<0.01. DG, dentate gyrus; OPC, oligodendrocyte precursor cell; SGZ, subgranular zone.
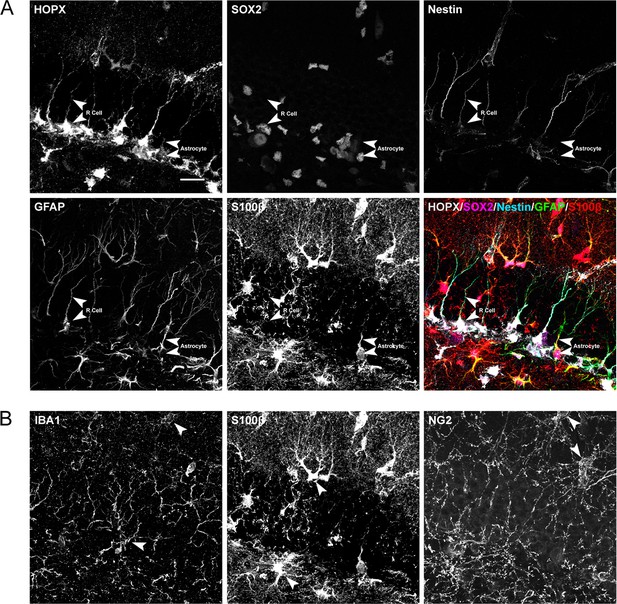
Expression of stem cell and glial cell markers in the DG.
(A) High-power views of markers for R cells (upper panels, arrowheads) and classical astrocytes (lower panels, arrowhedas). Right lower panel shows merged images with the markers indicated. (B) High-power views of markers for microglial (left panel arrowheads), astroglial (middle panel arrowheads), and oligodendroglial cells (right panel arrowheads). Scale bar represents 25 μm.
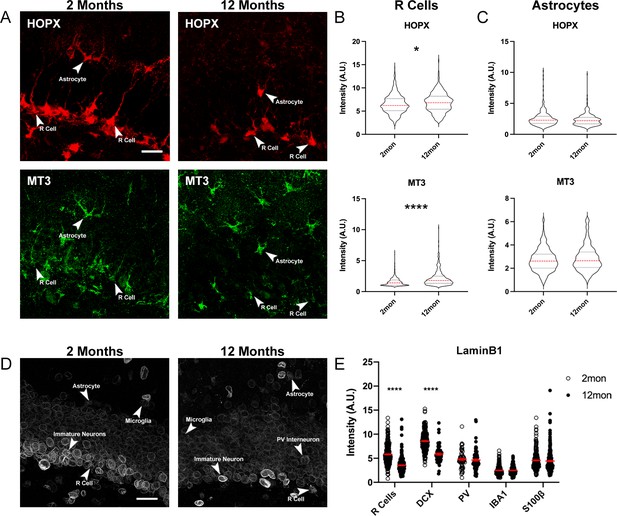
Age-dependent changes of protein expression in the DG.
(A) Representative images of 2- and 12-month-old DG sections labeled with quiescent markers HOPX and MT3. Arrowheads indicate the same cell across stains. (B) Quantification of the fluorescent intensities of HOPX and MT3 in R cells normalized to background. (C) Quantification of the fluorescent intensities of HOPX and MT3 in astrocytes normalized to background. For details of statistics please refer to Supplementary file 1. (D) Images showing LaminB1 in DGs from 2- and 112-month-old mice with different cell types indicated by arrows. (E) Quantification of the fluorescent intensities normalized to background. For details of statistics please refer to Supplementary file 1. Scale bars represent 25 μm. ****p<0.0001. DG, dentate gyrus.
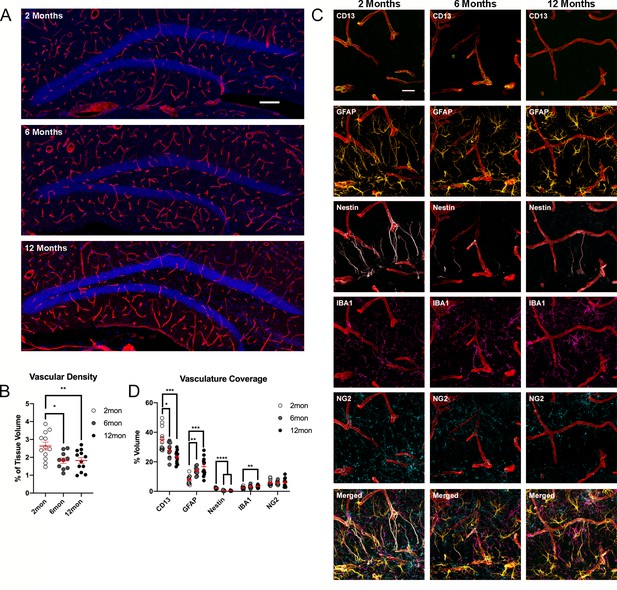
Changes of DG vasculature with advancing age.
(A) Images of CollagenIV+ blood vessels in the DGs at 2, 6, and 12 months. Nuclei were counterstained with DAPI. (B) Quantification of vascular density in the DGs of 2-, 6-, and 12-month-old mice. For details of statistics please refer to Supplementary file 1. (C) Representative images showing the interactions of pericytes, glia, and R cells with the vasculature in the DG. (D) Quantification of colocalization of pericytes, astrocytes, R cells, microglia, and OPCs with CollagenIV+ represented a percentage of total vascular volume. For details of statistics please refer to Supplementary file 1. Scale bars represent 100 µm (A), 25 µm (C). **p<0.01, ***p<0.001, ****p<0.0001. DG, dentate gyrus; OPC, oligodendrocyte precursor cell.
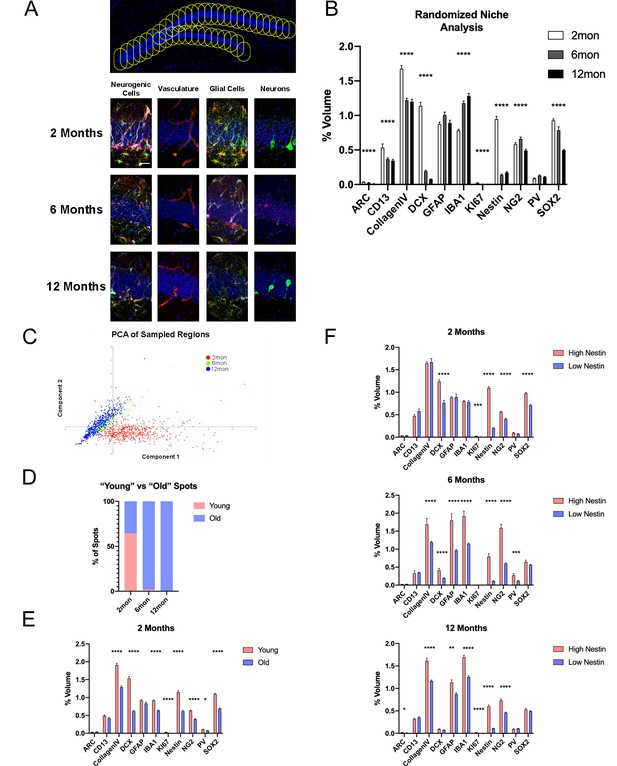
Microniche analysis reveals features of the neurogenic niche with advancing age.
(A) A representative overview of the arrangement of randomized sampling spots, with close-up examples of the cellular contents within a single sampled spot for each age group. (B) Quantification of volumes of cell types within the random sampled spots normalized to the sampled area. For details of statistics please refer to Supplementary file 1. (C) Principle component analysis for dimensional reduction and clustering of the random sampled spots from 2-, 6-, and 12-month groups based on cellular content. (D) Percentage of spots identified as either ‘young’ or ‘old’ separated through k-means analysis. (E) Comparison of cell volumes in spots identified as young and old in the 2-month-old group. (F) Comparison of cell volumes in spots identified as high and low R cell populated as neurogenic classified of age in the 2- (upper graph), 6- (middle graph), and 12-month group (lower graph). Scale bars represent 25 µm (A, lower panels) and 100 µm (upper panel). *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
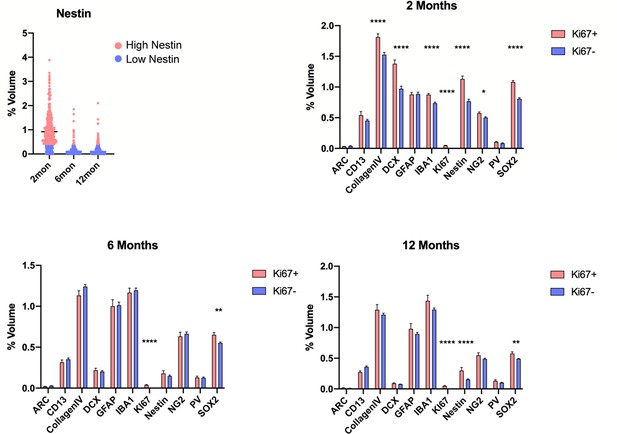
Quantification of proliferating and non-proliferating cellular microenvironments in the DG.
(A) The volume of Nestin-labeled radial processes within the random sampled spots. Spots in red were classified as high Nestin populated while blue represents low Nestin spots. (B–D) Comparison of cell volumes in spots containing and lacking Ki67+ cells in the 2 month (B). For details of statistics please refer to Supplementary file 1. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
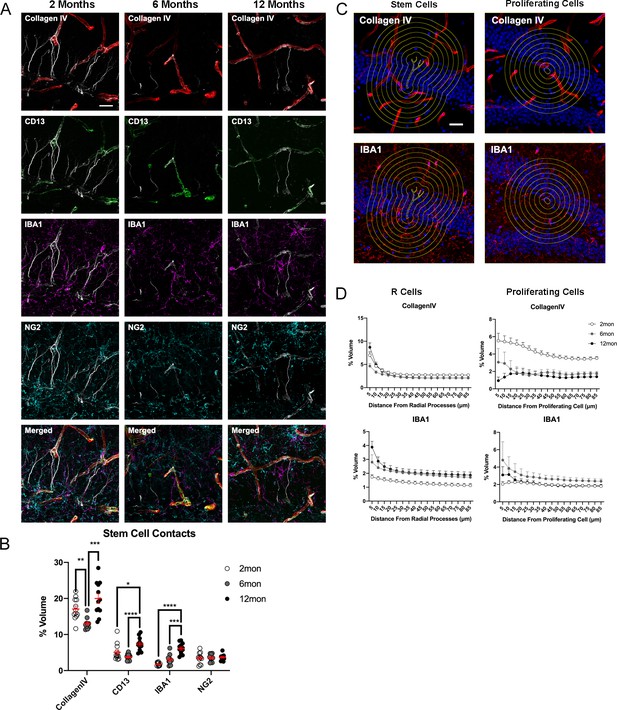
Age-dependent dynamics of the niche surrounding adult stem cells.
(A) Representative images showing the interactions of R cells with vascular and glial cells in the surrounding niche in the DG in 2-, 6-, and 12-month-old mice. (B) Quantification of colocalization of blood vessels, pericytes, microglia, and OPCs with CollagenIV+ represented a percentage of total vascular volume. For details of statistics please refer to Supplementary file 1. (C) Representative images of areas measured for volumes of vasculature and microglia at radiating distances around Nestin+ R cells and Ki67+ proliferating cells. (D) Quantification of volumes of vasculature and microglia at radiating distances from R cells and Ki67+ proliferating cells. For details of statistics please refer to Supplementary file 1. Scale bars represent 25 μm. Scale bar represents 100 µm. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. DG, dentate gyrus; OPC, oligodendrocyte precursor cell.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Biological samples (Mus musculus) | C57BL/6JRj | Janvier Labs | https://www.janvier-labs.com/en/fiche_produit/c57bl-6jrj_mouse/ | |
| Antibody | ARC (Guinea pig Polyclonal) | Synaptic Systems | RRID:AB_2619853 | Dilution: (1:500) |
| Antibody | BLBP (Rabbit Polyclonal) | Abcam | RRID:AB_880078 | Dilution: (1:200) |
| Antibody | CD13 (Goat Polyclonal) | Novus | RRID:AB_2227288 | Dilution: (1:500) |
| Antibody | Cleaved Caspase 3 (Rabbit Polyclonal) | Cell Signaling Technology | RRID:AB_2341188 | Dilution: (1:150) |
| Antibody | CollagenIV (Rabbit Polyclonal) | Bio-Rad | RRID:AB_2082660 | Dilution: (1:750) |
| Antibody | CTIP2 (Rat Monoclonal) | Abcam | RRID:AB_2064130 | Dilution: (1:250) |
| Antibody | Doublecortin (Goat Polyclonal) | Santa Cruz | RRID:AB_2088491 | Dilution: (1:300) |
| Antibody | GFAP (Chicken Polyclonal) | Novus | RRID:AB_1556315 | Dilution: (1:750) |
| Antibody | Histone H3 Phospho S10 (Mouse Monoclonal) | Abcam | RRID:AB_443110 | Dilution: (1:250) |
| Antibody | HOPX (Mouse Monoclonal) | Santa Cruz | RRID:AB_2687966 | Dilution: (1:500) |
| Antibody | IBA1 (Rabbit Polyclonal) | WAKO | RRID:AB_839504 | Dilution: (1:500) |
| Antibody | ID4 (Rabbit Monoclonal) | Biocheck | RRID:AB_2814978 | Dilution: (1:250) |
| Antibody | KI67 (Rat Monoclonal) | Bioscience | RRID:AB_10854564 | Dilution: (1:250) |
| Antibody | LaminB1 (Rabbit Polyclonal) | Abcam | RRID:AB_10107828 | Dilution: (1:500) |
| Antibody | MT3 (Rabbit Monoclonal) | Abcam | RRID:AB_2297959 | Dilution: (1:300) |
| Antibody | Nestin (Mouse Monoclonal) | BD | RRID:AB_396354 | Dilution: (1:250) |
| Antibody | NeuroD1 (Goat Polyclonal) | Santa Cruz | RRID:AB_630922 | Dilution: (1:250) |
| Antibody | NG2 (Rabbit Polyclonal) | Millipore | RRID:AB_11213678 | Dilution: (1:500) |
| Antibody | OLIG2 (Rabbit Polyclonal) | Millipore | RRID:AB_570666 | Dilution: (1:500) |
| Antibody | Parvalbumin (Mouse Monoclonal) | Sigma-Aldrich | RRID:AB_477329 | Dilution: (1:250) |
| Antibody | S100β (Rabbit Monoclonal) | Abcam | RRID:AB_882426 | Dilution: (1:500) |
| Antibody | SOX2 (Rat Monoclonal) | Invitrogen | RRID:AB_11219471 | Dilution: (1:250) |
| Antibody | Anti-Chicken IgY (IgG) 488 (Donkey Polyclonal) | Jackson Laboratory | RRID:AB_2340375 | Dilution: (1:250) |
| Antibody | Anti-Chicken IgY (IgG) 647 (Donkey Polyclonal) | Jackson Laboratory | RRID:AB_2340379 | Dilution: (1:250) |
| Antibody | Anti-Goat IgG 488 (Donkey Polyclonal) | Jackson Laboratory | RRID:AB_2336933 | Dilution: (1:250) |
| Antibody | Anti-Goat IgG 647 (Donkey Polyclonal) | Jackson Laboratory | RRID:AB_2340437 | Dilution: (1:250) |
| Antibody | Anti-Goat IgG Cy3 (Donkey Polyclonal) | Jackson Laboratory | RRID:AB_2307351 | Dilution: (1:250) |
| Antibody | Anti-Guinea pig IgG488 (Donkey Polyclonal) | Jackson Laboratory | RRID:AB_2340472 | Dilution: (1:250) |
| Antibody | Anti-Guinea pig IgG647 (Donkey Polyclonal) | Jackson Laboratory | RRID:AB_2340476 | Dilution: (1:250) |
| Antibody | Anti-Mouse IgG 488 (Donkey Polyclonal) | Jackson Laboratory | RRID:AB_2341099 | Dilution: (1:250) |
| Antibody | Anti-Mouse IgG 647 (Donkey Polyclonal) | Jackson Laboratory | RRID:AB_2340863 | Dilution: (1:250) |
| Antibody | Anti-Mouse IgG Cy3 (Donkey Polyclonal) | Jackson Laboratory | RRID:AB_2315777 | Dilution: (1:250) |
| Antibody | Anti-Rabbit IgG 488 (Donkey Polyclonal) | Jackson Laboratory | RRID:AB_2313584 | Dilution: (1:250) |
| Antibody | Anti-Rabbit IgG 647 (Donkey Polyclonal) | Jackson Laboratory | RRID:AB_2492288 | Dilution: (1:250) |
| Antibody | Anti-Rabbit IgG Cy3 (Donkey Polyclonal) | Jackson Laboratory | RRID:AB_2307443 | Dilution: (1:250) |
| Antibody | Anti-Rat IgG 488 (Donkey Polyclonal) | Jackson Laboratory | RRID:AB_2340684 | Dilution: (1:250) |
| Antibody | Anti-Rat IgG 647 (Donkey Polyclonal) | Jackson Laboratory | RRID:AB_2340694 | Dilution: (1:250) |
| Antibody | Anti-Rat IgG Cy3 (Donkey Polyclonal) | Jackson Laboratory | RRID:AB_2340667 | Dilution: (1:250) |
| Chemical compound, drug | Donkey serum | Millipore | Cat#: 530-100 ML | |
| Chemical compound, drug | Ethylene glycol | Sigma-Adrich | Cat#: 324558 | |
| Chemical compound, drug | Glycerol | Sigma-Aldrich | Cat#: G5516 | |
| Chemical compound, drug | Glycine | Biosolve | Cat#: 071323 | |
| Chemical compound, drug | Guanidinium chloride | Sigma-Aldrich | Cat#: G4505 | |
| Chemical compound, drug | Hydrochloric acid standard 33% solution | Sigma-Aldrich | Cat#: 71826 | |
| Chemical compound, drug | N-Acetyl- Cysteine | Sigma-Aldrich | Cat#: A9165 | |
| Chemical compound, drug | NaCl | Sigma-Aldrich | Cat#: S9625 | |
| Chemical compound, drug | O.C.T compound TissueTek | Sakura | Cat#: 4583 OCT 25608-930 | |
| Chemical compound, drug | Para formaldehyde | Sigma-Aldrich | Cat#: 441244 | |
| Chemical compound, drug | Poly-D-Lysine | Sigma-Aldrich | Cat#: P6407 | |
| Chemical compound, drug | Potassium chloride | Sigma-Aldrich | Cat#: P9333 | |
| Chemical compound, drug | Potassium phosphate monobasic | Sigma-Aldrich | Cat#: P8709 | |
| Chemical compound, drug | Sodium hydroxide | Sigma-Aldrich | Cat#: S5881 | |
| Chemical compound, drug | Sodium phosphate dibasic dehydrate | Sigma-Aldrich | Cat#: 30435 | |
| Chemical compound, drug | Sodium phosphate monobasic monohydrate | Sigma-Aldrich | Cat#: S9638 | |
| Chemical compound, drug | Sucrose | Sigma-Aldrich | Cat#: 84100 | |
| Chemical compound, drug | TCEP-HCl | Sigma-Aldrich | Cat#: C4706 | |
| Chemical compound, drug | Triton X-100 | Sigma-Aldrich | Cat#: 93443 | |
| Chemical compound, drug | Urea | Sigma-Aldrich | Cat#: U1250 | |
| Software, algorithm | GraphPad Prism | GraphPad | RRID:SCR_002798 | http://www.graphpad.com/ |
| Software, algorithm | Fiji/ImageJ | Fiji | RRID:SCR_002285 | http://fiji.sc |
| Software, algorithm | ZEN Blue | Carl Zeiss AG | RRID:SCR_013672 | http://www.zeiss.com/microscopy/en_us/products/microscope-Software, Algorithm/zen. html#introduction |
| Software, algorithm | Past4.03 | Oyvind Hammer | RRID:SCR_019129 | http://folk.uio.no/ohammer/past/ |
| Other | DAPI | Sigma- Aldrich | Cat#: D9542 | Dilution: (1:1000) |
Additional files
-
Supplementary file 1
Values and statistics for graphs shown in main Figures 1 and 3—7 and Figure 6—figure supplement 1 of Figure 6.
- https://cdn.elifesciences.org/articles/68000/elife-68000-supp1-v1.xlsx
-
Supplementary file 2
Primary and secondary antibody combinations used for antigenicity testing.
- https://cdn.elifesciences.org/articles/68000/elife-68000-supp2-v1.xlsx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/68000/elife-68000-transrepform1-v1.docx





