Myosin-based regulation of twitch and tetanic contractions in mammalian skeletal muscle
Figures
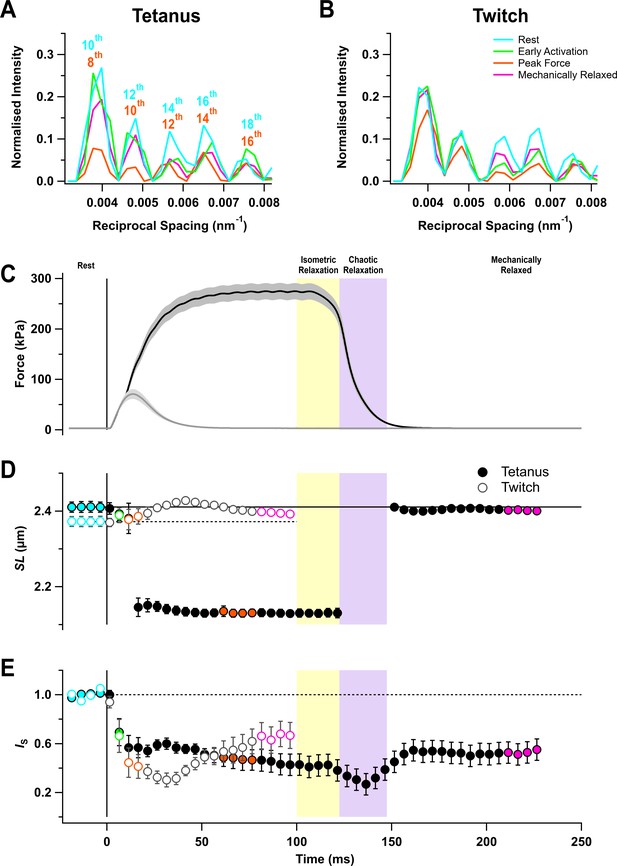
Force and sarcomere length changes in fixed-end twitch and tetanus.
(A) Ultra-low-angle X-ray reflections from the sarcomere periodicity with indicated orders of the fundamental sarcomere length repeat in a tetanus. Cyan, rest; green, early activation; orange, tetanus plateau; magenta, mechanically relaxed. (B) Corresponding results for the twitch, with orange denoting the peak force in the twitch. (C) Time course of changes in force with SEM indicated by grey shading. (D) Sarcomere length (SL). (E) Intensity of sarcomere reflections (IS) determined from the average intensities of the third and fourth peaks in the region shown in (A) and (B) normalised by the mean resting value. Filled and open symbols in (D) and (E) denote tetanus and twitch, respectively; coloured symbols denote the time periods used to calculate the profiles in (A) and (B); error bars denote SEM for n = 5 muscles for tetanus and n = 4 muscles for twitch. Yellow- and purple-shaded panels denote isometric and chaotic relaxation. Black horizontal continuous and dashed lines denote resting values.
-
Figure 1—source data 1
An excel file containing the data for individual muscles from which the mean and SEM shown in Figure 1 were calculated.
- https://cdn.elifesciences.org/articles/68211/elife-68211-fig1-data1-v2.xlsx
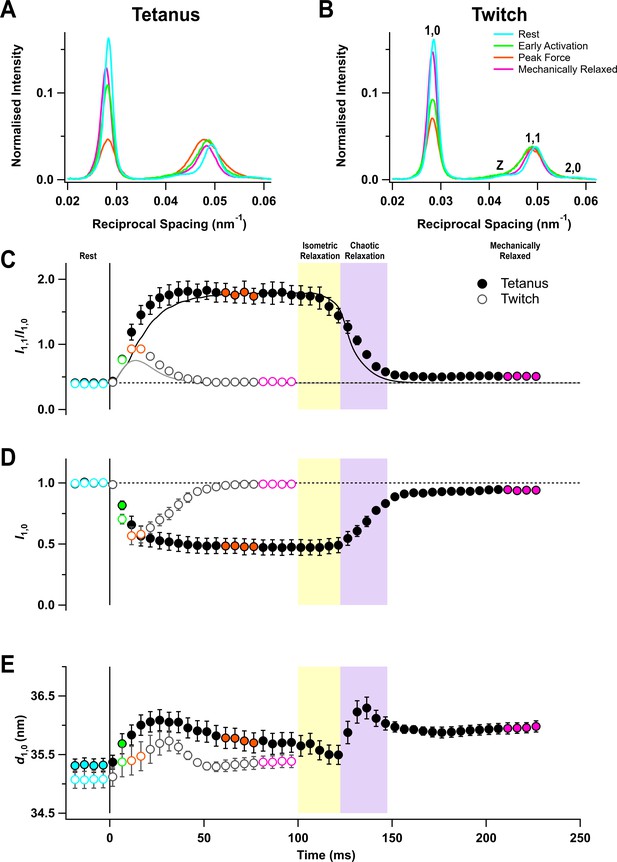
Equatorial X-ray reflections.
(A) Distribution of intensity along the equator of the diffraction pattern, perpendicular to the muscle long axis, in different phases of a tetanus. Cyan, rest; green, early activation; orange, tetanus plateau; magenta, mechanically relaxed. (B) Corresponding results for the twitch, with orange denoting the peak force in the twitch. (C) Time course of the ratio of the intensities of (1,0) and (1,1) reflections (I1,1/I1,0) superimposed on the force in the tetanus (black) and twitch (grey). (D) Time course of the intensity of (1,0) reflection (I1,0) normalised by the mean resting value. (E) Time course of the filament lattice spacing parameter d1,0. Filled and open symbols in (C), (D), and (E) denote tetanus and twitch respectively; coloured symbols denote the time periods used to calculate the profiles in (A) and (B); error bars denote SEM for n = 5 muscles for tetanus and n = 4 muscles for twitch. Yellow- and purple-shaded panels denote isometric and chaotic relaxation. Black horizontal dashed lines denote resting values.
-
Figure 2—source data 1
An excel file containing the data for individual muscles from which the mean and SEM shown in Figure 2 were calculated.
- https://cdn.elifesciences.org/articles/68211/elife-68211-fig2-data1-v2.xlsx
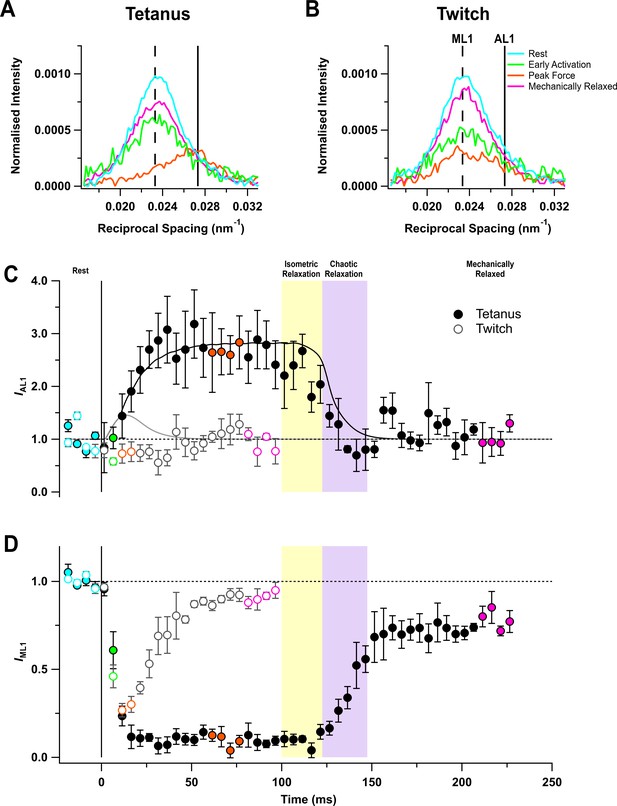
Myosin- and actin-based layer line reflections.
(A) Axial distribution of diffracted intensity in the region of the mixed myosin/actin first layer line in different phases of a tetanus. Cyan, rest; green, early activation; orange, tetanus plateau; magenta, mechanically relaxed. The vertical dashed and continuous lines denote the global best-fit reciprocal spacings of the myosin- and actin-based layer lines, respectively. (B) Corresponding results for the twitch, with orange denoting the peak force in the twitch. (C) Time course of the intensity of the first actin layer line (IAL1) normalised to the mean resting value and superimposed on the force in tetanus (black) and twitch (grey). (D) Time course of the intensity of the first myosin layer line (IML1) normalised to the mean resting value. Filled and open symbols in (C) and (D) denote tetanus and twitch, respectively; coloured symbols denote the time periods used to calculate the profiles in (A) and (B); error bars denote SEM for n = 5 muscles for tetanus and n = 4 muscles for twitch. Yellow- and purple-shaded panels denote isometric and chaotic relaxation. Black horizontal dashed lines denote resting values.
-
Figure 3—source data 1
An excel file containing the data for individual muscles from which the mean and SEM shown in Figure 3 were calculated.
- https://cdn.elifesciences.org/articles/68211/elife-68211-fig3-data1-v2.xlsx

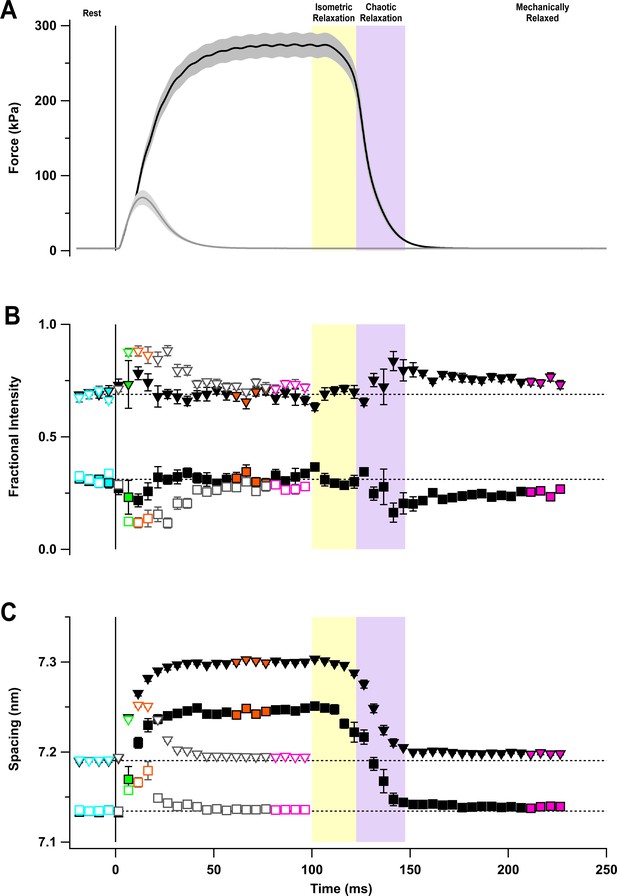
The M6 reflection.
(A) Axial distribution of diffracted intensity in the region of the M6 reflection in different phases of a tetanus corrected by the cross-meridional width. Cyan, rest; green, early activation; orange, tetanus plateau; magenta, mechanically relaxed. (B) Corresponding results for the twitch, with orange denoting the peak force in the twitch. (C) Time course of the width-corrected intensity of the M6 reflection (IM6) normalised to the mean resting value. (D) Time course of the spacing of the M6 reflection (SM6) superimposed on the force in tetanus (black) and twitch (grey). Filled and open symbols in (C) and (D) denote tetanus and twitch, respectively. Coloured symbols denote the time periods used to calculate the profiles in (A) and (B); error bars denote SEM for n = 5 muscles for tetanus and n = 4 muscles for twitch. Yellow- and purple-shaded panels denote isometric and chaotic relaxation. Black horizontal dashed lines denote resting values.
-
Figure 4—source data 1
An excel file containing the data for individual muscles from which the mean and SEM shown in Figure 4 were calculated.
- https://cdn.elifesciences.org/articles/68211/elife-68211-fig4-data1-v2.xlsx
Fractional intensities and spacings of the low-angle and high-angle components of the M6 reflection.
(A) Time course of changes in force with SEM indicated by grey shading. (B) Fractional intensities of the lower (inverted triangles) and higher angle (squares) components of the M6 reflection. (C) Spacings of the lower (inverted triangles) and higher angle (squares) components of the M6 reflection. Filled and open symbols in panels (B) and (C) denote tetanus and twitch, respectively. Coloured symbols denote the time periods used to calculate the profiles in Figure 4A and B; error bars denote SEM for n = 5 muscles for tetanus and n = 4 muscles for twitch. Yellow- and purple-shaded panels denote isometric and chaotic relaxation. Black horizontal dashed lines denote resting values.

The M3 reflection.
(A) Axial distribution of diffracted intensity in the region of the M3 reflection in different phases of a tetanus corrected by the cross-meridional width. Cyan, rest; green, early activation; orange, tetanus plateau; magenta, mechanically relaxed. (B) Corresponding results for the twitch, with orange denoting peak force in the twitch. The star, low-angle (LA), mid-angle (MA), and high-angle (HA) sub-peaks are indicated. (C) Time course of the width-corrected intensity of the M3 reflection (IM3) normalised to the mean resting value and superimposed on the force in tetanus (black) and twitch (grey). (D) Time course of the spacing of the M3 reflection (SM3) with force superimposed. (E) Fractional intensities of the star (triangles), low-angle (LA; inverted triangles), mid-angle (MA; squares), and high-angle (HA; diamonds) sub-peaks with symbols defined in panel (B). (F) Spacings of the star, LA, MA, and HA sub-peaks with symbols defined in panel (B). Filled and open symbols in (C–F) denote tetanus and twitch, respectively. Coloured symbols denote the time periods used to calculate the profiles in (A) and (B); error bars denote SEM for n = 5 muscles for tetanus and n = 4 muscles for twitch. Yellow- and purple-shaded panels denote isometric and chaotic relaxation. Black horizontal dashed lines denote resting values.
-
Figure 5—source data 1
An excel file containing the data for individual muscles from which the mean and SEM shown in Figure 5 were calculated.
- https://cdn.elifesciences.org/articles/68211/elife-68211-fig5-data1-v2.xlsx
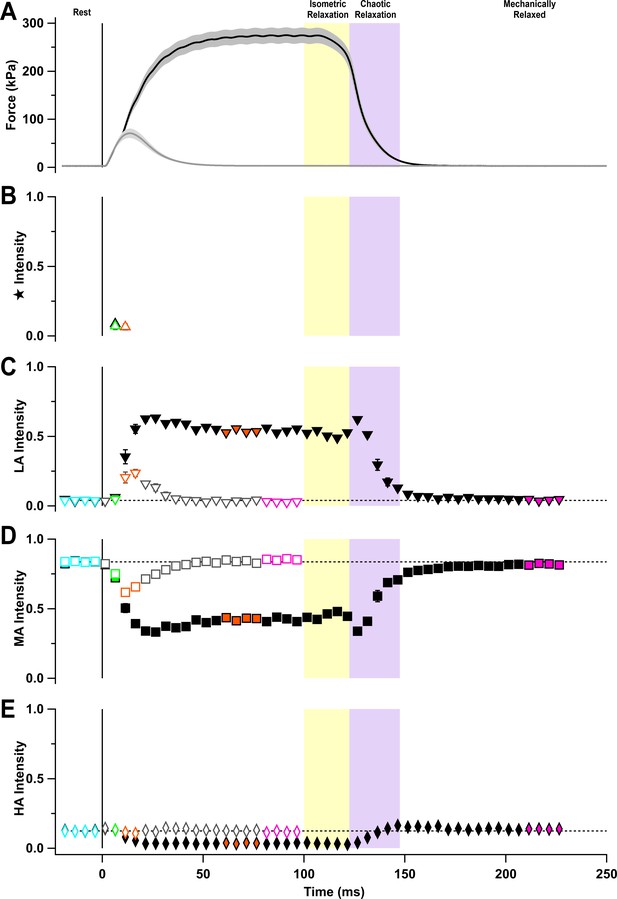
Fractional intensities of the sub-peak components of the M3 reflection.
(A) Time course of changes in force with SEM indicated by grey shading. Fractional intensities of the (B) star (triangles), (C) low-angle (LA; inverted triangles), (D) mid-angle (MA; squares), and (E) high-angle (HA; diamonds) sub-peaks for twitch (open symbols) and tetanus (filled symbols). Coloured symbols denote the time periods used to calculate the profiles in Figure 5A and B; error bars denote SEM for n = 5 muscles for tetanus and n = 4 muscles for twitch. Yellow- and purple-shaded panels denote isometric and chaotic relaxation. Black horizontal dashed lines denote resting values.
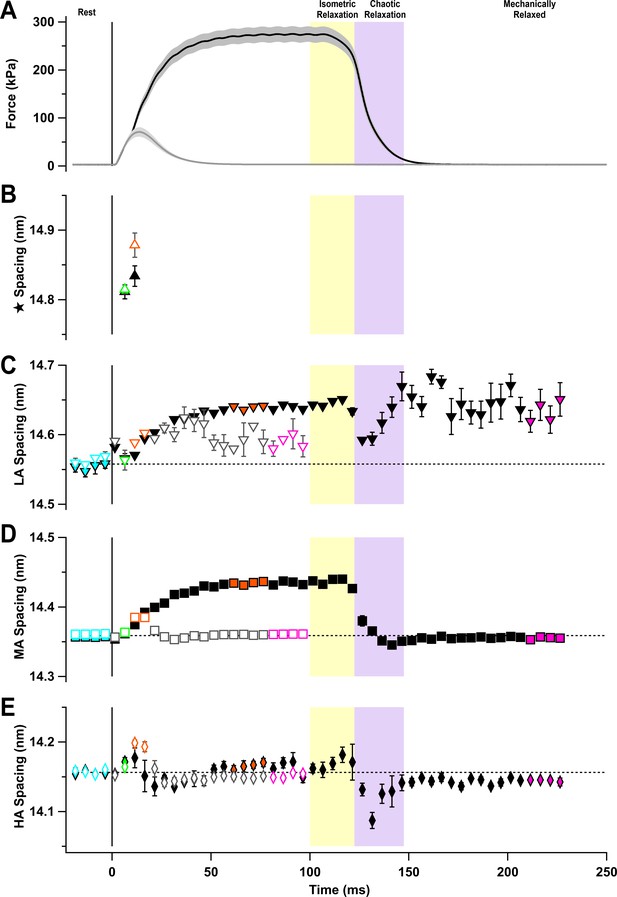
Spacings of the sub-peak components of the M3 reflection.
(A) Time course of changes in force with SEM indicated by grey shading. Spacings of the (B) star (triangles), (C) low-angle (LA; inverted triangles), (D) mid-angle (MA; squares), and (E) high-angle (HA; diamonds) sub-peaks for twitch (open symbols) and tetanus (filled symbols). Coloured symbols denote the time periods used to calculate the profiles in Figure 5A and B; error bars denote SEM for n = 5 muscles for tetanus and n = 4 muscles for twitch. Yellow- and purple-shaded panels denote isometric and chaotic relaxation. Black horizontal dashed lines denote resting values.

Fitting the axial profile of the M3 reflection with a structural model of the thick filament.
(A) Schematic diagram of the sarcomere for the best-fit model parameters at peak force in the twitch. The sarcomere is delimited by Z-disks (black) and contains overlapping actin (dark grey) and myosin (white) filaments. Myosin filament midpoint, M; layers of myosin motors, vertical lines on myosin filament; bare zone at the centre of the sarcomere lacking myosin motors, purple; half-bare zone, hbz; zone of the thick filament containing titin C-type repeats, magenta; myosin-binding protein-C-containing C-zone, green. Ordered layers of myosin motors (blue vertical bars between medial layer nm and distal layer nd) in the two half-thick filaments have a centre-to-centre or interference distance D, shown in blue. Disordered layers are shown in grey. (B–G) Experimental meridional intensity distribution in the region of the M3 reflection (black) with superimposed best fits from the model (orange). Rest (B); early activation (C); twitch peak force (D); twitch mechanical relaxation (E); tetanus plateau (F); tetanus mechanical relaxation (G). Shaded grey areas indicate ± SD from nine (B, C), four (D, E), or five (F, G) muscles.
-
Figure 6—source data 1
An excel file containing the data for individual muscles from which the mean and SEM shown in Figure 6 were calculated.
- https://cdn.elifesciences.org/articles/68211/elife-68211-fig6-data1-v2.xlsx
Tables
Force, sarcomere length, and X-ray parameters at rest and peak force in twitch and tetanus.
Rest, average of four frames from −18.5 ms to −3.5 ms for twitch and tetanus; PF tetanus, average of four frames from 61.5 ms to 76.5 ms; PF twitch, average of two frames at 11.5 ms and 16.5 ms. All X-ray intensities have been normalised by their resting values. Mean ± SEM for n = 5 for tetanus and n = 4 for twitch. SL, sarcomere length.
| Tetanus | Twitch | ||||
|---|---|---|---|---|---|
| Rest | PF | Rest | PF | ||
| Force (kPa) | - | 273 ± 16 | - | 68 ± 10 | |
| SL (µm) | 2.41 ± 0.01 | 2.13 ± 0.01 | 2.37 ± 0.01 | 2.38 ± 0.03 | |
| d1,0 (nm) | 35.32 ± 0.11 | 35.74 ± 0.17 | 35.07 ± 0.15 | 35.44 ± 0.25 | |
| I1,1/I1,0 | 0.41 ± 0.02 | 1.77 ± 0.14 | 0.39 ± 0.01 | 0.93 ± 0.05 | |
| IAL1 | 1 | 2.66 ± 0.43 | 1 | 0.74 ± 0.11 | |
| IML1 | 1 | 0.09 ± 0.03 | 1 | 0.28 ± 0.04 | |
| AML1 | 1 | 0.36 ± 0.06 | 1 | 0.53 ± 0.04 | |
| AAL1 | 1 | 1.61 ± 0.13 | 1 | 0.84 ± 0.07 | |
| IM3 | 1 | 3.21 ± 0.26 | 1 | 0.69 ± 0.13 | |
| SM3 (nm) | 14.339 ± 0.003 | 14.535 ± 0.002 | 14.344 ± 0.004 | 14.425 ± 0.004 | |
| IM6 | 1 | 1.12 ± 0.26 | 1 | 0.81 ± 0.15 | |
| SM6 (nm) | 7.173 ± 0.002 | 7.283 ± 0.001 | 7.173 ± 0.002 | 7.241 ± 0.002 | |
Half-times of force and X-ray parameters during activation and relaxation.
Half-times (t1/2) for the rising phase of the tetanus (activation) and for relaxation following tetanus and twitch. t1/2 values for X-ray parameters were determined by fitting sigmoidal curves to the 5-ms time interval data. t1/2 values for tetanus activation and twitch relaxation are reported with respect to the first stimulus at time 0 and those for tetanus relaxation with respect to the last stimulus at 100 ms. Rows are sorted by t1/2 for tetanus activation, with the fastest signals at the top. No clear change in AAL1 was observed during the twitch. LM3, MM3, and HM3 are, respectively, the fractional intensities of the low-, mid- and high-angle peaks of the M3 reflection in Figure 5E. n = 5 muscles for tetanus and n = 4 muscles for twitch. Data presented are mean ± SEM. *A significant difference in t1/2 for the X-ray parameter with respect to force in that phase. *p<0.05; **p<0.001; ***p<0.0001.
| Tetanus activation t1/2 (ms) | Tetanus relaxation t1/2 (ms) | Twitch relaxation t1/2 (ms) | |
|---|---|---|---|
| SM6 (nm) | 8.1 ± 0.7*** | 32.4 ± 0.9* | 25.7 ± 0.6 |
| AML1 | 8.6 ± 0.9*** | 41.8 ± 2.6** | 29.4 ± 2.3 |
| MM3 | 8.8 ± 0.5*** | 39.5 ± 1.4*** | 25.3 ± 1.7 |
| SM3 (nm) | 10.9 ± 1.0** | 33.6 ± 1.2* | 20.1 ± 0.8* |
| LM3 | 11.0 ± 0.6** | 37.7 ± 1.1*** | 25.8 ± 2.8 |
| I1,1/I1,0 | 11.3 ± 0.7** | 30.3 ± 0.8* | 28.4 ± 0.8* |
| AAL1 | 15.3 ± 1.2 | 23.4 ± 1.9 | - |
| Force (kPa) | 17.0 ± 0.7 | 27.3 ± 0.6 | 24.4 ± 1.3 |
| AM3 | 21.4 ± 1.3* | 20.9 ± 0.7** | 40.0 ± 1.7** |
Interpretation of the axial profile of the M3 reflection: best-fit model parameters.
The rows denote the different phases of the tetanus and twitch defined in Figure 6. Half-bare zone, hbz; medial and distal layers marking the ends of the region of ordered myosin motors, nm and nd, respectively; axial periodicity between adjacent layers of myosin motors, d; intensity scaling factor, y; interference distance, ID. Mean ± SD from N muscles.
| N | hbz (nm) | nm | nd | d (nm) | y | ID (nm) | |
|---|---|---|---|---|---|---|---|
| Rest | 9 | 79.51 ± 0.14 | 2 ± 1 | 46 ± 3 | 14.348 ± 0.010 | 4.5 ± 0.6 | 814 ± 39 |
| Early activation | 9 | 79.40 ± 0.22 | 3 ± 1 | 42 ± 3 | 14.362 ± 0.008 | 4.9 ± 1.0 | 776 ± 53 |
| Twitch PF | 4 | 85.65 ± 0.37 | 9 ± 2 | 43 ± 2 | 14.416 ± 0.013 | 5.1 ± 1.8 | 881 ± 25 |
| Twitch relax | 4 | 79.52 ± 0.12 | 2 ± 1 | 45 ± 3 | 14.348 ± 0.009 | 4.7 ± 0.8 | 790 ± 35 |
| Tetanus PF | 5 | 90.58 ± 0.10 | 1 ± 0 | 49 ± 1 | 14.552 ± 0.003 | 2.0 ± 0.7 | 874 ± 8 |
| Tetanus relax | 5 | 79.61 ± 0.07 | 2 ± 1 | 46 ± 0 | 14.340 ± 0.003 | 5.5 ± 1.3 | 822 ± 12 |





