Tonic interferon restricts pathogenic IL-17-driven inflammatory disease via balancing the microbiome
Figures
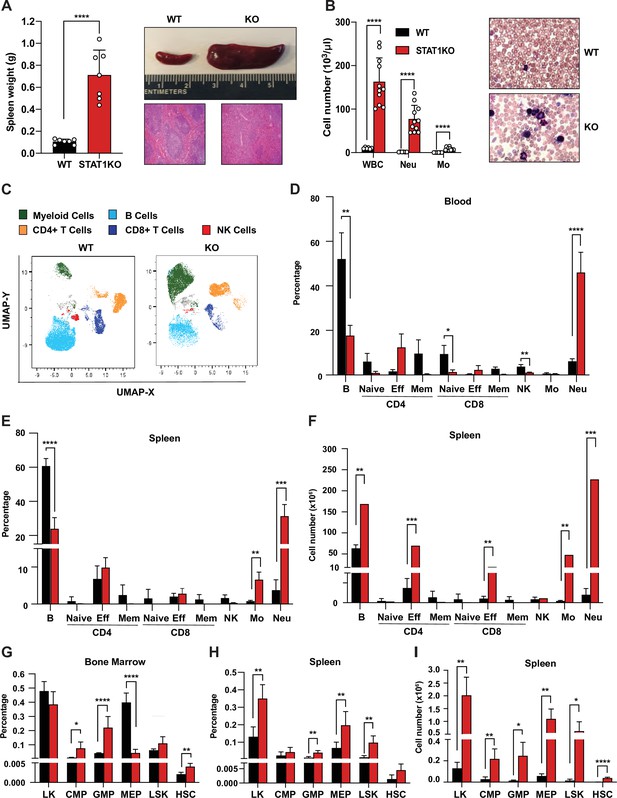
Characterization of STAT1 KO hematopoietic defects.
(A) Spleen weights in g (n = 7), mean ± SD (left). Representative spleen of a STAT1 KO mouse and its age- and sex-matched WT littermate (top right). Representative histology of STAT1 KO and WT spleens, stained with H&E (bottom right). (B) Total WBC, neutrophil and monocyte counts (n = 6 WT, n = 11 KO), mean ± SD (left). Representative blood smear from WT and STAT1 KO blood after Giemsa staining (right). (C) Uniform Manifold Approximation and Projection (UMAP) plots of blood from WT and STAT1 KO littermates after 18-color multi-parameter flow cytometry staining. Clusters were annotated using known markers. Flow cytometric analysis of blood (D), bone marrow (G), spleen (E–H) of STAT1 KO, and WT littermates (n = 4–5). Values represent mean ± SD of live cells, percentage or number, as indicated. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 by Student’s t-test. NK, natural killer; LK, lin−Sca1−c-Kit+; CMP, common myeloid progenitor; GMP, granulocyte-macrophage progenitor; MEP, megakaryocyte-erythroid progenitor; LSK, lin-Sca1+c-Kit+; HSC, hematopoietic stem cells. Each dot represents an individual animal (A, B).
-
Figure 1—source data 1
Source data for Figure 1A, B, D–I in Excel format.
- https://cdn.elifesciences.org/articles/68371/elife-68371-fig1-data1-v2.xlsx
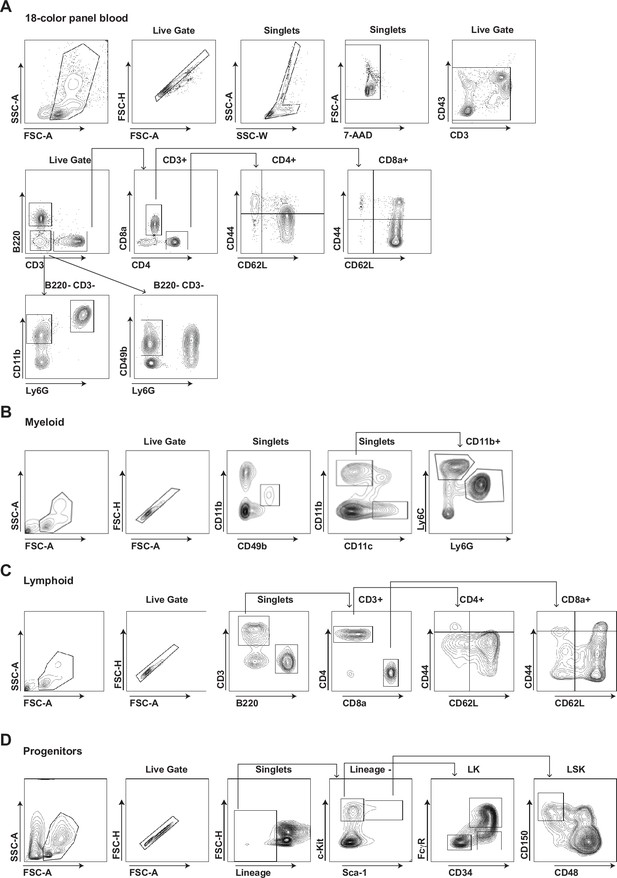
Gating strategies of multi-parameter flow cytometry analysis.
Gating strategies of multi-parameter flow cytometry analysis on blood (A) and for myeloid (B), lymphoid (C), and progenitor subsets (D) from bone marrow and spleen.
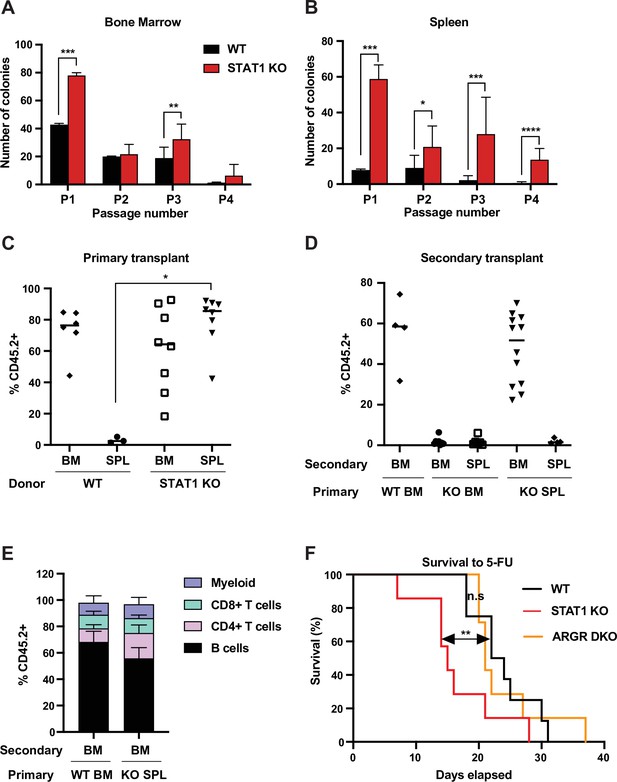
STAT1-deficient spleens harbor a large number of definitive HSCs.
In vitro self-renewal colony forming assay of hematopoietic progenitors from bone marrow (A) or spleen (B) after serial plating (n = 3 mice, each plating in triplicate). Values represent mean number of colonies ± SD. Each replating culture was inoculated with equal numbers of cells. Quantification of donor-derived (CD45.2) cells, provenance as indicated, in the peripheral blood of recipient CD45.1 animals 1 month after primary transplant (C) or 4 months after secondary transplant (D). Values represent individual animals. (E) Quantification of donor-derived (CD45.2) B (B220+) cells, T (CD4+ or CD8+), and myeloid (CD11b+) cells in the peripheral blood of recipient animals 4 months after secondary transplant ; values represent mean ± SD; WT n = 4 mice; KO n = 11 mice. (F) Kaplan–Meier survival curve of WT, STAT1 KO, and ARGR DKO mice after weekly injections of 5-fluorouracil (5-FU). WT n = 5 mice, STAT1 KO and ARGR DKO n = 7 mice. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 by Student’s t-test or Gehan–Breslow–Wilcoxon test for Kaplan–Meier.
-
Figure 2—source data 1
Source data for Figure 2A–F in Excel format.
- https://cdn.elifesciences.org/articles/68371/elife-68371-fig2-data1-v2.xlsx
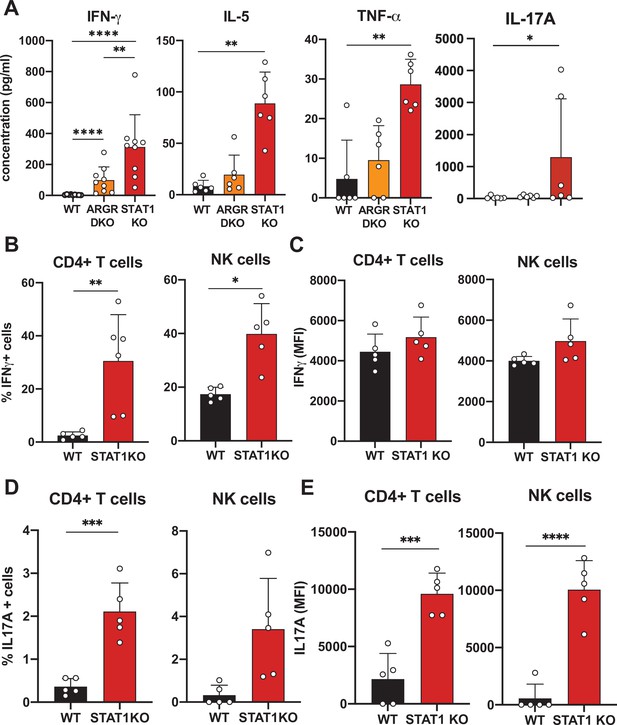
Cytokine profiling of STAT1 KO blood.
(A) Cytokine concentration in blood of WT, ARGR DKO, and STAT1 KO mice in pg/ml (n = 6–9 mice for each group). Data are representative of two experiments. Percentage of IFN-γ (B) or IL-17A-producing (D) CD4+ T cells (left) or NK cells (right) in STAT1 KO and WT mice (n = 5 or 6) with corresponding MFI (C, E). Values represent mean ± S.D. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 by Mann–Whitney (A) or Student’s t-test (B–E). Each dot represents an individual animal.
-
Figure 3—source data 1
Source data for Figure 3A–E in Excel format.
- https://cdn.elifesciences.org/articles/68371/elife-68371-fig3-data1-v2.xlsx
-
Figure 3—source data 2
Comprehensive cytokine and chemokine quantification data related to Figure 3 in Excel format.
- https://cdn.elifesciences.org/articles/68371/elife-68371-fig3-data2-v2.xlsx
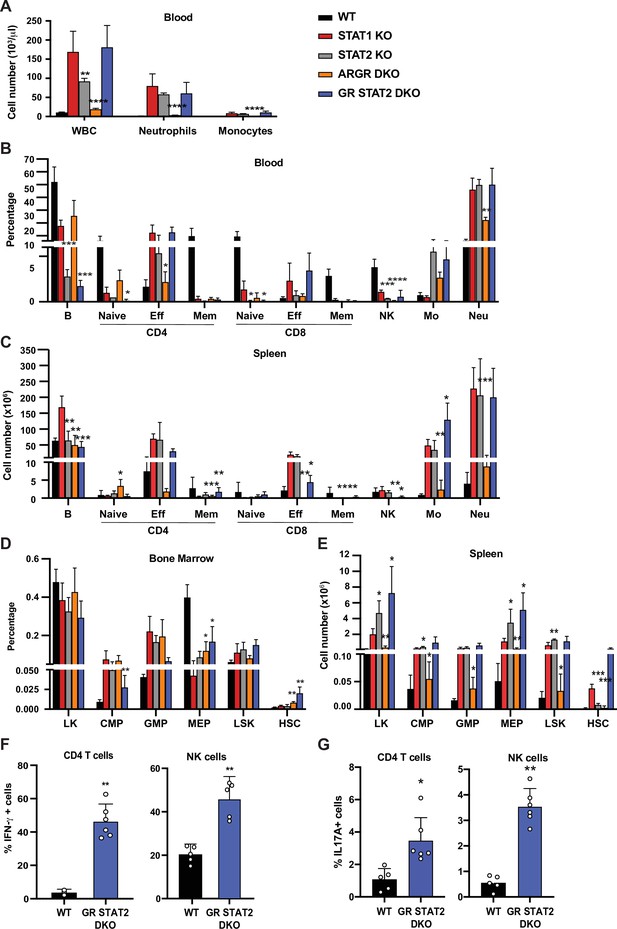
Combined IFN type I, II, III response deficiency recapitulates STAT1 deficiency.
(A) Total WBC, neutrophils, and monocytes counts using Hemavet in blood from WT, STAT1 KO, STAT2 KO, ARGR DKO, and GR STAT2 DKO mice (n = 5–10 mice/group). Quantification by flow cytometry of mature cell populations from blood (B), spleen (C), and progenitor populations from bone marrow (D) and spleen (E) from WT, STAT1 KO, STAT2 KO, ARGR DKO, and GRSTAT2 DKO (n = 4 or 5). Same legend as in (A). Statistical comparison is between each genotype and STAT1 KO. Percentage of IFN-γ- (F) or IL-17A-producing (G) CD4+ T cells (left) or NK cells (right) in GR STAT2 DKO and WT mice (n = 4–6). Values represent mean ± SD of live cells, percentage or number, as indicated. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 by Student’s t-test. NK, natural killer; LK, lin−Sca1−c-Kit+; CMP, common myeloid progenitor; GMP, granulocyte-macrophage progenitor; MEP, megakaryocyte-erythroid progenitor; LSK, lin−Sca1+c-Kit+; HSC, hematopoietic stem cells. Each dot represents an individual animal (F, G).
-
Figure 4—source data 1
Source data for Figure 4A–G in Excel format.
- https://cdn.elifesciences.org/articles/68371/elife-68371-fig4-data1-v2.xlsx
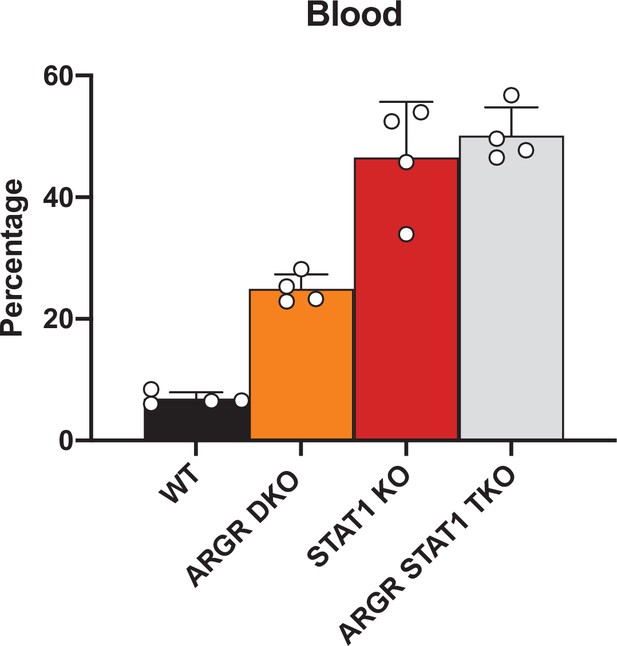
Flow cytometric analysis of myeloid cells in WT, STAT1 KO, ARGR DKO, and ARGR STAT1 TKO animals.
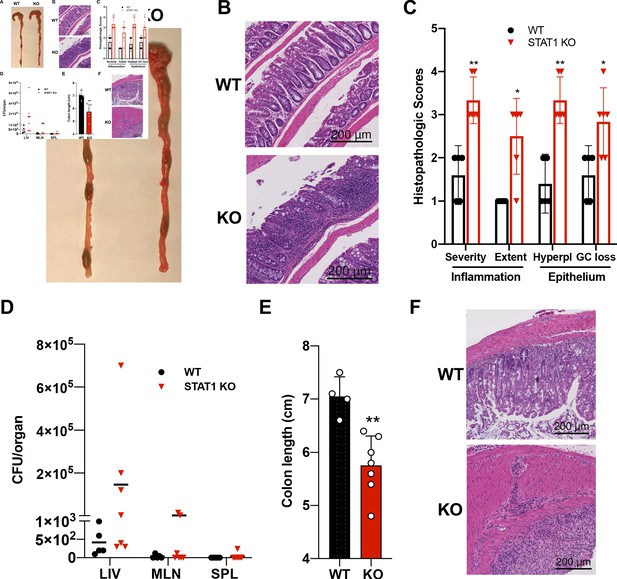
STAT1KO mice are prone to develop spontaneous colitis.
(A) Representative colon gross anatomy of WT and STAT1 KO mice. (B) Representative histology of WT and STAT1 KO colons, stained with H&E (scale bar = 200 μm). (C) Histopathologic scores of WT and STAT1 KO colons. Scoring was performed for severity and extent of inflammatory cell infiltrates and for epithelial changes (hyperplasia [hyperpl] and goblet cell loss [GC loss]) (n = 5 for WT, n = 6 for STAT1 KO) *p<0.05, **p<0.01 by Mann−Whitney U test. (D) Bacterial leakage (CFU/organ) into liver (LIV), mesenteric lymph nodes (MLN) and spleen (SPL), as described in materials and methods (n = 5 for WT, n = 7 for STAT1 KO). (E) Colon length after 7 days of DSS treatment in drinking water (n = 4 for WT, n = 7 for STAT1 KO) **p<0.01 by Student’s t-test. (F) Representative histology of WT and STAT1 KO colons, stained with H&E, after 7 days of DSS treatment (scale bar = 200 μm). Each symbol represents an individual animal (C–E).
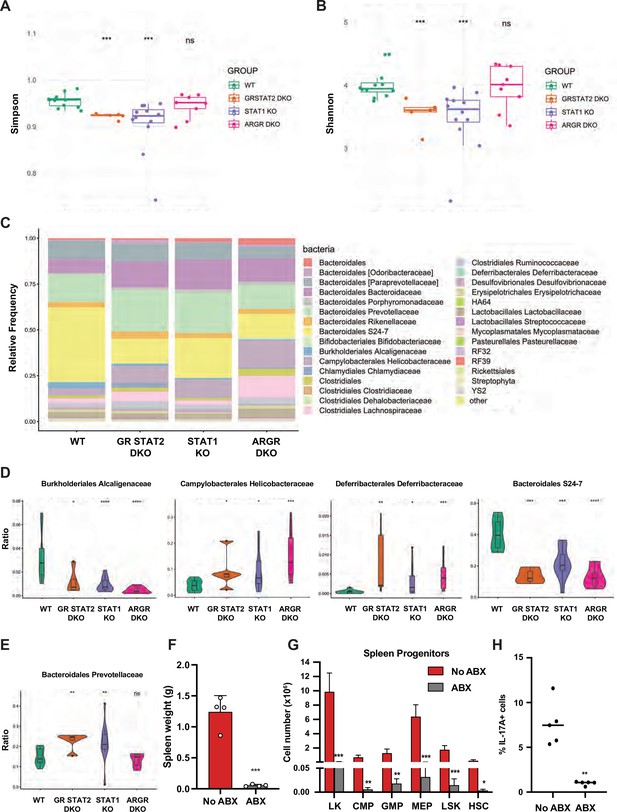
Tonic IFN controls gut microbiota 16 S rRNA sequencing of fecal DNA samples from WT, GR STAT2 DKO, STAT1 KO, and ARGR DKO mice (n = 8–12 animals/strain).
(A) Alpha diversity as estimated by Simpson index (Kruskal–Wallis p-value = 0.00068). (B) Alpha diversity as estimated by Shannon index (Kruskal–Wallis p-value = 0.00082). (C) Family level microbiota composition. (D) Relative abundance of the total microbial load of four families of bacteria. (E) Relative abundance of the total microbial load of Bacteroidales Prevotellaceae. (F) Spleen weights in g of antibiotics (ABX)-treated STAT1 KO animals compared to untreated animals (No ABX) (n = 4). (G) Flow cytometric analysis of splenic progenitors of antibiotics (ABX)-treated STAT1 KO animals compared to untreated animals (No ABX) (n = 4). (H) Percentage of IL-17A-producing CD4+ T cells in antibiotics (ABX)-treated animals compared to untreated animals (No ABX). Values represent mean ± SD of live cells, percentage or number, as indicated. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 by Student’s t-test. LK, lin-Sca1-c-Kit+; CMP, common myeloid progenitor; GMP, granulocyte-macrophage progenitor; MEP, megakaryocyte-erythroid progenitor; LSK, lin-Sca1+ c-Kit+; HSC, hematopoietic stem cells. Each dot represents an individual animal (A, B, F, H).
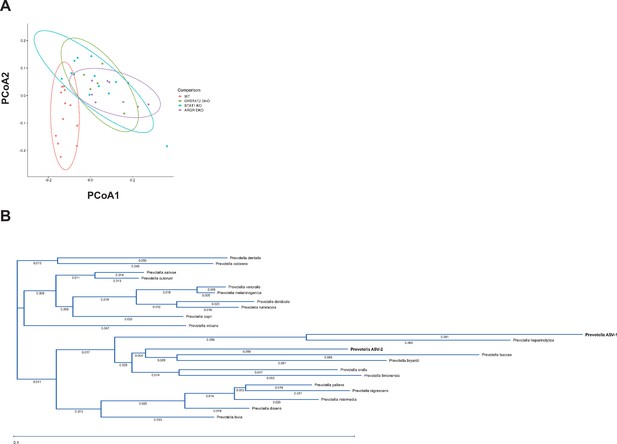
Bioinformatic analysis of beta diversity and phylogeny of Prevotella species.
(A) Principal component analysis. (B) Phylogenetic tree of Prevotella species.
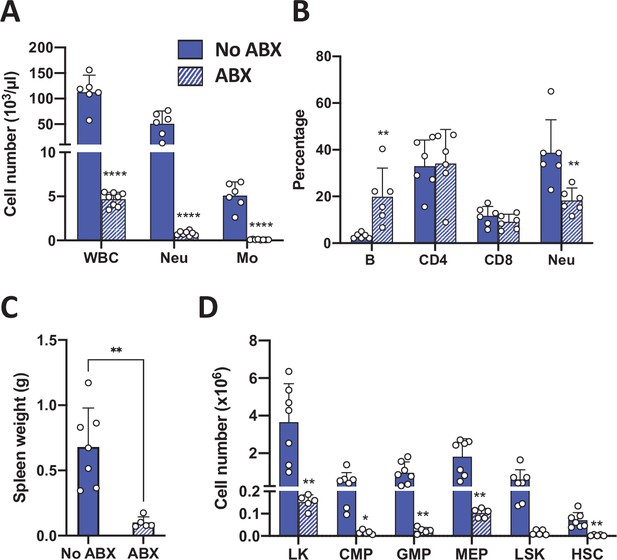
Antibiotic treatment of GR STAT2 DKO mice.
(A) Total WBC, neutrophil, and monocyte counts of GR STAT2 DKO mice untreated (No ABX, n = 6) or treated with antibiotics (ABX, n = 8). (B) Flow cytometric quantification of leukocyte subsets in peripheral blood of untreated and antibiotic-treated GR STAT2 DKO mice (n = 6). (C) Spleen weights of untreated and antibiotic-treated GR STAT2 DKO mice (n = 5–7). (D) Flow cytometric analysis of splenic progenitors of of untreated and antibiotic-treated GR STAT2 DKO mice (n = 5–7). *p<0.05, **p<0.01, ****p<0.0001 by Student’s t-test. LK, lin-Sca1-c-Kit+; CMP, common myeloid progenitor; GMP, granulocyte-macrophage progenitor; MEP, megakaryocyte-erythroid progenitor; LSK, lin-Sca1+ c-Kit+; HSC, hematopoietic stem cells.
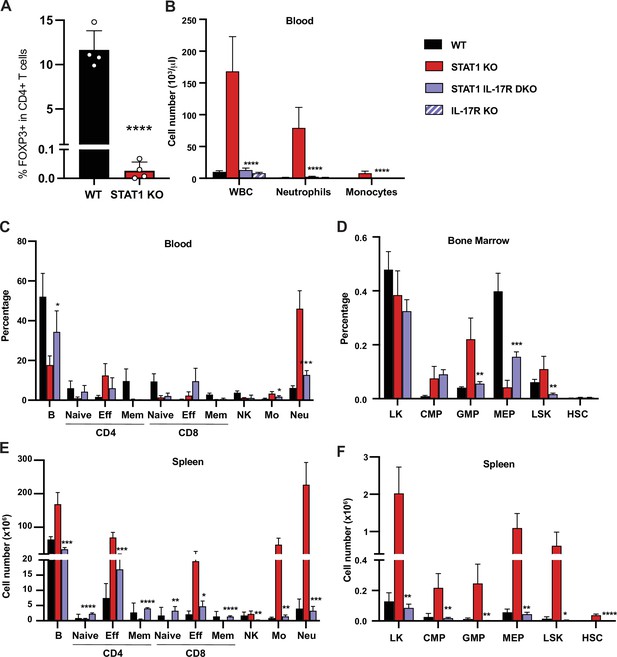
IL-17R deficiency rescues hematopoietic anomalies of STAT1 KO mice.
(A) Percentage of FOXP3+ Treg cells in splenic CD4+ T cells of WT and STAT1 KO littermates (n = 4). (B) Total WBC, neutrophil, and monocyte counts of WT, STAT1 KO, IL-17R STAT1 DKO, and IL-17R KO, mean ± SD (n = 6 for WT, n = 9 or 10 for other groups). Quantification by flow cytometry of mature cell populations from blood (C), spleen (E), and progenitor populations from bone marrow (D) and spleen (F) from WT, STAT1 KO, IL-17R STAT1 DKO (n = 4 or 5). Values represent mean ± SD of live cells, percentage or number, as indicated. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 by Student’s t-test. NK, natural killer; LK, lin-Sca1− c-Kit+; CMP, common myeloid progenitor; GMP, granulocyte-macrophage progenitor; MEP, megakaryocyte-erythroid progenitor; LSK, lin−Sca1+c-Kit+; HSC, hematopoietic stem cells. Each dot represents an individual animal (A).
-
Figure 7—source data 1
Source data for Figure 7A–F in Excel format.
- https://cdn.elifesciences.org/articles/68371/elife-68371-fig7-data1-v2.xlsx
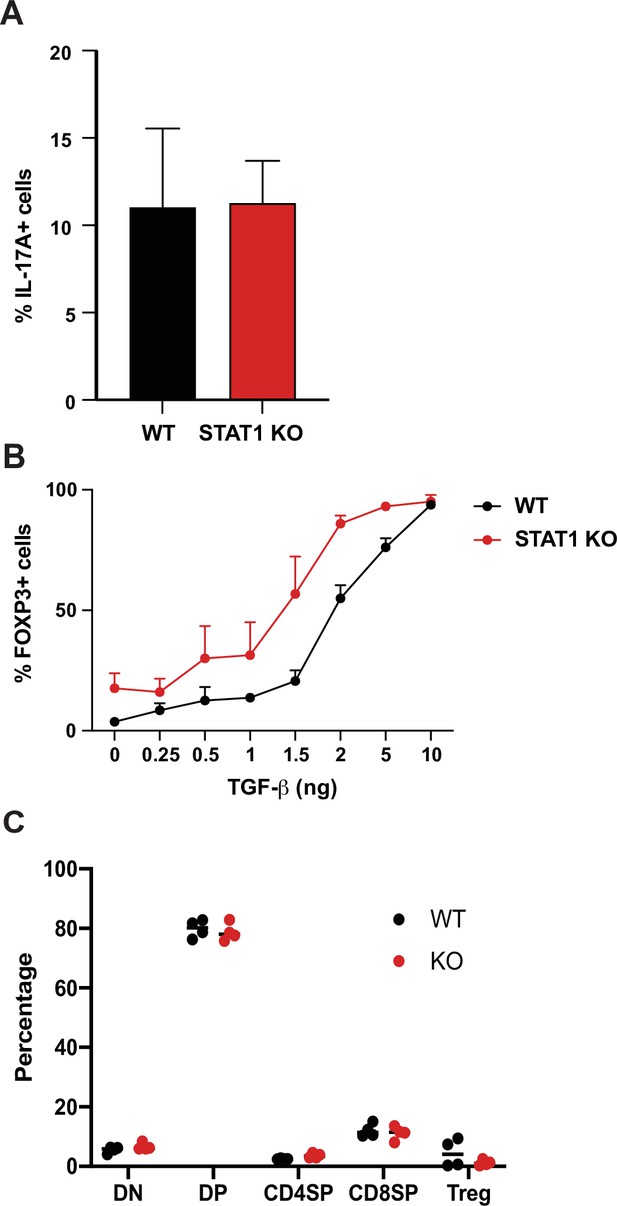
Analysis of STAT1 KO and WT TH17 and Treg cells.
(A) In vitro TH17 differentiation in response to IL-6 and TGF−β of WT and STAT1 KO T cells (n = 3 mice). (B) In vitro Treg differentiation in response to TGF−β of WT and STAT1 KO T cells (n = 3 mice). (C) Quantification of thymic T cell populations in WT and STAT1 KO mice (n = 4 mice).
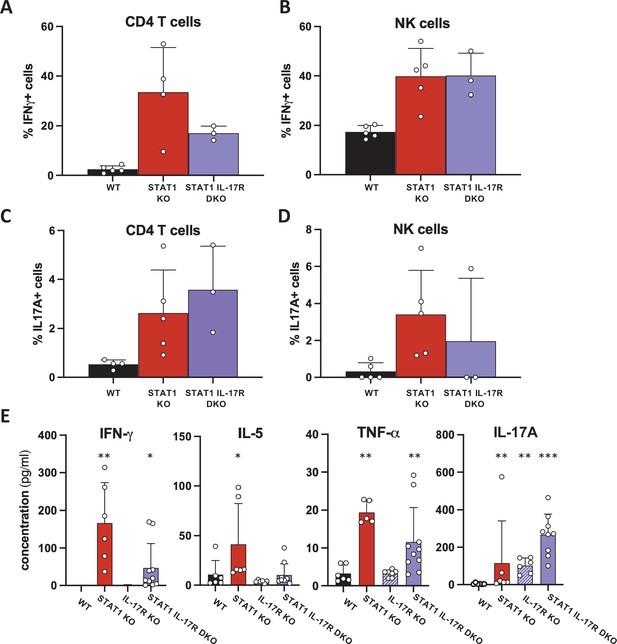
Cytokine production by STAT1 IL-17R DKO lymphocytes.
Percentage of IFN-γ- (A) or IL-17A-producing (C) CD4+ T cells or NK cells (B, D) in WT, STAT1 KO, and IL-17Ra STAT1 DKO mice (n = 4 or 5). (E) Concentration of IFN-γ, IL-5, TNF-α, and IL-17A in WT, STAT1 KO, IL-17R KO, and STAT1 IL-17R DKO blood (n = 5–10). *p<0.05, **p<0.01, ***p<0.001 by Mann–Whitney.
-
Figure 7—figure supplement 2—source data 1
Comprehensive cytokine and chemokine quantification data related to Figure 7—figure supplement 2 in Excel format.
- https://cdn.elifesciences.org/articles/68371/elife-68371-fig1-data1-v2.xlsx
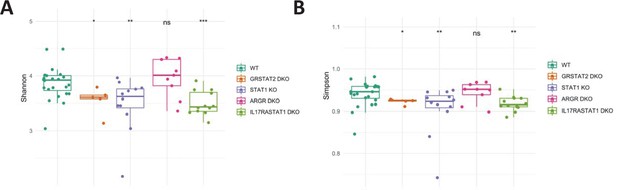
Microbiome diversity 16 S rRNA sequencing of fecal DNA samples from WT, GR STAT2 DKO, STAT1 KO, ARGR DKO, and IL17R STAT1 DKO mice (n = 5–21 animals/strain).
Each symbol represents an individual animal. (A) Alpha diversity as estimated by Shannon index (Kruskal–Wallis p=0.00025). (B) Alpha diversity as estimated by Simpson index (Kruskal–Wallis p=0.0037).
Additional files
-
Supplementary file 1
List of antibodies used for flow cytometry.
- https://cdn.elifesciences.org/articles/68371/elife-68371-supp1-v2.docx
-
Supplementary file 2
Summary of mutant phenotypes.
- https://cdn.elifesciences.org/articles/68371/elife-68371-supp2-v2.docx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/68371/elife-68371-transrepform1-v2.docx





