The molecular appearance of native TRPM7 channel complexes identified by high-resolution proteomics
Figures
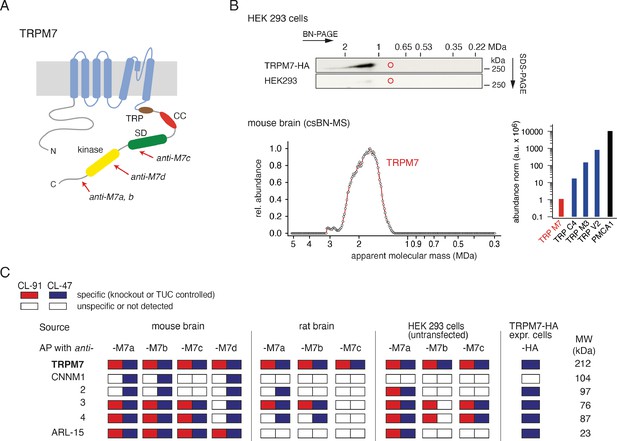
Protein constituents of native transient receptor potential melastatin-subfamily member 7 (TRPM7) channels identified by multi-epitope antibody-based affinity purification (ME-AP) proteomics.
(A) Topology and localisation of the anti-TRPM7 antibodies used for ME-APs. Established hallmark domains of TRPM7 are colour-coded, TRP (transient receptor potential domain, brown), CC (coiled-coil domain, red), kinase (kinase domain, yellow), SD (serine/threonine-rich substrate domain of kinase(s), green). (B) Upper panel: Two-dimensional gel separation of TRPM7 channels in CL-47 solubilised membrane fractions of HEK293 cells with (upper panel) or without (lower panel) transfection of HA-tagged Trpm7, Western-probed with an anti-TRPM7 antibody (Materials and methods). Size (blue native polyacrylamide gel electrophoresis [BN-PAGE]) and molecular weight (SDS-PAGE) are as indicated. Lower panel: Abundance-mass profile of TRPM7 obtained by cryo-slicing blue native mass spectrometry (csBN-MS) in a CL-47 solubilised membrane fraction from adult mouse brain (a total of 192 gel slices). Inset: Abundance of the indicated proteins in the mouse brain. Note the large apparent molecular mass of the native TRPM7 channel in both culture cells and mouse brain, markedly exceeding the mass calculated for tetrameric channel assemblies (about 850 kDa, red circles). (C) Table summarising the results of all anti-TRPM7 APs performed with the indicated antibodies on membrane fractions prepared from rodent brain and cultured HEK293 cells. Solubilisation conditions and specificity of purification of the listed proteins determined by comparison with stringent negative controls are colour-coded as given in the upper left; MW is indicated on the right. TUC refers to series of APs with target-unrelated control antibodies. Note that TRPM7 channels co-assemble with all CNNM family members and ADP-ribosylation factor-like protein 15 (ARL15) in the brain and HEK293 cells.
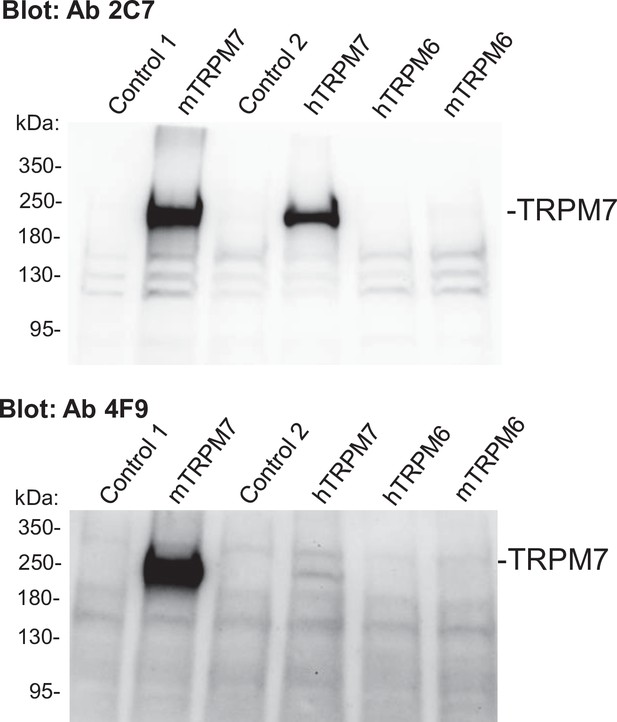
The specificity of an anti-transient receptor potential melastatin-subfamily member 7 (TRPM7) mouse monoclonal antibody in Western blot assessment of the recombinant TRPM6 and TRPM7 proteins.
0.8 µg/ml of 2C7 IgG (upper panel) or 1.3 µg/ml of 4F9 IgG (lower panel) were used for Western blot analysis of untransfected HEK293 cells (Control 1) or cells transfected with mouse Trpm7 cDNA (mTRPM7), uninduced (Control 2) or induced HEK293-Rex cells expressing the human TRPM7 (hTRPM7), HEK293 cells transfected with human TRPM6 (hTRPM6), or mouse Trpm6 cDNA (mTRPM6). Representative results of two independent experiments are shown.
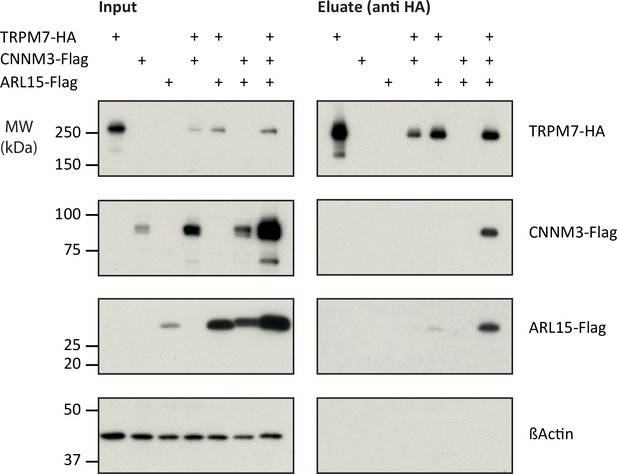
Heterologous reconstitution of transient receptor potential melastatin-subfamily member 7 (TRPM7) complexes in HEK293 cells.
Affinity purifications (APs) with anti-HA antibody from CL-47 solubilised membrane fractions of TRPM7-/- HEK293 cells transiently expressing the proteins indicated above. Input and eluates of the distinct APs were separated by SDS-PAGE and Western-probed with anti-Flag, anti-HA, and anti-β-actin antibodies. Molecular weight (MW) is marked on the left.
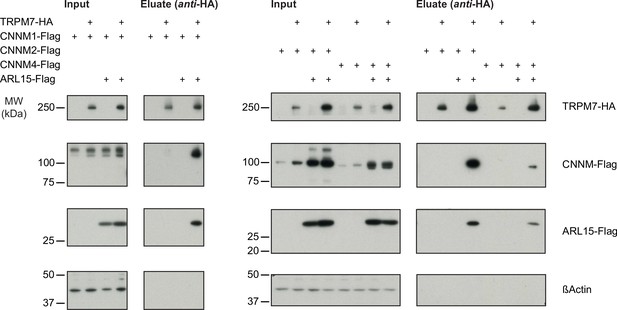
Heterologous reconstitution of transient receptor potential melastatin-subfamily member 7 (TRPM7) complexes in HEK293 cells.
Affinity purifications (APs) with anti-HA antibody from CL-47 solubilised membrane fractions of TRPM7-/- HEK293 cells transiently expressing the indicated combinations of proteins. Input and eluates of the distinct APs were separated by SDS-PAGE and Western-probed with anti-Flag, anti-HA, and anti-β-actin antibodies. Molecular weight (MW) is marked on the left.
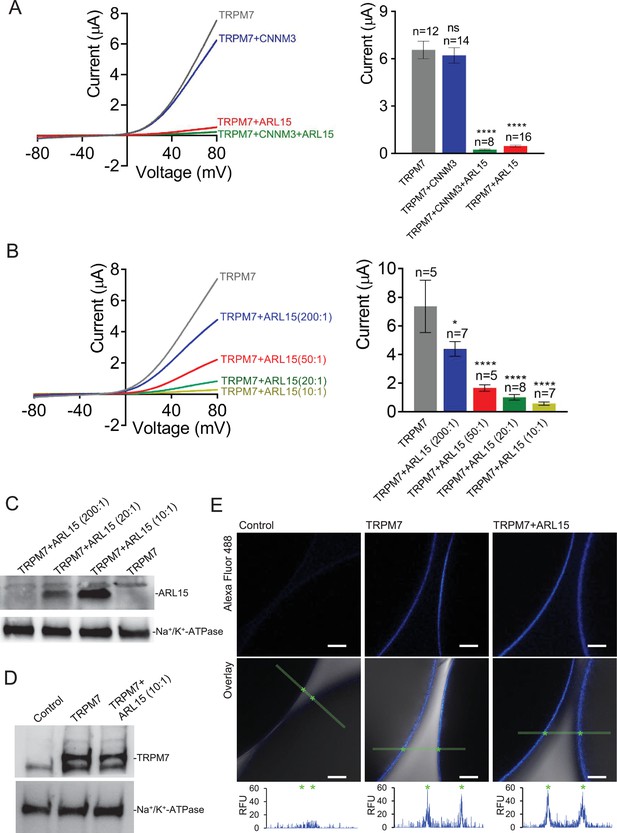
Heterologous expression of transient receptor potential melastatin-subfamily member 7 (TRPM7) in Xenopus oocytes.
(A, B) Two-electrode voltage clamp (TEVC) measurements of TRPM7 currents. (A) Left panel: Representative current-voltage (I-V) relationships of TRPM7 currents measured in oocytes expressing TRPM7 alone or TRPM7 with CNNM3 or ADP-ribosylation factor-like protein 15 (ARL15) (cRNAs ratio 2:1), and TRPM7 with CNNM3 and ARL15 (cRNAs ratio 2:1:1). Right panel: Current amplitudes (mean ± standard error of the mean [SEM]) at +80 mV in measurements shown on the left. Two independent batches of injected oocytes (n = 8–16) were examined. *p < 0.05; ****p < 0.0001 (ANOVA). (B) Left panel: Representative I-V relationships of TRPM7 currents measured in oocytes expressing TRPM7 or co-expressing TRPM7 with ARL15 at the indicated ratios of injected cRNAs. Right panel: Current amplitudes (mean ± SEM) at +80 mV in measurements shown on the left. Two independent batches of injected oocytes (n = 5–7) were examined. *p < 0.05; ****p < 0.0001 (ANOVA). (C) Western blot analysis of ARL15 expression using the anti-Myc antibody in total lysates of oocytes injected with Trpm7 or Trpm7 and Arl15 cRNAs (ratios 200:1, 20:1, and 10:1). Representative results are shown for two independent experiments. Anti-Na+/K+-ATPase antibody was used for loading controls. (D) Western blot analysis of TRPM7 expression using the anti-M7d antibody in total lysates of oocytes injected with Trpm7 or Trpm7 and Arl15 cRNAs (ratio 10:1). Anti-Na+/K+ ATPase antibody was used for loading controls. Representative results are shown for two independent experiments. (E) Immunofluorescence staining of un-injected oocytes (control) or oocytes injected with Trpm7 (TRPM7) or Trpm7 and Arl15 cRNAs (TRPM7+ ARL15, ratio 10:1) using anti-M7d antibody and anti-mouse antibody conjugated with Alexa Fluor 488. Confocal images of Alexa Fluor 488 fluorescence (Alexa488) and overlays of Alexa488 with differential interference contrast images (overlay) are depicted for two independent oocytes per image; scale bars, 50 μm. The diagrams depict fluorescence intensity acquired along the green bars shown in overlay images. The stars indicate the cell surface of two oocytes. Typical examples of two independent experiments (n = 10 oocytes) are shown.
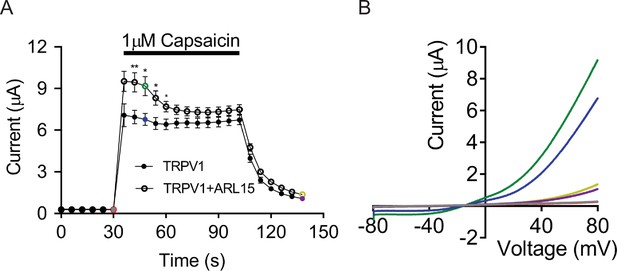
Two-electrode voltage clamp (TEVC) measurements of capsaicin-induced TRPV1 currents in Xenopus oocytes.
(A) Voltage ramps from –80 to +80 mV were applied every 6 s, and current amplitudes (mean ± standard error of the mean [SEM], n = 7 *p < 0.05; **p < 0.01 two-tailed t-test) were acquired at +80 mV in Xenopus oocytes expressing TRPV1 alone or TRPV1 with ADP-ribosylation factor-like protein 15 (ARL15) (cRNA ratio 2:1) and plotted over time. Oocytes were perfused with 1 µM capsaicin as indicated by the black bar. (B) Representative current-voltage (I-V) relationships of TRPV1 currents shown in (A) prior, during, and after exposure of oocytes to capsaicin as indicated by the correspondingly coloured data points.
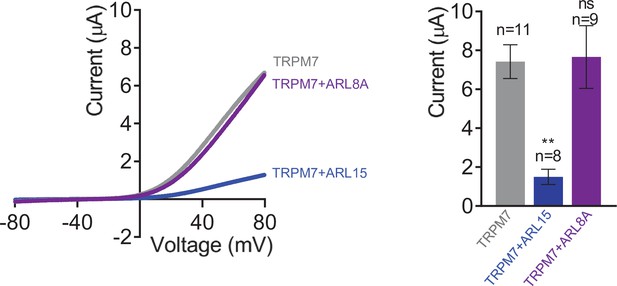
Heterologous expression of transient receptor potential melastatin-subfamily member 7 (TRPM7), ARL8A, and ADP-ribosylation factor-like protein 15 (ARL15) in Xenopus oocytes.
Two-electrode voltage clamp (TEVC) measurements were performed and analysed as explained in Figure 3A. Left panel: Representative current-voltage (I-V) relationships of TRPM7 currents measured in oocytes expressing TRPM7 or co-expressing TRPM7 with ARL8A or ARL15 (cRNA ratio 10:1). Right panel: Current amplitudes (mean ± standard error of the mean [SEM]) at +80 mV in measurements shown on the left. Two independent batches of injected oocytes (n = 8–11) were examined. ns, not significant; **p < 0.01 (ANOVA).
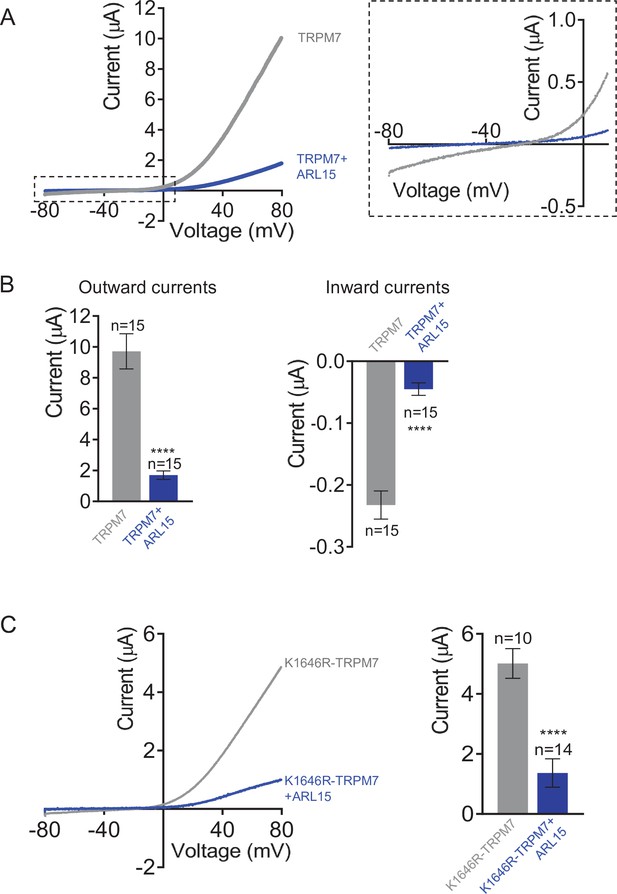
Assessment of the importance of the transient receptor potential melastatin-subfamily member 7 (TRPM7) kinase activity for the functional interplay between ADP-ribosylation factor-like protein 15 (ARL15) and TRPM7 by two-electrode voltage clamp (TEVC) measurements.
(A) Representative current-voltage (I-V) relationships of TRPM7 currents measured in oocytes expressing TRPM7 or co-expressing TRPM7 with ARL15 (cRNA ratio 10:1). The dashed box in left panel indicates the area of inward currents shown enlarged in the right panel. (B) Current amplitudes (mean ± standard error of the mean [SEM]) at +80 mV (outward currents) and –80 mV (inward currents) in measurements from (A). Two independent batches of injected oocytes (n = 15–21) were examined. ****p < 0.0001 (two-tailed t-test). (C) Left panel: Representative I-V relationships of TRPM7 currents measured in oocytes expressing the TRPM7 K1646R mutant without or with ARL15 (cRNA ratio 10:1). Right panel: Current amplitudes (mean ± SEM) at +80 mV in measurements shown on the left. Two independent batches of injected oocytes (n = 10–14) were examined. ****p < 0.0001 (two-tailed t-test).
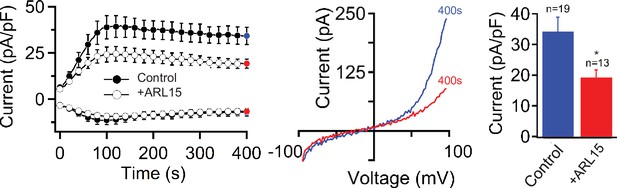
Impact of ADP-ribosylation factor-like protein 15 (ARL15) on endogenous transient receptor potential melastatin-subfamily member 7 (TRPM7) currents in HEK293 cells.
Whole-cell endogenous TRPM7 currents were recorded in untransfected cells (control) and cells transfected with Arl15 plasmid DNAs. Currents were induced using the Mg2+-free internal solution and the standard external solution containing 3 mM Ca2+ (no Mg2+). Left panel: Current amplitudes (mean ± standard error of the mean [SEM]) were acquired at –80 and +80 mV and plotted over time. Middle panel: Representative current-voltage (I-V) relationships of currents (at 400 s) shown in left panel. Right panel: Bar graphs of outward currents (mean ± SEM) in (A) at 400 s shown in left panel. n, number of cells measured. ns, not significant; *p ≤ 0.05 (two-tailed t-test).
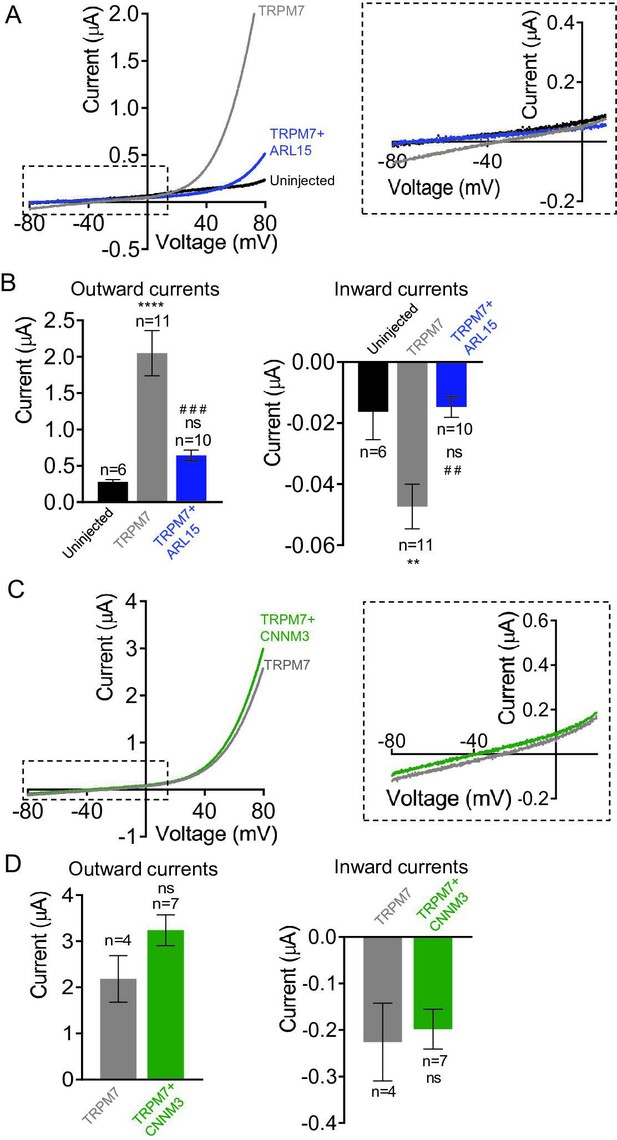
Effects of ADP-ribosylation factor-like protein 15 (ARL15) and CNNM3 on Mg2+ currents of the transient receptor potential melastatin-subfamily member 7 (TRPM7) channel expressed in Xenopus oocytes.
TEVC measurements were performed using the external ND96 solution containing 3 mM Mg2+and no other divalent cations. (A, B) Assessment of oocytes expressing TRPM7 or co-expressing TRPM7 with ARL15 (cRNA ratio 10:1). (A) Representative I-V relationships ofTRPM7 currents. The dashed box in Left panelindicates the area of inward currents enlarged in the Right panel. (B) Current amplitudes (mean ± SEM) at+80 mV (Outward currents) and at -80 mV (Inward currents) in measurements from (A). Two independent batches of injected oocytes (n=6-11) were examined. ns, not 36significant; ** P < 0.01, **** P < 0.0001 significant to the Uninjected group (ANOVA). # # P < 0.01, # # # P < 0.001 significant to the TRPM7 group (ANOVA). (C, D) Examination of oocytes expressing TRPM7 or co-expressing TRPM7 with CNNM3 (cRNA ratio 2:1). Data were produced and analyzed as explained in (A, B). Two independent batches of injected oocytes (n=4-7) were examined. ns, not significant (two-tailed t-test).
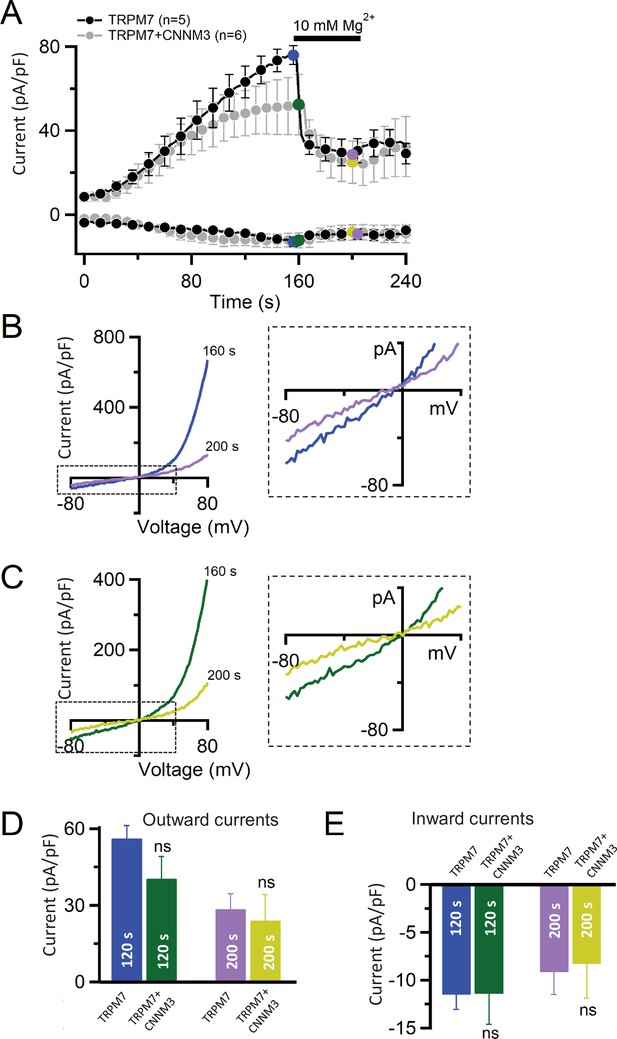
Heterologous expression of transient receptor potential melastatin-subfamily member 7 (TRPM7) and CNNM3 in HEK293T cells.
(A) Whole-cell currents in cells transfected with Trpm7 or Trpm7 and Cnnm3 were recorded using the standard Mg2+-free internal solution and standard external solution. When currents were developed, the cells were exposed to the external solution containing 10 mM Mg2+ as indicated by the black bar. Current amplitudes (mean ± standard error of the mean [SEM]) were acquired at –80 and +80 mV and plotted over time. (B, C) Representative current-voltage (I-V) relationships of currents in (A) at 160 and 200 s in cells transfected with Trpm7 (B) or Trpm7 and Cnnm3 (C). The dashed boxes in the left panels indicate areas of inward currents enlarged in the right panels. (D) Bar graphs of outward (+80 mV, mean ± SEM) and inward (–80 mV, mean ± SEM) currents were obtained before and during application of 10 mM Mg2+ as indicated in (A). n, number of cells measured. ns, not significant (two-tailed t-test).
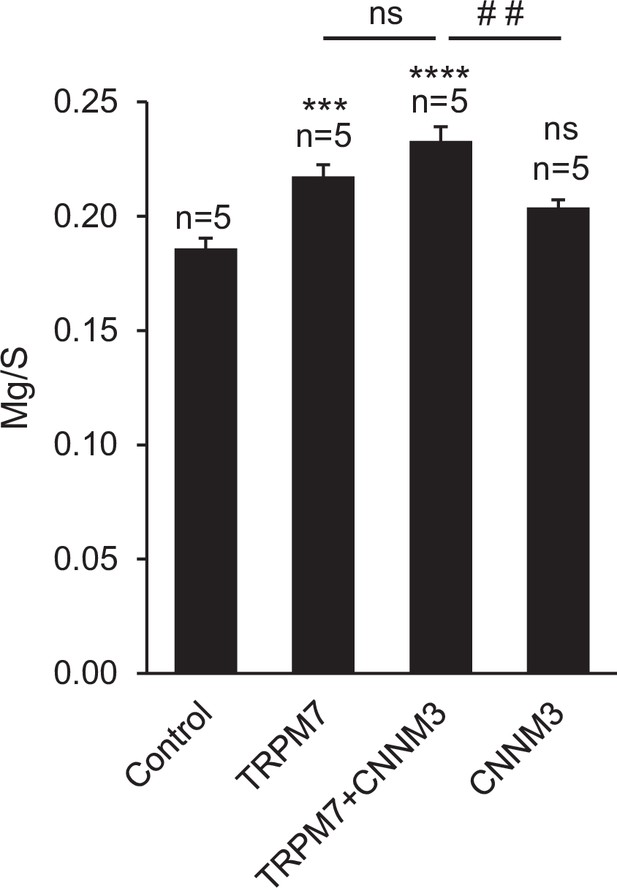
Assessment of total magnesium levels in TRPM7-/- HEK293T cells transiently transfected with Trpm7 and Cnnm3 plasmid cDNAs.
Frozen cell pellets were obtained from untransfected TRPM7-/- HEK293 cells (control) or cells transfected with Trpm7 and/or Cnnm3 cDNA plasmids and analysed by inductively coupled plasma mass spectrometry (ICP-MS). Total elementary Mg contents were normalised to elementary contents of sulphur (S) and represented as mean ± standard error of the mean [SEM] (n = number of independent cell pellets analysed). ns, not significant; ***p ≤ 0.001; ****p ≤ 0.0001 significant to the control group (ANOVA). ##p ≤ 0.01 significant to the TRPM7+ CNNM3 group (ANOVA).
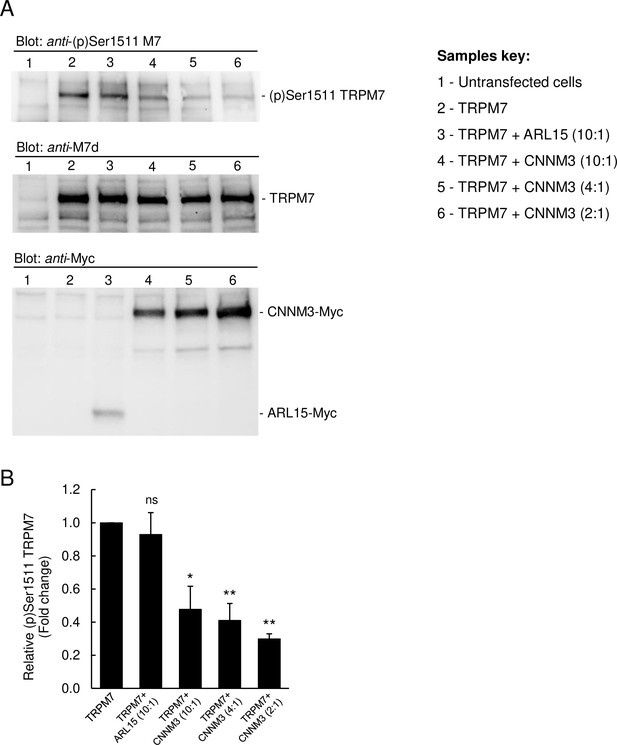
Impact of ADP-ribosylation factor-like protein 15 (ARL15) and CNNM3 on transient receptor potential melastatin-subfamily member 7 (TRPM7) autophosphorylation at Ser1511.
(A) HEK293 cells were transiently transfected with Trpm7, co-transfected with Trpm7 and Arl15, or with Trpm7 and different amounts of Cnnm3 plasmid cDNAs. Twenty-four hours after transfection, cell lysates were examined using an anti-(p)Ser1511 M7 antibody (upper panel). After a stripping step, the blot was probed with anti-M7d (middle panel) and anti-Myc antibodies (lower panel) to detect total levels of TRPM7, ARL15-Myc, and CNNM3-Myc, respectively. Representative results are shown from three independent experiments. (B) Quantification of (p)Ser1511 TRPM7 levels in Western blot experiments (n = 3) shown in (A). A relative band density for each sample was obtained by dividing the (p)Ser1511 signal (upper panel) by the corresponding anti-M7d value (middle panel). The relative density of Sample 2 (TRPM7) was set as a 1.0 to calculate changes in (p)Ser1511 TRPM7 (mean ± standard error of the mean [SEM]) caused by co-transfection of Arl15 or Cnnm3 as outlined in the bar graph. ns, not significant; *p ≤ 0.05, **p ≤ 0.01 significant to the control (ANOVA).
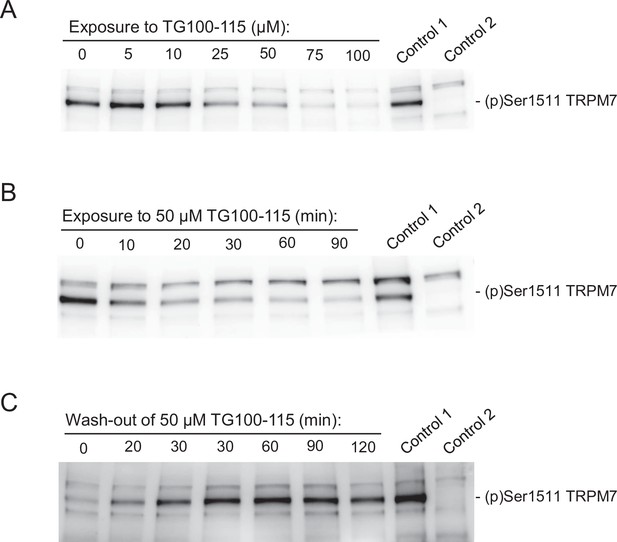
Effects of TG100-115 on transient receptor potential melastatin-subfamily member 7 (TRPM7) autophosphorylation.
(A) Concentration-dependent inhibitory effects of TG100-115 on the autophosphorylation of TRPM7. HEK293 cells were transiently transfected with Trpm7 cDNA. Twenty-four hours after transfection, the indicated concentrations of TG100-115 were added to the cell culture medium, and cells were cultured for an additional 12 hr and immunoreactivity of (p)Ser1511 TRPM7 was detected in cell lysates using the anti-(p)Ser1511 M7 antibody. (B) Time-dependent action of TG100-115 on (p)Ser1511-TRPM7 levels. Trpm7-transfected cells were exposed to the cell culture medium containing 50 µM TG100-115 during 10–90 min at room temperature, and cell lysates were examined as in (A). (C) Reversibility of TG100-115 effects on the autophosphorylation of TRPM7. Trpm7-transfected cells were exposed to cell culture medium containing 50 µM TG100-115 for 2 hr. Afterwards, the cells were washed with fresh medium and incubated without TG100-115 for 20–120 min at room temperature. Immunoreactivity of (p)Ser1511 TRPM7 was detected as in (A). To verify the specificity of the TRPM7 signal, lysates from Trpm7-transfected and -untransfected cells were used (correspondently, Control 1 and Control 2). Representative results are shown from two independent experiments. Note: In contrast to the (p)Ser1511 signal, unspecific bands were equally detectable in all samples examined.
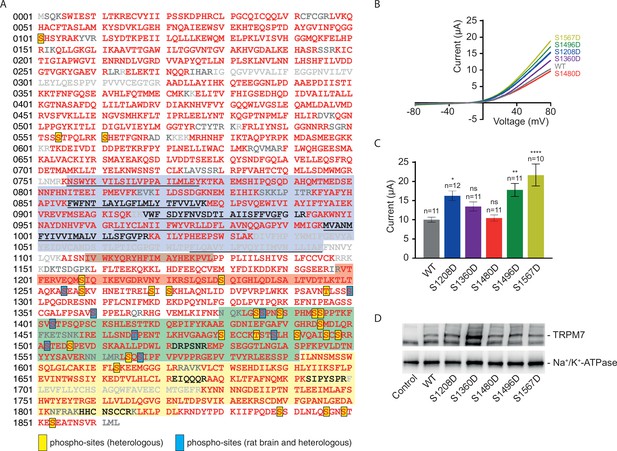
Identification of transient receptor potential melastatin-subfamily member 7 (TRPM7) phospho-sites and functional assessment of phosphomimetic TRPM7 mutants.
(A) Coverage of the primary sequence of TRPM7 and phosphorylation sites as identified by mass spectrometry (MS) analyses of affinity purifications (APs) from transfected HEK293 cells and rodent brain. Peptides identified by MS are in red; those accessible to but not identified in tandem mass spectrometry (MS/MS) analyses are in black, and peptides not accessible to the MS/MS analyses used are given in grey. Blue boxes indicate phospho-sites identified in the brain and transfected HEK293 cells; those uniquely seen in heterologous expressions are boxed in yellow. Colour coding of hallmark domains is as in Figure 1A; S1-S6 helices of TRPM7 are underlined. (B, C) Two-electrode voltage clamp (TEVC) measurements of phosphomimetic TRPM7 mutants performed and analysed as explained in Figure 3A. (B) Representative current-voltage (I-V) relationships of TRPM7 currents measured in oocytes expressing WT and mutant variants of TRPM7, as indicated. (C) Current amplitudes (mean ± standard error of the mean [SEM]) at +80 mV of measurements shown in (B). Two independent batches of injected oocytes (n = 10–12) were examined. ns, not significant; *p ≤ 0.05, **p ≤ 0.01, ****p ≤ 0.0001 (ANOVA). (D) Western blot analysis of TRPM7 variants with phosphomimetic mutations expressed in Xenopus oocytes. Lysates of un-injected oocytes (control) or oocytes injected with WT and indicated mutant variants of Trpm7 cRNAs were examined using the anti-M7d antibody. The anti-Na+/K+ ATPase antibody was used for loading controls. Representative results are shown for three independent experiments.
Tables
Protein constituents of native transient receptor potential melastatin-subfamily member 7 (TRPM7) channels identified by multi-epitope affinity purifications (ME-APs).
| Protein ID | Acc. No.UniProtKB | Name | Primary function | Rel. abundance | |
|---|---|---|---|---|---|
| CL-47 | CL-91 | ||||
| TRPM7 | Q923J1 | TRP channel M7 | Ion channel | = | = |
| CNNM1 | Q0GA42 | Transporter CNNM1, Cyclin-M1 | Potential transporter | << | |
| CNNM2 | Q5U2P1 | Transporter CNNM1, Cyclin-M2 | Potential transporter | < | << |
| CNNM3 | Q32NY4 | Transporter CNNM1, Cyclin-M3 | Potential transporter | < | << |
| CNNM4 | Q69ZF7 | Transporter CNNM1, Cyclin-M4 | Potential transporter | < | << |
| ARL15 | Q8BGR6 | ADP-ribosylation factor-like protein 15 | Unknown | = | << |
| TP4A1† | Q93096 | Protein tyrosine phosphatase type IVA 1 | Enzyme | <<< | << |
| TP4A3## | Q9D658 | Protein tyrosine phosphatase type IVA 3 | Enzyme | << | |
| TRPM6### | Q9BX84 | TRP channel M6 | Ion channel | <<< | << |
-
Notes: Relative abundance refers to the amount of TRPM7 as a reference and was classified as follows: = when between 0.33-fold and 3.3-fold of reference, < when between 0.033-fold and 0.33-fold of reference, << when between 0.0033-fold and 0.033-fold of reference, and <<< when less than 0.0033-fold of the reference amount.
-
 Transmembrane proteins;
Transmembrane proteins;  cytoplasmic proteins.
cytoplasmic proteins. -
†Co-purified from HEK293 cells with anti-M7a (CL-47) and with anti-M7c (CL-91); ##co-purified with anti-M7c from rat brain membranes (CL-91); ###co-purified with anti-M7a from HEK293 cells (CL-47, CL-91).
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Mus musculus) | C57BL/6 | Jackson Labs | JAX stock #000664 | Six weeks of age, equal numbers of male and female |
| Strain, strain background (Ratus norvegicus) | Wistar | Charles River | Strain code:003 | Six weeks of age, equal numbers of male and female |
| Strain, strain background (Xenopus laevis) | Xenopus laevis | NASCO | Cat#:LM00535 | |
| Cell line (human) | HEK293T | Sigma | Cat#:96121229; RRID:CVCL_2737 | |
| Cell line (human) | TRPM7-/- HEK293T | DOI:10.1073/pnas.1707380114 | ||
| Cell line (human) | HEK293T-Rex cells stably expressing TRPM7 | 10.1016/s0092-8674(03)00556–7 | ||
| Antibody | Anti-HA (rat monoclonal) | Roche | Cat#:11867423001; RRID:AB_390918 | IP (3–15 µg per IP), WB (0.2 µg/ml) |
| Antibody | Anti-HA (mouse monoclonal) | Invitrogen | Cat#:26183; RRID:AB_2533056 | IP (3 µg per IP) |
| Antibody | Normal rabbit IgG | Millipore | Cat#:12–370; RRID:AB_145841 | IP (15 µg per IP) |
| Antibody | Anti-βArrestin 2 (mouse monoclonal) | Santy Cruz Biotechnology | Cat#:sc-13140; RRID:AB_626701 | WB (1 µg/ml) |
| Antibody | Anti-TRPC1 (rabbit polyclonal) | Other | 4921 | Gift from Veit Flockerzi Immunogen: N-terminus of mouse TRPC1, IP (15 µg per IP) |
| Antibody | Anti-TRPC3 (rabbit polyclonal) | Other | 1378 | Gift from Veit Flockerzi Immunogen:N-terminus of mouse TRPC3, IP (15 µg per IP) |
| Antibody | Anti-NMDAR1 (mouse monoclonal) | Millipore | Cat#:MAB1586; RRID:AB_11213180 | IP (15 µg per IP) |
| Antibody | Anti-LRRTM2 (rabbit polyclonal), | ProteinTech | Cat#:23094–1-AP; RRID:AB_2879209 | IP (15 µg per IP) |
| Antibody | Anti-DPP10 (mouse monoclonal) | Santa Cruz Biotechnology | sc-398108 | IP (15 µg per IP) |
| Antibody | Anti-RGS9 (goat polyclonal) | Santa Cruz Biotechnology | sc-8143; RRID:AB_655555 | IP (15 µg per IP) |
| Antibody | Anti-TRPM7 (mouse monoclonal) | Thermo Fisher Scientific | Cat#:MA5-27620; RRID:AB_2735401 | IP (15 µg per IP) |
| Antibody | Anti-TRPM7 (mouse monoclonal) | NeuroMab | Cat#:75–114; RRID:AB_2877498 | IP (15 µg per IP) |
| Antibody | Anti-(p)Ser1511 TRPM7 (mouse monoclonal) | DOI:10.1038/s41467-017-01960-z | Affinity purified with peptide H2N-DSPEVD(p)SKAALLPC-NH2, WB (2 µg/ml) | |
| Antibody | Anti-M7c (rabbit polyclonal) | DOI:10.1038/s41467-017-01960-z | Affinity purified with peptide H2N-DSPEVDSKAALLPC-NH2, IP (15 µg per IP) | |
| Antibody | Anti-M7d (2C7, mouse monoclonal) | This paper | See ‘Materials and methods, Antibodies’, IP (15 µg per IP), WB (0.8 µg/ml),IF (1.6 µg/ml) | |
| Antibody | Anti-TRPM7 (4F9, mouse monoclonal) | This paper | See ‘Materials and methods, Antibodies’, WB (1.4 µg/ml) | |
| Antibody | Anti-TRPM7 (rabbit polyclonal) | Millipore | Cat#:AB15562; RRID:AB_805460 | WB (1 µg/ml) |
| Antibody | Anti-Flag (mouse monoclonal) | Sigma | Cat#:F3165; RRID:AB_259529 | WB (1 µg/ml) |
| Antibody | Anti-βActin (rabbit polyclonal) | Bioss Inc | Cat#:bs-0061R; RRID:AB_10855480 | WB (0.5 µg/ml) |
| Antibody | Anti-rabbit IgG (goat polyclonal, HRP conjugate) | abcam | ab7090 | WB (1:30000) |
| Antibody | Anti-mouse IgG (goat polyclonal, HRP conjugate) | abcam | ab7068 | WB (1:10000) |
| Antibody | Anti-mouse IgG (horse polyclonal, HRP conjugate) | Cell Signaling Technology | Cat#:7076 | WB (1:1000) |
| Antibody | Anti-Na+/K+ ATPase (rabbit monoclonal, HRP conjugate) | Abcam | Cat#:ab185065 | WB (1:1000) |
| Antibody | Anti-Myc (mouse monoclonal, clone 9B11) | Cell Signaling Technology | Cat#:2276 | WB (1:1000) |
| Antibody | Anti-mouse IgG- Alexa Fluor 488 (goat IgG, Alexa Fluor 488 conjugate) | Thermo Fisher Scientific | Cat#:A11029 | 2 μg/ml |
| Recombinant DNA reagent | pT7-His6-Trpm7-KD(plasmid) | This paper | See ‘Materials and methods, Antibodies’ | |
| Peptide, recombinant protein | His6-TRPM7-KD(purified protein) | This paper | See ‘Materials and Methods, Antibodies’ | |
| Peptide, recombinant protein | TRPM7-KD(purified protein) | This paper | See ‘Materials and methods, Antibodies’ | |
| Recombinant DNA reagent | Mouse Trpm7 cDNA in pIRES2-EGFP vector (plasmid) | DOI: https://doi.org/10.1038/s41598-017-08144-1 | Expression in mammalian cells | |
| Recombinant DNA reagent | Mouse Trpm6 cDNA in pIRES2-EGFP vector (plasmid) | DOI: https://doi.org/10.1038/s41598-017-08144-1 | Expression in mammalian cells | |
| Recombinant DNA reagent | Human TRPM6 cDNA in pIRES2-EGFP vector (plasmid) | DOI: https://doi.org/10.1038/s41598-017-08144-1 | Expression in mammalian cells | |
| Recombinant DNA reagent | Mouse Trpm7 cDNA in pOG1 vector (plasmid) | DOI: 10.1073/pnas.0305252101 | cRNA synthesis | |
| Recombinant DNA reagent | Mouse Trpm7-Myc cDNA in pcDNA3.1/V5-His TA-TOPO vector (plasmid) | DOI: 10.1073/pnas.0305252101 | Expression in mammalian cells | |
| Recombinant DNA reagent | Mouse Trpm7-HA cDNA in pcDNA3.1/V5-His TA-TOPO vector (plasmid) | DOI:10.1073/pnas.0305252101 | Expression in mammalian cells | |
| Recombinant DNA reagent | Human TRPV1-His cDNA in pNKS2 vector (plasmid) | This paper | See ‘Materials and methods, Antibodies, Molecular biology’ cRNA synthesis | |
| Recombinant DNA reagent | Mouse Cnnm1-Myc-Flag in pCMV6-Entry (plasmid) | OriGene | Cat#:MR218318 | Expression in mammalian cells |
| Recombinant DNA reagent | Mouse Cnnm2-Myc-Flag in pCMV6-Entry (plasmid) | OriGene | Cat#:MR218370 | Expression in mammalian cells |
| Recombinant DNA reagent | Mouse Cnnm3-Myc-Flag in pCMV6-Entry (plasmid) | OriGene | Cat#:MR224758 | Expression in mammalian cells, cRNA synthesis |
| Recombinant DNA reagent | Mouse Cnnm4-Myc-Flag in pCMV6-Entry (plasmid) | OriGene | Cat#:MR215721 | Expression in mammalian cells |
| Recombinant DNA reagent | Mouse Arl15-Myc-Flag in pCMV6-Entry (plasmid) | OriGene | Cat#:MR218657 | Expression in mammalian cells, cRNA synthesis |
| Recombinant DNA reagent | Mouse Arl8a-Myc-Flag in pCMV6-Entry (plasmid) | OriGene | Cat#:MR201740 | Expression in mammalian cells, cRNA synthesis |
| Commercial assay or kit | Bio-Rad Protein Assay | Bio-Rad | Cat#:5000006 | Protein concentration determination |
| Chemical compound, drug | ComplexioLyte CL-47 | Logopharm | Cat#:CL-47–01 | Mild detergent buffer |
| Chemical compound, drug | ComplexioLyte CL-91 | Logopharm | Cat#:CL-91–01 | Detergent buffer with intermediate stringency |
| Chemical compound, drug | Trypsin, sequencing grade modified | Promega | Cat#:V5111 | |
| Chemical compound, drug | Leupeptin | Sigma | Cat#:L2884 | |
| Chemical compound, drug | Pepstatin A | Sigma | Cat#:P5318 | |
| Chemical compound, drug | Aprotinin | Roth | Cat#:A162.2 | |
| Chemical compound, drug | Phenylmethylsulfonyl fluoride | Roth | Cat#:6367.3 | |
| Chemical compound, drug | Iodoacetamide | Sigma | I6125 | |
| Chemical compound, drug | Aminocaproic acid | Roth | 3113.3 | |
| Chemical compound, drug | TG100-115 | Selleck Chemicals | Cat#:S1352 | |
| Software, algorithm | msconvert.exe | http://proteowizard.sourceforge.net/ | ||
| Software, algorithm | MaxQuant v1.6.3 | http://www.maxquant.org | ||
| Software, algorithm | Mascot 2.6 | Matrix Science, UK | ||
| Software, algorithm | CellWorks 5.5.1 | npi electronic https://www.npielectronic.com | ||
| Software, algorithm | ZEN 2.3 | Carl Zeiss https://www.zeiss.de | ||
| Software, algorithm | PatchMaster 2 × 90 | Harvard Bioscience https://www.heka.com | ||
| Software, algorithm | Studio Lite 4.0 | https://www.licor.com/bio/image-studio-lite | ||
| Other | Dynabeads Protein A | Invitrogen | Cat#:10002D | |
| Other | Dynabeads Protein G | Invitrogen | Cat#:10004D | |
| Other | Tissue embedding media | Leica | Cat#:14020108926 | Used to support gel slices during cryotomy |
Additional files
-
Supplementary file 1
Numerical data for peak volumes, abundance norm values, relative abundance, and ratio distance values obtained through analysis of the mass spectrometry (MS) data.
- https://cdn.elifesciences.org/articles/68544/elife-68544-supp1-v2.xlsx
-
Supplementary file 2
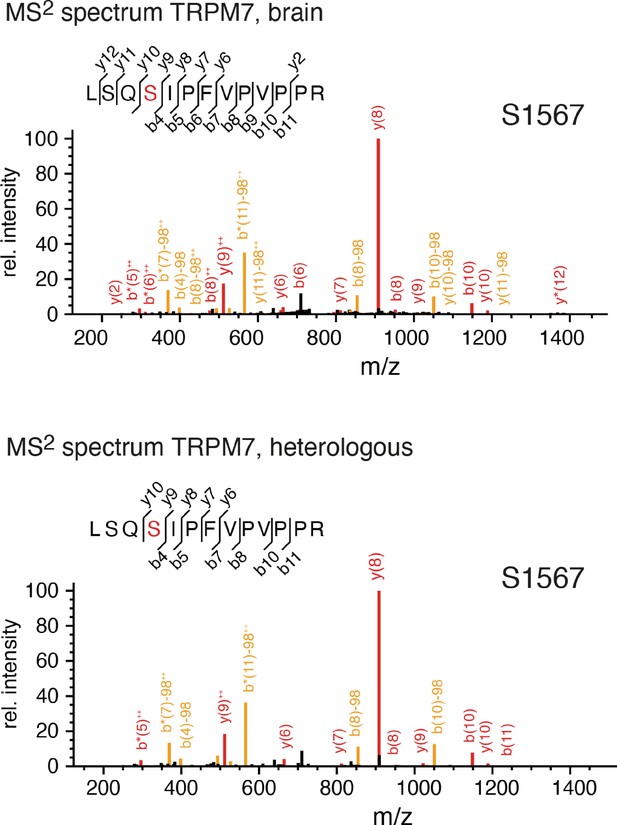
Mass spectrometry (MS) spectra of phosphorylated transient receptor potential melastatin-subfamily member 7 (TRPM7), CNNM3, and CNNM4 peptides identified in affinity purifications (APs) from HEK293 and rodent brain.
- https://cdn.elifesciences.org/articles/68544/elife-68544-supp2-v2.xlsx
-
Supplementary file 3
Phosphorylation sites in transient receptor potential melastatin-subfamily member 7 (TRPM7), CNNM3, and CNNM4 identified in affinity purifications (APs) from transfected HEK293 cells and rodent brain.
Excel file contains one worksheet: The phosphorylated residues of TRPM7, CNNM3, and CNNM4 identified by mass spectrometry (MS) in the present study are outlined in conjunction with previously published data (Nguyen et al., 2019; Zhou et al., 2013; Cai et al., 2017; Huttlin et al., 2010).
- https://cdn.elifesciences.org/articles/68544/elife-68544-supp3-v2.docx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/68544/elife-68544-transrepform1-v2.docx