Condensin DC loads and spreads from recruitment sites to create loop-anchored TADs in C. elegans
Figures
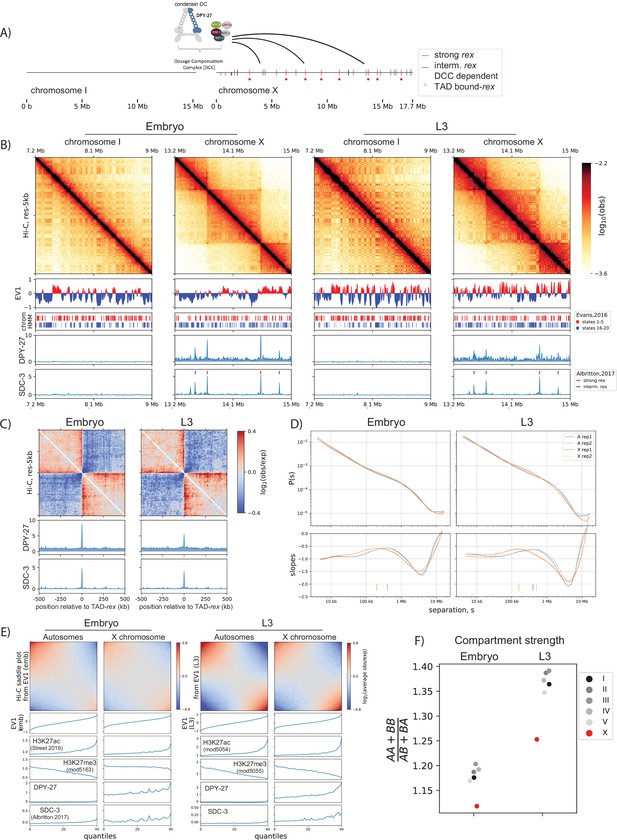
Developmentally conserved evidence of X-chromosome enriched loop extruding factor, condensin dosage compensation (DC).
(A) Cartoon of the dosage compensation complex in C. elegans. Strong and intermediate recruitment elements on the X-chromosome (rex) sites (Albritton et al., 2017) and DC complex (DCC)-dependent topologically associating domain (TAD) boundary rex sites (Anderson et al., 2019) are annotated. (B) Snapshot of chromosome-I and X for embryo and L3. EV1 is computed using the center regions of chromosomes as defined in Ikegami et al., 2010. The center regions have lower LEM-2 association, higher gene-density, and lower repeat density than the arms. Shown below are annotated ChromHMM states 1–5 and 16–20 that are associated with highest and lowest quantiles of gene expression, respectively (Evans et al., 2016). SDC-3 and DPY-27 binding is plotted as normalized Chromatin immunoprecipitation followed by high-throughput sequencing (ChIP-seq) scores. (C) Meta-plot of observed Hi-C interactions compared to expected, centered across strong rex sites. The expected values are computed using only the X-chromosome due to X and autosomes having distinct P(s) curves. (D) Hi-C relative contact probability, P(s), as a function of genomic separation, s, and the log-derivative of P(s) for two biological reps. The local-maxima of slopes or inferred mean loop size is annotated as vertical ticks. (E) Compartmentalization of all autosomes and the X-chromosomes at the center region of the chromosomes. The saddle plot indicates the level of intrachromosomal interactions between and within A and B compartments defined as top and bottom halves of EV1 values. (F) The strength of compartmentalization was calculated for each chromosome by taking a ratio of the sum of interaction within the same compartment to the sum of interactions across compartment as described in Nuebler et al., 2018. Compartmentalization is weaker in mixed developmental stage embryos, compared to L3 larvae. In both developmental stages, compartmentalization is weaker on the X. See Figure 1—figure supplement 1 for an alternative method of computing compartment strength.
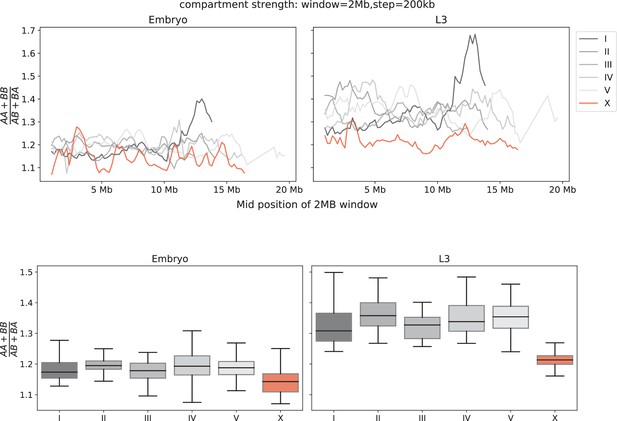
The strength of compartmentalization is computed using a sliding window submatrix (related to Figure 1E, F).
Chromosomes have different total, arm, and center lengths (Crane et al., 2015; Bian et al., 2020; Ikegami et al., 2010), therefore using only the center regions to draw conclusions about the entire chromosome could potentially be misleading. To control for the size of the submatrix and the chromosomal region, we used a 2 kb-resolution matrix with a fixed submatrix size of 2 MB with step size of 200 kb to generate a saddle plot, from which the strength is computed. The strength per 2 MB submatrix is plotted across the entire chromosome (top panel) or plotted as a boxplot per chromosome (bottom panel). Consistent with the conclusion drawn from the center region (Figure 1F), the compartmentalization is weaker on the X.
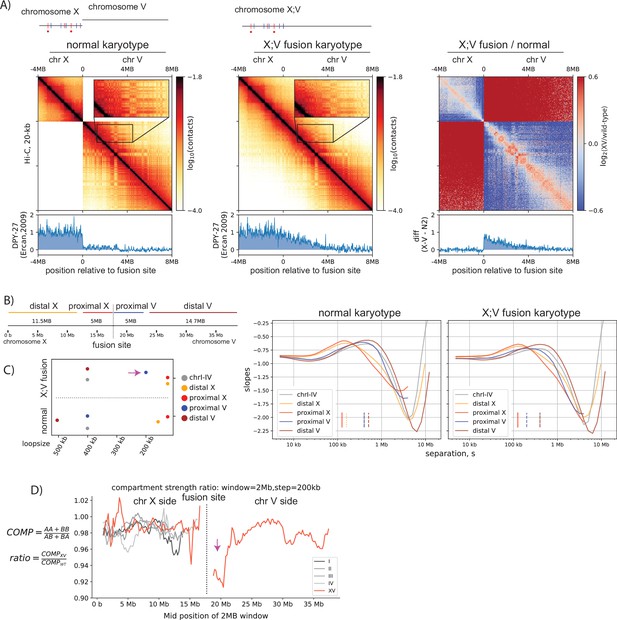
Spreading of condensin dosage compensation (DC) coincides with decrease in loop size and weakening of compartments.
(A) Condensin DC spreads ~3 MB into the autosomal region of X;V fusion chromosome. 12-MB region snapshot of Hi-C contacts in the wildtype karyotype and X;V fusion strain. Hi-C and ChIP-seq data are from embryos. The inset zoom (10 kb-binned matrix) highlights weakening of the checker-board pattern indicating compartmentalization. Log2ratio plot highlights enrichment (red) of ‘shorter-range’ interactions at the spreading region of X;V. (B) To compare the local effect of spreading, chromosome X;V is divided into four regions surrounding the fusion site and log-derivative of P(s) is plotted. (C) Inferred mean loop size of each region from the derivative plot is plotted. Purple arrow highlights the decrease in mean loop size for chromosome-V side of the fusion (proximal V). (D) Difference in local compartment strength in X;V compared to normal karyotype. EV1 and saddle strength are computed for each 2-MB submatrix. The ratio of the same region between two conditions highlights distance-dependent decrease (purple arrow) in compartment strength on chromosome-V side of the fusion.
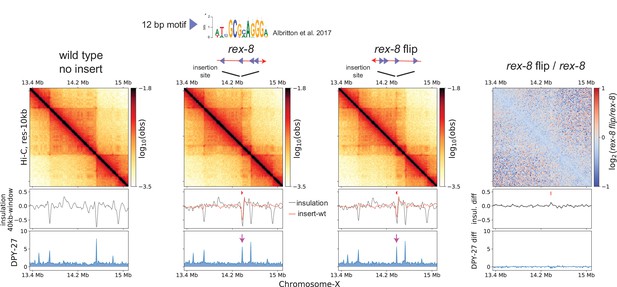
Ectopically inserted rex-8 in two different orientations act as a bidirectional barrier.
A strong recruitment elements on the X-chromosome (rex) site with four motifs in the same orientation acts as a topologically associating domain (TAD) boundary on the X. The 358-bp rex-8 was inserted in two orientations at the same site on X-chromosome. Hi-C interactions surrounding the insertion sites along with the insulation score (black) and its subtraction to that of wild type (red), and DPY-27 ChIP-seq are plotted. A dip in the insulation score coincides with enrichment of DPY-27 ChIP (purple arrow) and supports barrier activity. Log2ratio plot highlights the lack of noticeable Hi-C difference between insertion of two different orientations.
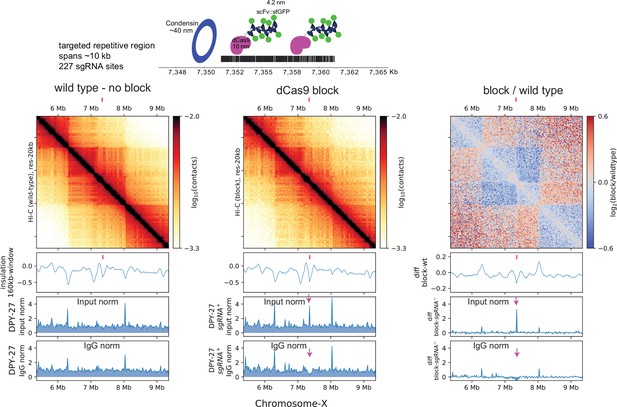
dCas9 block fail to sufficiently recapitulate rex-like boundary on the X-chromosome.
Top: schematic depicting the multi-protein block and the approximate size of the components utilized to prevent condensin from translocating linearly along chromatin. Bottom: Hi-C of wildtype and block strain expressing all components of dCas9-SunTag and single guide RNA (sgRNA). The tick marks point to the block target. The below are ChIP-seq data in dCas9-SunTag expressing strain with (first panel) and without (middle panel) sgRNA. The two ChIP-seq tracks show normalization by input and IgG. Arrows pointing down to the DPY-27 ChIp signal that is apparent when data is normalized to input but not to IgG.
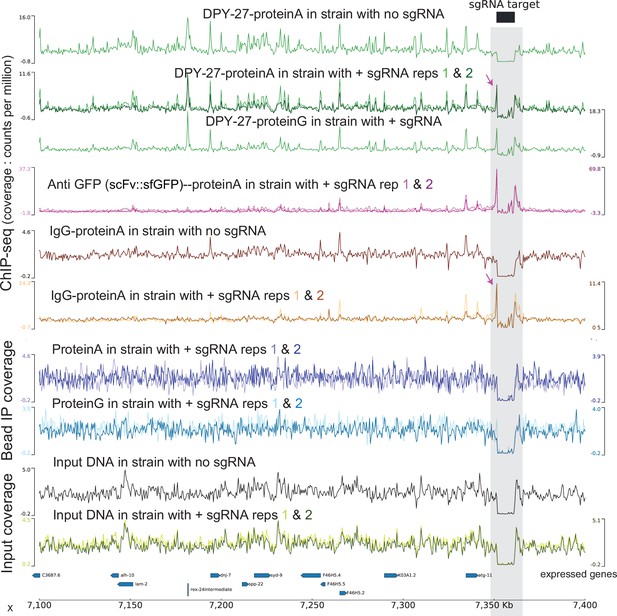
Increased ‘chippability’ due to dCas9-SunTag targeting.
Counts per million (CPM) normalized tracks of various ChIP and input data in conditions with (JZ2005) or without (JZ1973) sgRNA expression construct. The mapping quality (MAPQ) score threshold is increased to 20 to prevent mapping at the repetitive target locus. sgRNA expression results in an increased signal at/near the locus when chipped with DPY-27 or IgG. This is not an artifact of cross-reactivity of suntag with protein A/G or changes in sequencing bias of input as exclusion of antibody does not affect the observed tracks. The artifactual signal at the targeted locus only arises in the presence of antibodies.
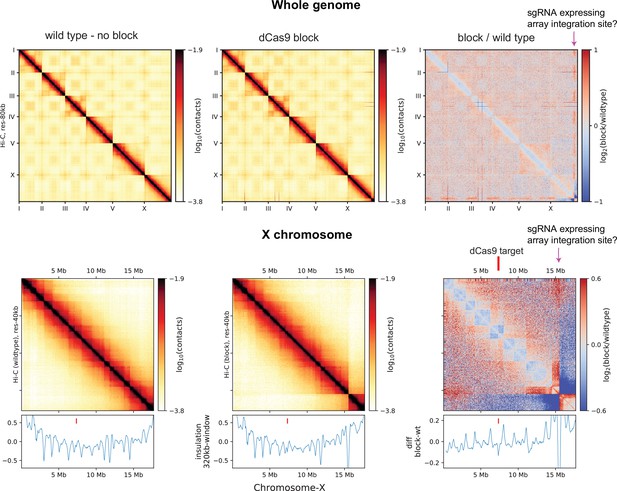
Global effect of Hi-C data in dCas9-SunTag targeting strain.
JZ2005 strain has a global effect on topologically associating domains (TADs) and likely a multi-megabase insertion near the end of chromosome-X.
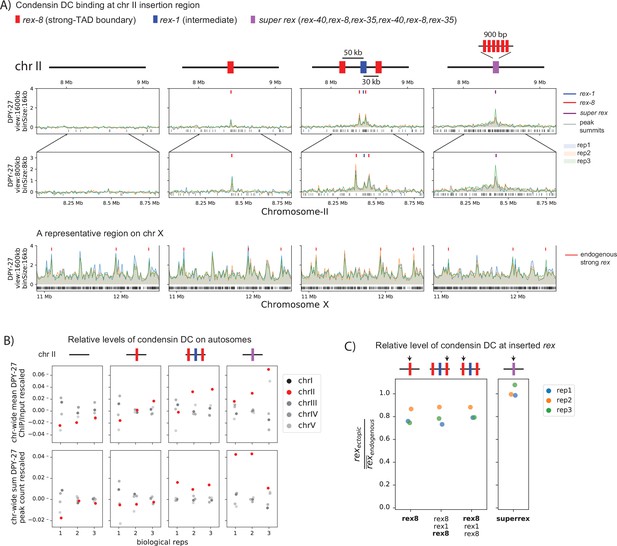
Condensin dosage compensation (DC) is recruited to and spreads in either direction from the ectopically inserted recruitment elements on the X-chromosome (rex) sites on chromosome-II in L3.
(A) Snapshot of a region on chromosome-II where rex sites are inserted. Shown are four different conditions in L3 stage: wild-type, ERC61 (single rex-8 insertion) (Albritton et al., 2017), ERC63 (rex-8, rex-1, rex-8) [33], and ERC90 super rex, an array of six truncated, midsection (150 bp) of three strong rex’s: rex-40, rex-8, rex-35, repeated. Binding surrounding the insertion sites in both directions increases with increased number and strength of inserted rexes. The levels of condensin DC ectopically recruited to chromosome-II remain weaker than the endogenous binding at X-chromosome (lower panel). (B) The enrichment of condensin DC on each autosome plotted as chromosome-wide mean enrichment and total number of binding peaks calculated from DPY-27 ChIP-seq data. The ChIP-seq values or the total number of peaks are rescaled for each data, such that the mean of autosomes excluding chromosome-II is fixed to 0 and that of X-chromosome is at 1. The plot highlights weak but reproducible recruitment of more condensin DC to chromosome-II by rex insertion. (C) The level of condensin DC binding at the inserted strong rex-8 (indicated by arrow in each strain) is comparable to the endogenous, as indicated by the ChIP-seq signal at inserted rex site (400 bp) normalized to the mean of all endogenous strong rex sites on the X.
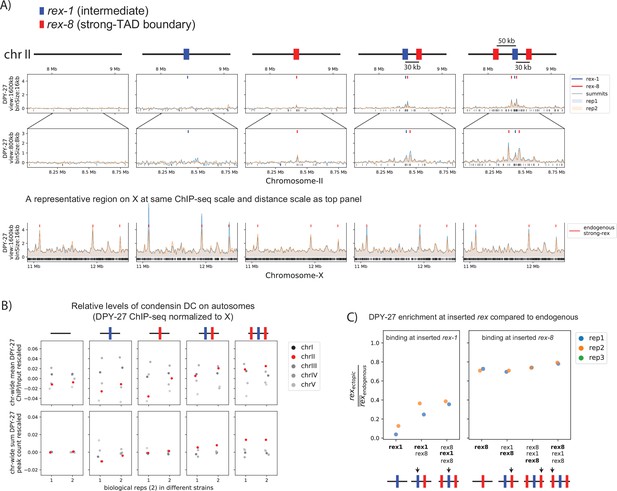
Condensin dosage compensation (DC) is recruited to and spreads in either direction from the ectopically inserted recruitment elements on the X-chromosome (rex) sites on chromosome-II in embryo.
(A) Snapshot of a region on chromosome II where rex sites are inserted. Shown are four different conditions in embryo stage: wild-type, ERC06 (single rex-1 insertion) (Albritton et al., 2017), ERC61 (single rex-8 insertion) [33], ERC62 (rex-1, rex-8) [33], and ERC63 (rex-8, rex-1, rex-8) (Albritton et al., 2017). Binding surrounding the insertion sites in both directions increases with increased number and strength of inserted rexes. The levels of condensin DC ectopically recruited to chromosome-II remain weaker than the endogenous binding at X-chromosome (lower panel). (B) The enrichment of condensin DC on each autosome plotted as chromosome-wide mean enrichment and total number of binding peaks calculated from DPY-27 ChIP-seq data. The ChIP-seq values or the total number of peaks are rescaled for each data, such that the mean of autosomes excluding chromosome-II is fixed to 0 and that of X-chromosome is at 1. The plot highlights weak but reproducible recruitment of more condensin DC to chromosome-II by rex insertion. (C) The level of condensin DC binding at the inserted rex-1 or strong rex-8 (indicated by arrow in each strain) is comparable to the endogenous, as indicated by the ChIP-seq signal at inserted rex site (400 bp) normalized to the mean of all endogenous strong rex sites on the X.
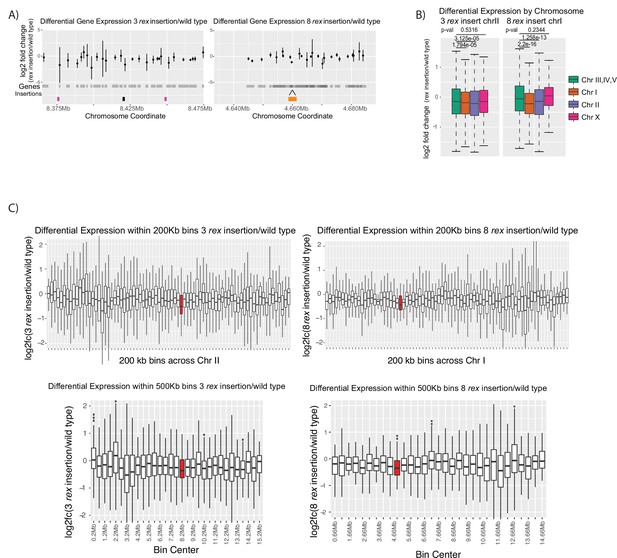
mRNA-seq data comparing recruitment elements on the X-chromosome (rex) insertion strains to wildtype.
(A) Log2 fold change between the insertion and wildtype for each gene in the 100 kb region surrounding the insertion sites. Two insertion conditions used are ERC63 (rex-8, rex-1, rex-8 on chromosome-II) [33] (left panel) and ERC08 (eight rex-1 on chromosome-I) (right panel). The gray shading underneath the points indicates gene bodies. (B) Boxplots of the log2 fold change in the two strains. Autosomes III, IV, and V are grouped as autosomal control. Two-tailed student’s t-test p-values are given. (C) Boxplots log2 fold change values are binned into 200 kb (top panels) or 500 kb (bottom panels) domains tiled across the chromosome where the rex sites are inserted. Bins containing a significant level of repression are marked with asterisks (Fisher exact test; * indicates p-value<0.05, ** indicates p-value<0.01, *** indicates p-value<0.001). The red bins indicate bins that contain an inserted rex site.
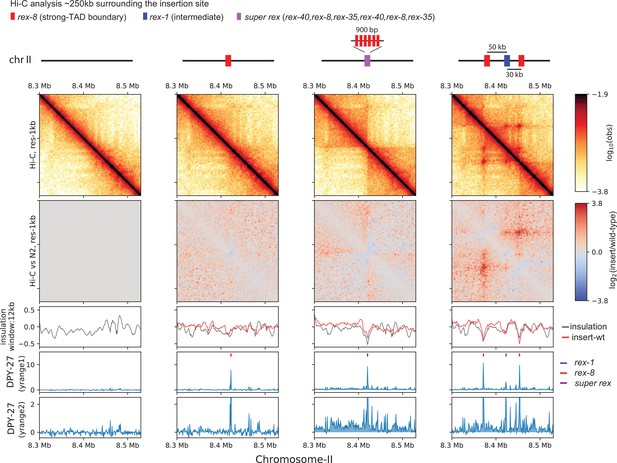
Ectopic insertion of rex elements that lead to condensin dosage compensation (DC) spreading form loop-anchored topologically associating domains (TADs).
Snapshot of a region on chromosome-II where recruitment elements on the X-chromosome (rex) sites are inserted. Shown are four different conditions in L3 stage: wild-type, single rex-8 insertion at one site, six concatenated strong rex (super rex) insertion at the same site and insertion of rex-8, rex-1, and rex-8 at a distance from each other spanning ~80 kb. Two different y-range cutoffs for ChIP-seq data are provided to highlight the level of condensin DC spreading in each strain. Log2ratio Hi-C plots or subtraction of insulation score (insertion-wild type) plots are generated using wild-type data mapped to the corresponding insertion genome in comparison.
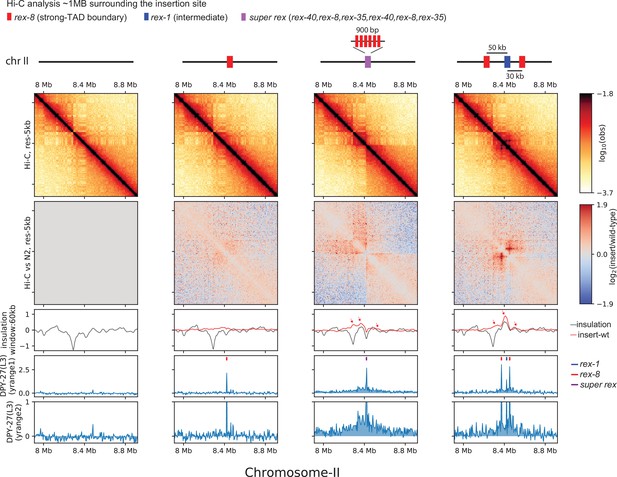
Spreading coincides with changes in 3D contact.
Zoomed-out snapshot of a region on chromosome-II where recruitment elements on the X-chromosome (rex) sites are inserted.
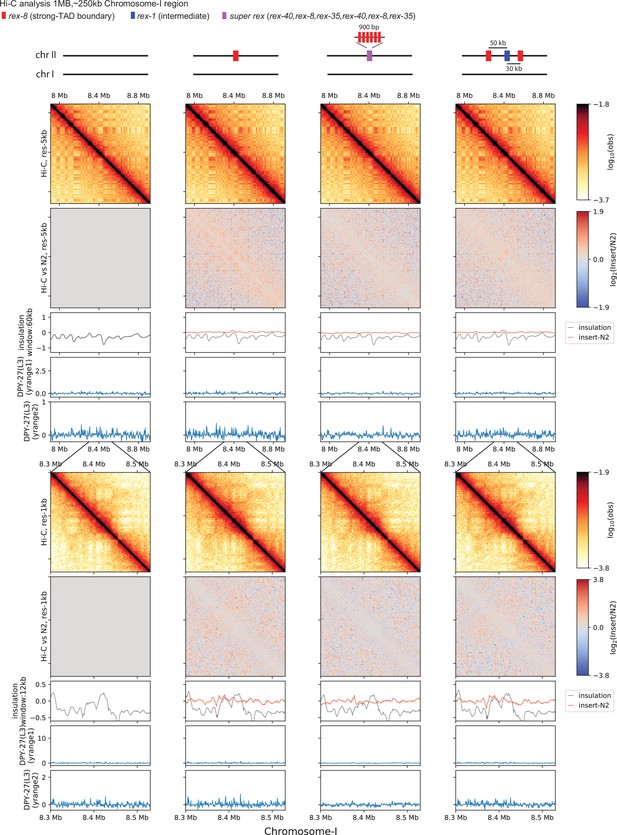
Control regions for ectopic recruitment elements on the X-chromosome (rex) insertion experiment.
Snapshot of a control region on chromosome-I showing negligible technical variability. The same parameters are used as Figure 6 and Figure 6—figure supplement 1 for the top and the bottom panels, respectively.
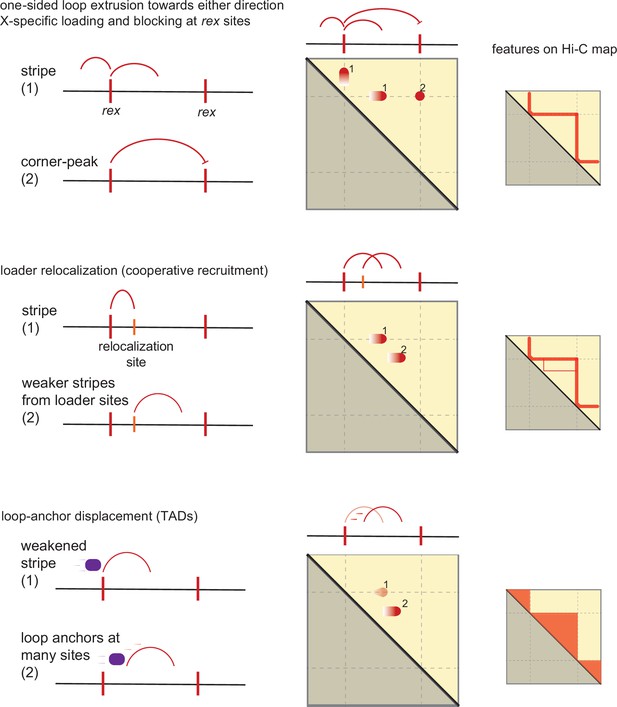
Model of condensin dosage compensation (DC) recruitment and spreading forming loop-anchored topologically associating domains (TADs).
Shown are three conceptually distinct features that contribute to TAD formation in C. elegans dosage compensation complex (DCC) system. The model as a group contains the following properties: (i) rex is a bidirectional barrier, (ii) rex is a loading site, (iii) rex can activate/enhance weaker ‘secondary loading sites,’ (iv) condensin DC is a one-sided loop extruder, and (v) condensin DC’s loop-anchor is prone to displacement.
Additional files
-
Supplementary file 1
Data summary and statistics.
ChIP-seq mapping statistics, Hi-C juicer statistics, and RNA-seq Transcript per million (TPM) values for each replicate are provided. The description of strains and the primers used to generate the strains are also provided.
- https://cdn.elifesciences.org/articles/68745/elife-68745-supp1-v1.xlsx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/68745/elife-68745-transrepform1-v1.docx





