Edge-strand of BepA interacts with immature LptD on the β-barrel assembly machine to direct it to on- and off-pathways
Figures
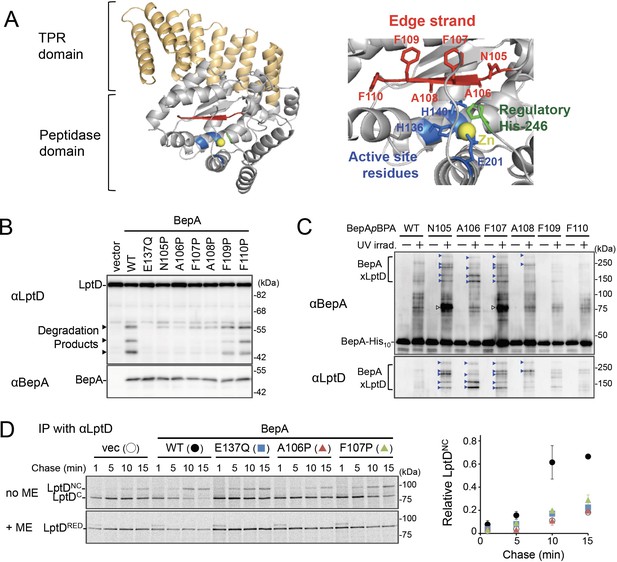
The edge-strand of BepA is crucial for functional interaction with LptD.
(A) Crystal structure of BepA (PDB code: 6AIT). The peptidase and the TPR domains of BepA are shown in gray and orange, respectively. The edge-strand, the proteolytic active site (the HExxH motif and the third zinc ligand, Glu-201), and the regulatory His-246 residue (His switch) in the peptidase domain are shown in red, blue, and green, respectively, and the coordinated zinc atom is shown in yellow. An enlarged view of the active site region is shown in right. (B) Protease activities of the BepA edge-strand mutants. Cells of SN56 (ΔbepA) carrying pTWV228-lptD-his10 and either pSTD689 or pSTD689‐bepA plasmids were grown at 30°C in L-medium until early log phase and induced with 1 mM IPTG for 1 hr. Total cellular proteins were acid-precipitated and analyzed by 7.5 or 10% Laemmli SDS‐PAGE and immunoblotting with the indicated antibodies. (C) In vivo photo-crosslinking analysis of the BepA edge-strand. Cells of SN56 carrying pEVOL-pBpF and pUC18‐bepA(E137Q, amb)-his10 plasmids were grown at 30°C in L-medium containing 0.02% arabinose and 0.5 mM pBPA until early log phase, and induced with 1 mM IPTG for 1 hr to express the indicated BepA(pBPA) variants. The cultures were divided into two portions, each of which was treated with or without UV-irradiation for 10 min at 4°C. Proteins of the total membrane fractions were subjected to pull-down with Ni-NTA agarose. Purified proteins were analyzed by 7.5% Laemmli SDS‐PAGE and immunoblotting with the indicated antibodies. Open triangles indicate unknown crosslinked products. (D) Chaperone-like activities of the BepA edge-strand mutants. Cells of SN56 carrying pSTD689 or a pSTD689‐bepA plasmid were grown at 30°C in M9-based medium until early log phase, induced with 1 mM IPTG for 15 min, pulse-labeled with 35S-Met for 1 min and chased for the indicated periods. At each time point, total cellular proteins were acid-precipitated, subjected to IP with an anti-LptD antibody, and analyzed by 7.5% Laemmli SDS‐PAGE followed by phosphorimaging. The ratio of the band intensities of LptDNC at each time point to that of total LptD (LptDC+LptDNC) at 5 min was quantitated and the mean values were plotted with S.D. (n=2). The result shown is a representative of two independent experiments that were conducted using the same transformants (i.e., two technical replicates). See Figure 1—source data 1 for gel images and quantitated band intensities data for (D).
-
Figure 1—source data 1
A Zip file containing gel images.
(B–D) For the immunoblotting experiments using the anti-BepA and anti-LptD antibodies and quantified band intensity data for the pulse-chase experiments using the anti-LptD antibody.
- https://cdn.elifesciences.org/articles/70541/elife-70541-fig1-data1-v2.zip
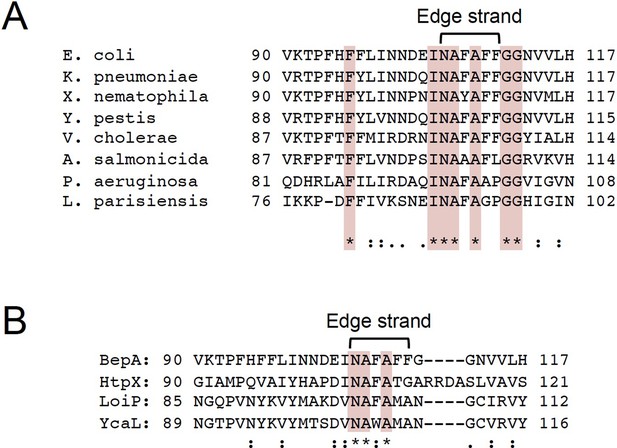
Sequence alignment of BepA homologs and Escherichia coli M48 family peptidases.
(A) Alignment of the edge-strand amino acid sequences of BepA homologs of E. coli, Klebsiella pneumoniae, Xenorhabdus nematophila, Yersinia pestis, Vibrio cholerae, Aeromonas salmonicida, Pseudomonas aeruginosa, and Legionella parisiensis. (B) Alignment of the edge-strand amino acid sequences of BepA and other E. coli M48 family peptidase (HtpX, LoiP, and YcaL). The alignments were conducted by using the Clustal Omega program. Conserved residues are marked in red.
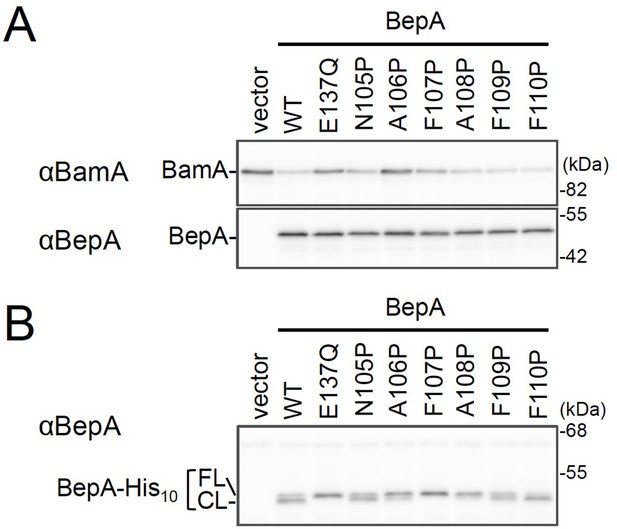
Effects of the BepA edge-strand Pro mutations on the BamA degradation and the BepA self-cleavage.
(A) Degradation of BamA in a ΔsurA strain expressing the BepA edge-strand Pro mutants. Cells of SN259 (ΔbepA, ΔsurA) carrying either pSTD689 or pSTD689‐bepA plasmids were grown at 30°C in M9-based medium supplemented with 1 mM IPTG for 4 hr. Total cellular proteins were acid-precipitated and analyzed by 7.5 or 10% Laemmli SDS‐PAGE and immunoblotting with the indicated antibodies. (B) C-terminal self-cleavages of the BepA edge-strand mutants. Cells of SN56 (ΔbepA) carrying either pUC18 or pUC18‐bepA-his10 plasmids were grown at 30°C in L-medium until early log phase and induced with 1 mM IPTG for 1 hr. Total cellular proteins were acid-precipitated and analyzed by 7.5% Laemmli SDS‐PAGE and immunoblotting with an anti-BepA antibody. The result shown is a representative of two independent experiments that were conducted using the same transformants (i.e., two technical replicates). See Figure 1—figure supplement 2—source data 1 for gel images for Figure 1—figure supplement 1A and B.
-
Figure 1—figure supplement 2—source data 1
A Zip file containing gel images (A–C) for the immunoblotting experiments using the anti-BepA and anti-LptD antibodies.
- https://cdn.elifesciences.org/articles/70541/elife-70541-fig1-figsupp2-data1-v2.zip
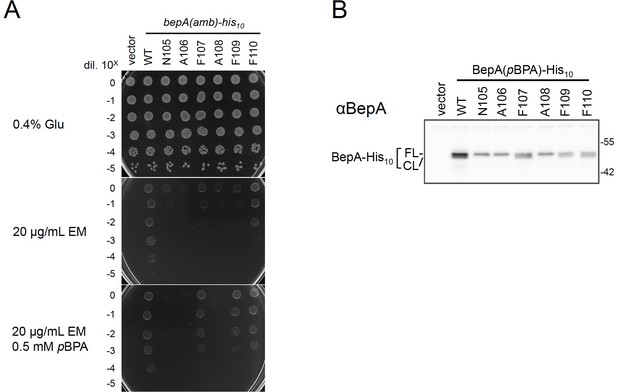
Functionality of the BepA derivatives having pBPA in the edge-strand.
(A) Complementation activity of the BepA(pBPA) derivatives. Cells defective in the BepA functions exhibit elevated sensitivity to high-molecular-mass antibiotics such as erythromycin (EM) (Narita et al., 2013; Nichols et al., 2011) possibly due to an impaired barrier function of the OM. Thus, the functions of the BepA mutants (including BepA(pBPA)) can be evaluated by examining their ability to suppress the EM sensitivity of a ΔbepA strain (Daimon et al., 2017). Cells from 30 μL of overnight cultures of SN56 (ΔbepA) carrying pEVOL-pBpF and either pUC18 or pUC18-bepA(amb)-his10 plasmids were washed, suspended in saline, and serially diluted with saline (to about 109 cells/mL). 2.5 μL each of the diluted cells were spotted on L-0.4% Glucose agar plate (a positive control without EM; glucose was included to minimize the expression of the BepA(amber) mutants) or L-agar plates supplemented with 20 μg/mL EM with or without 0.5 mM pBPA. Plates were incubated at 30°C for 22 hr. Growth of the cells in the presence of EM, IPTG, and pBPA indicates the expressed BepA(pBPA) is functional. (B) Self-cleavage of the BepApBPA derivatives. Cells of the same strains in (A) were grown at 30°C in L-medium supplemented with 0.02% arabinose (included to maximize the expression of evolved tRNA/aminoacyl tRNA synthetase for incorporation of pBPA at an amber site in BepA) and 0.5 mM pBPA until early log phase and induced with 1 mM IPTG (for the induction of the BepA(amber) mutants) for 1 hr. Total cellular proteins were acid-precipitated and analyzed by 7.5% Laemmli SDS‐PAGE and immunoblotting with an anti-BepA antibody. The result shown is a representative of two technical replicates. See Figure 1—figure supplement 3—source data 1 for plate and gel images for (A, B).
-
Figure 1—figure supplement 3—source data 1
A Zip file containing plate images (A) and gel images (B) for the immunoblotting experiments using the anti-BepA antibody.
- https://cdn.elifesciences.org/articles/70541/elife-70541-fig1-figsupp3-data1-v2.zip
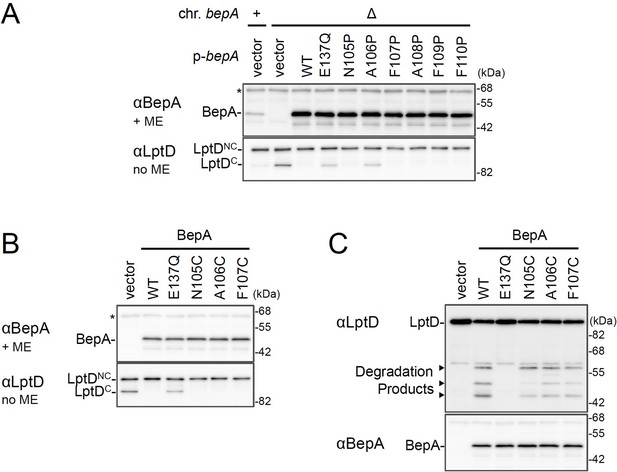
The chaperone-like and proteolytic activities of the BepA edge-strand mutants.
(A, B) The accumulation of the BepA mutants and LptDC in the ΔbepA cells expressing the BepA mutants. Cells of AD16 (bepA+) carrying pSTD689 (vector) or SN56 (ΔbepA) carrying either pSTD689 or pSTD689‐bepA plasmids were grown at 30°C in L-medium until early log phase and induced with 1 mM IPTG for 1 hr. Total cellular proteins were acid-precipitated, and analyzed by 10 or 7.5% Laemmli SDS-PAGE under a reducing (+ME) or non-reducing (no ME) condition and immunoblotting with anti-BepA (upper panels) or anti-LptD (lower panel) antibodies. (C) Protease activities of the BepA edge-strand Cys mutants against overproduced LptD. Cells of SN56 (ΔbepA) carrying pTWV228-lptD-his10 and either pSTD689 or pSTD689‐bepA plasmids were grown at 30°C in L-medium until early log phase and induced with 1 mM IPTG for 1 hr. Total cellular proteins were analyzed as in Figure 1B. The result shown is a representative of two technical replicates. See Figure 1—figure supplement 4—source data 1 for gel images for (A–C).
-
Figure 1—figure supplement 4—source data 1
A Zip file containing gel images (A, B) for the immunoblotting experiments using the anti-BepA and anti-BamA antibodies.
- https://cdn.elifesciences.org/articles/70541/elife-70541-fig1-figsupp4-data1-v2.zip
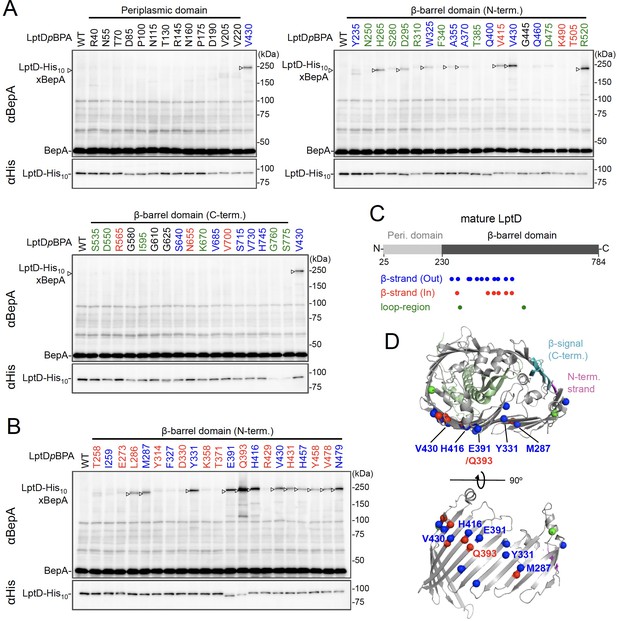
Photo-crosslinking of the β-barrel forming domain of LptD with BepA.
(A, B) In vivo photo-crosslinking between LptD and BepA. Cells of RM2243 (bepA(E137Q)) carrying pEVOL-pBpF, pMW118‐bepA(E137Q), and pRM294-lptD(amb)-his10 plasmids were grown at 30°C in L-medium containing 0.02% and 0.5 mM pBPA until early log phase and induced with 1 mM IPTG for 3 hr to express the indicated LptD(pBPA) variants. The cultures were then divided into two portions, each of which was UV-irradiated for 10 min at 4°C. Total cellular proteins were acid-precipitated and analyzed by 7.5% Laemmli SDS‐PAGE and immunoblotting with the indicated antibodies. Most of the LptD mutants were accumulated in comparable amounts. LptD-His10xBepA crosslinked products were not detectable with an anti-His antibody due to its low reactivity to LptD-His10 in this and the following experiments. Amino acid residues shown in red and blue indicate the ones whose side chain is pointing inward and outward, respectively. Amino acid residues shown in green indicate the ones located in the loop regions. The result shown is a representative of two technical replicates. (C) Summary of the BepA crosslinked positions in LptD. Positions where the crosslinking with BepA was clearly and reproducibly detected are indicated by colored dots. (D) Mapping of the BepA crosslinked positions on the barrel domain of LptD in the Escherichia coli LptD–LptE structure (PDB code: 4RHB). LptD and LptE are shown in gray and light green, respectively. The N-terminal strand and the β-signal (C-terminal region) in the LptD β-barrel domain are shown in magenta and light blue, respectively. The top view of the LptD/E structure from extracellular space (upper) and the side view of the N-terminal region of LptD β-domain (lower) are shown. The positions where the crosslinking with BepA was observed were indicated by spheres colored as above. See Figure 2—source data 1 for gel images for (A, B).
-
Figure 2—source data 1
A Zip file containing gel images (A, B) for the immunoblotting experiments using the anti-BepA and anti-His tag antibodies.
- https://cdn.elifesciences.org/articles/70541/elife-70541-fig2-data1-v2.zip
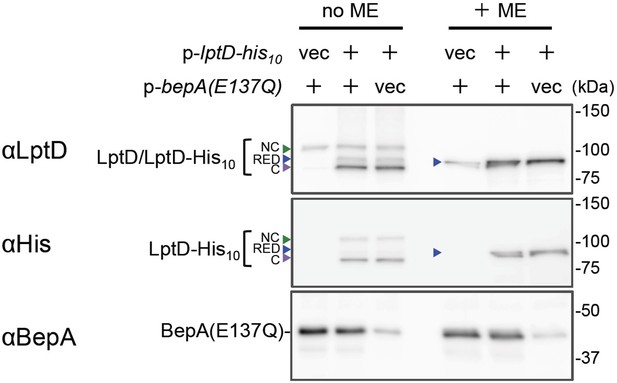
Overexpressed LptD molecules mainly accumulate as LptDC.
Cells of RM2243(bepA(E137Q))/pEVOL-pBpF carrying either pMW118 or pMW118-bepA(E137Q) and either pRM294 or pRM294-lptD-his10 were grown in L-medium containing 0.02% arabinose and 0.5 mM pBPA until early log phase at 30°C and induced with 1 mM IPTG for 3 hr. Total cellular proteins were acid-precipitated and analyzed by 7.5 or 10% Laemmli SDS-PAGE under a reducing (+ME) or non-reducing (no ME) condition and immunoblotting with the indicated antibodies. The result shown is a representative of two biological replicates. See Figure 2—figure supplement 1—source data 1 for gel images.
-
Figure 2—figure supplement 1—source data 1
A Zip file containing gel images for the immunoblotting experiments using the anti-LptD, anti-BepA, and anti-BamA antibodies.
- https://cdn.elifesciences.org/articles/70541/elife-70541-fig2-figsupp1-data1-v2.zip
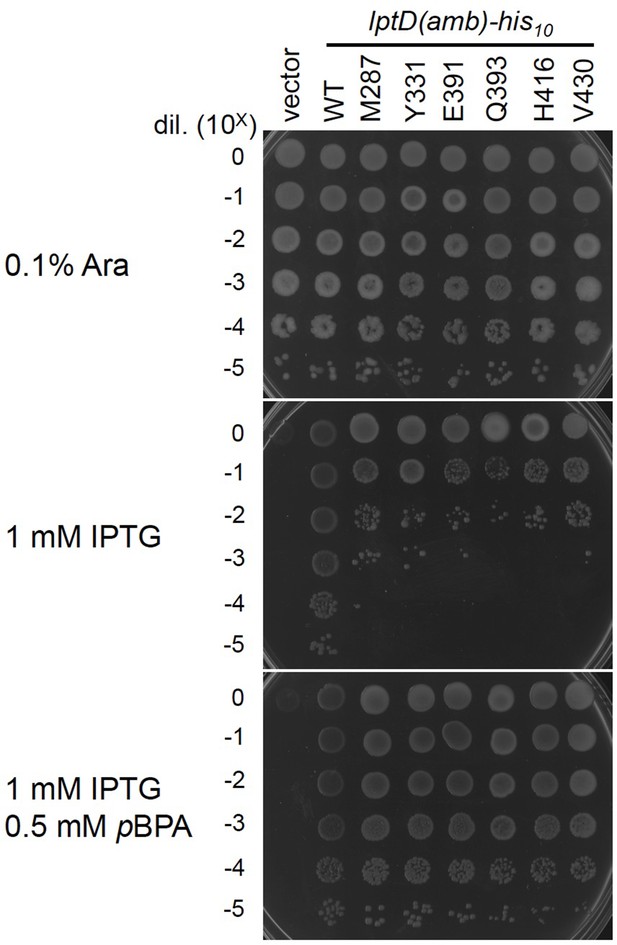
Complementation activity of the LptD(pBPA) derivatives.
Cells of RM3588 (Para-lptD) carrying pEVOL-pBpF and either pRM294 or pRM294-lptD(amb)-his10 plasmids were grown at 30°C in L-medium supplemented with 0.1% arabinose for 2.5 hr. The expression of the chromosomally located Para-lptD(WT) is induced by the addition of arabinose. RM3588 (Para-lptD) cells (without pEVOL-pBpF and an lptD(amb) plasmid) cannot grow in the absence of arabinose as the growth-essential LptD protein is depleted in an arabinose-free condition. Cells were washed, suspended in saline, and serially diluted with saline (to about 109 cells/mL). 2.5 μL each of the diluted cells were spotted on an L-agar plate containing 0.1% arabinose (positive control) or L-medium-based agar plates supplemented with 1 mM IPTG (for the expression of the LptD(amber) mutants from a plasmid) with or without 0.5 mM pBPA. Plates were incubated at 30°C for 22 hr. Growth of the cells in the absence of arabinose and in the presence of IPTG and pBPA indicates the plasmid-expressed LptD(pBPA) is functional. The result shown is a representative of two technical replicates. See Figure 2—figure supplement 2—source data 1 for plate images.
-
Figure 2—figure supplement 2—source data 1
A Zip file containing plate images for the complementation assays for the LptD(pBPA) derivatives.
- https://cdn.elifesciences.org/articles/70541/elife-70541-fig2-figsupp2-data1-v2.zip
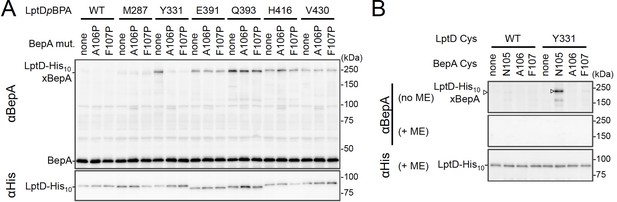
BepA edge-strand directly contacts with the Tyr-331 residue in the N-terminal half region of the LptD β-barrel forming domain.
(A) Effect of the BepA edge-strand mutations on the crosslinking between BepA and the LptD derivatives having pBPA in the N-terminal half region of the LptD β-barrel-forming domain. Cells of SN56 (ΔbepA) carrying pEVOL-pBpF, pMW118‐bepA(E137Q, mut), and pRM294-lptD(amb)-his10 were grown, induced to express a BepA and a LptDpBPA derivative, and subjected to photo-crosslinking analysis as described in Figure 2. (B) Disulfide crosslinking between the Cys residues in the edge-strand of BepA and the N-terminal half region of the LptD β-barrel-forming domain. Cells of SN56 (ΔbepA) carrying a combination of plasmids encoding WT or a Cys-introduced mutant of BepA and LptD-His10 as indicated were grown in L-medium and induced with 1 mM IPTG for 3 hr to express BepA(Cys) and LptD(Cys)-His10. Total cellular proteins were acid-precipitated, solubilized with SDS buffer containing NEM (for blocking free thiol groups), and subjected to pull-down with Ni-NTA agarose. The purified proteins were treated with or without 2-mercaptoethanol (ME) and analyzed by 7.5% Laemmli SDS-PAGE and immunoblotting with the indicated antibodies. The result shown is a representative of two technical replicates. See Figure 3—source data 1 for gel images for (A, B).
-
Figure 3—source data 1
A Zip file containing gel images (A, B) for the immunoblotting experiments using the anti-BepA and anti-His tag antibodies.
- https://cdn.elifesciences.org/articles/70541/elife-70541-fig3-data1-v2.zip
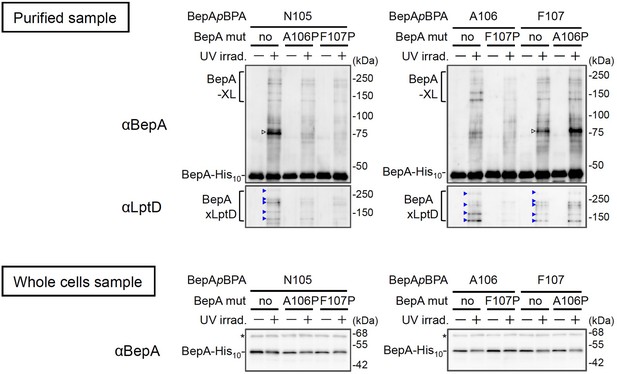
Effects of the BepA edge-strand mutations on the crosslinking of the BepA edge-strand to LptD.
Cells SN56 (ΔbepA) carrying pEVOL-pBpF and pUC18‐bepA(E137Q, mut., amb)-his10 plasmids were grown in L-medium containing 0.02% arabinose and 0.5 mM pBPA until early log phase at 30°C and induced with 1 mM IPTG for 1 hr to express the indicated BepA(pBPA) variants. The cultures were divided into two portions, each of which was treated with or without UV-irradiation for 10 min at 4°C. Proteins of the total membrane fractions were subjected to pull-down with Ni-NTA agarose. Purified proteins were analyzed by 7.5% Laemmli SDS‐PAGE and immunoblotting with the indicated antibodies. For analysis of the cellular accumulation of each BepA derivative, total cellular proteins of cells of the same cultures were acid-precipitated, and analyzed by 10% Laemmli SDS‐PAGE and immunoblotting with the indicated antibodies. Open triangles indicate unknown crosslinked products. The result shown is a representative of two technical replicates. See Figure 3—figure supplement 1—source data 1 for gel images.
-
Figure 3—figure supplement 1—source data 1
A Zip file containing gel images for the immunoblotting experiments using the anti-LptD, anti-BepA, and anti-BamA antibodies.
- https://cdn.elifesciences.org/articles/70541/elife-70541-fig3-figsupp1-data1-v2.zip
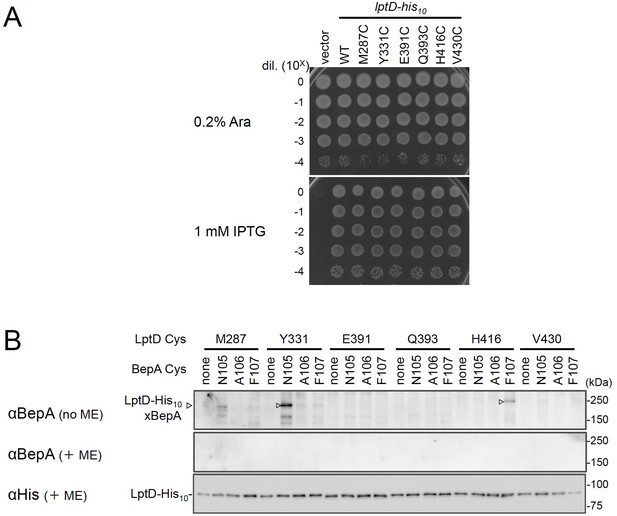
Disulfide crosslinking between BepA and LptD.
(A) Complementation activity of the LptD derivatives with an engineered Cys residue. Cells of RM3588 (Para-lptD) carrying pRM294 or pRM294-lptD(Cys)-his10 plasmids were grown at 30°C in L-medium supplemented with 0.1% arabinose for 2.5 hr. Cells were washed, suspended in saline, and serially diluted with saline (to about 109 cells/mL). 2.5 μL each of the diluted cells were spotted on an L-agar plate containing 0.1% arabinose (positive control) or 1 mM IPTG (for the induction of LptD(Cys) from a plasmid). Plates were incubated at 30°C for 22 hr. (B) BepA–LptD disulfide crosslinking. Cells of SN56 (ΔbepA) carrying a combination of plasmids encoding WT or a Cys-mutant form of BepA and LptD-His10 as indicated were grown in L-medium and induced with 1 mM IPTG for 3 hr to express BepA(Cys) and LptD(Cys)-His10. Total cellular proteins were acid-precipitated, solubilized with SDS buffer containing NEM and subjected to pull-down with Ni-NTA agarose. The purified proteins were treated with or without 2-mercaptoethanol (ME) and analyzed by 7.5% Laemmli SDS-PAGE and immunoblotting with the indicated antibodies. The result shown is a representative of two technical replicates. See Figure 3—figure supplement 2—source data 1 for plate gel images for (A, B).
-
Figure 3—figure supplement 2—source data 1
A Zip file containing plate images (A) for the complementation assays for the LptD(Cys) derivatives and gel images (B) for the immunoblotting experiments using the anti-BepA and anti-His antibodies.
- https://cdn.elifesciences.org/articles/70541/elife-70541-fig3-figsupp2-data1-v2.zip
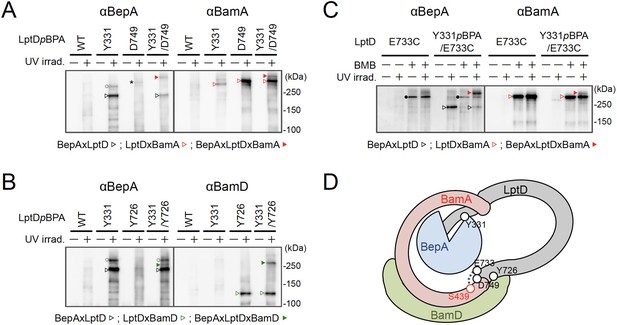
BepA interacts with an LptD intermediate assembling on the BAM complex.
(A, B) In vivo photo-crosslinking of an LptD mutant having pBPA at two positions with BepA. Cells of RM2243 (bepA(E137Q)) carrying pEVOL-pBpF, pMW118‐bepA(E137Q) and pRM294-lptD(amb)-his10 plasmids were grown at 30°C in L-medium containing 0.5 mM pBPA until early log phase and induced with 1 mM IPTG for 3 hr to express the indicated LptD(pBPA) variants. The cultures were divided into two portions, each of which was treated with or without UV-irradiation for 30 min at 4°C. Proteins of the total membrane fractions were subjected to pull-down with Ni-NTA agarose. Purified proteins were analyzed by 7.5% Laemmli SDS‐PAGE by immunoblotting with the indicated antibodies. Asterisk in the anti-BepA blots possibly indicates an LptD-BamA crosslinked product that was detected due to the apparent cross-reactivity of the anti-BepA antibody with the LptDxBamA crosslinked product (see below). (C) Simultaneous crosslinking of LptD having Y331pBPA and E733C with the BepA edge-strand and the seam region of BamA(S439C). Cells of RM3655 (bamA(S439C), ΔbepA)/pEVOL-pBpF/pMW118‐bepA(E137Q) carrying pRM294-lptD(E733C)-his10, or pRM294-lptD(Y331amb, E733C)-his10 were grown and induced as in (A). After treatment with or without BMB and the following quenching of BMB by addition of excess cysteine, the cultures were divided into two portions, each of which was treated with or without UV-irradiation for 30 min at 4°C. Total cellular proteins were acid-precipitated, solubilized with SDS buffer containing NEM, and subjected to pull-down with Ni-NTA agarose. Purified proteins were analyzed by 7.5% Laemmli SDS‐PAGE and immunoblotting with the indicated antibodies. The anti-BamA immunoblotting showed that the amount of the BepAxLptDxBamA crosslinked product was much lower than that of the LptDxBamA crosslinked product. Although the anti-BepA antibodies apparently cross-reacted weakly with the LptDxBamA crosslinked products (closed circles), the higher signal intensity of the BepAxLptDxBamA crosslinked product band as compared with the intensity of the LptDxBamA band (closed circles) indicate that the detection of the former band with the anti-BepA antibodies cannot be ascribed to this cross-reactivity. The identities of the bands marked by open circles in (A, B) are unclear; they might represent BepA-LptD crosslinked products or BepA-BamA crosslinked products (detected due to the cross-reactivity of anti-BepA antibodies with LptDxBamA crosslinked products as described above). In (A–C), we confirmed that the amounts of the isolated non-crosslinked LptD-His10 derivatives were roughly equal by CBB staining or anti-His immunoblotting (Figure 4—figure supplement 3). The result shown is a representative of two technical replicates. (D) A schematic cartoon of the interaction of the LptD assembly intermediate with BepA and BamA/D on the BAM complex. See Figure 4—source data 1 for gel images for (A–C).
-
Figure 4—source data 1
A Zip file containing gel images (A–C) for the immunoblotting experiments using the anti-BepA, anti-BamA, and anti-BamD antibodies.
- https://cdn.elifesciences.org/articles/70541/elife-70541-fig4-data1-v2.zip
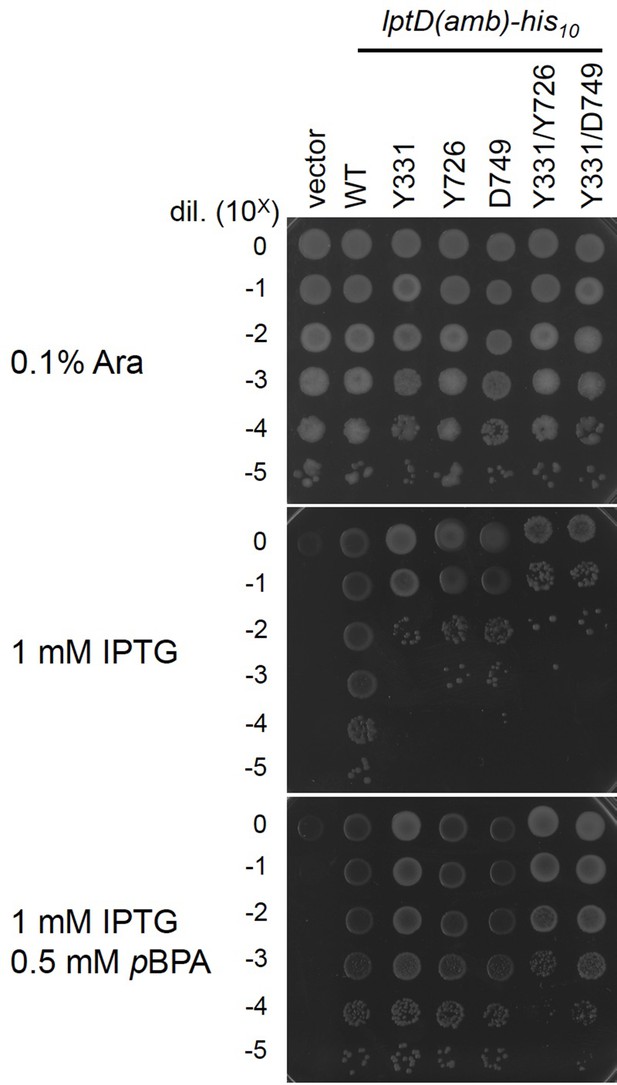
Complementation activity of the LptD derivatives having pBPA at one or two positions.
Cells of RM3588 (Para-lptD) carrying pEVOL-pBpF and either pRM294 or pRM294-lptD(amb)-his10 plasmids were grown and analyzed as in Figure 2—figure supplement 2. The result shown is a representative of two technical replicates. See Figure 4—figure supplement 1—source data 1 for plate images.
-
Figure 4—figure supplement 1—source data 1
A Zip file containing plate images for the complementation assays for the LptD(pBPA) derivatives.
- https://cdn.elifesciences.org/articles/70541/elife-70541-fig4-figsupp1-data1-v2.zip
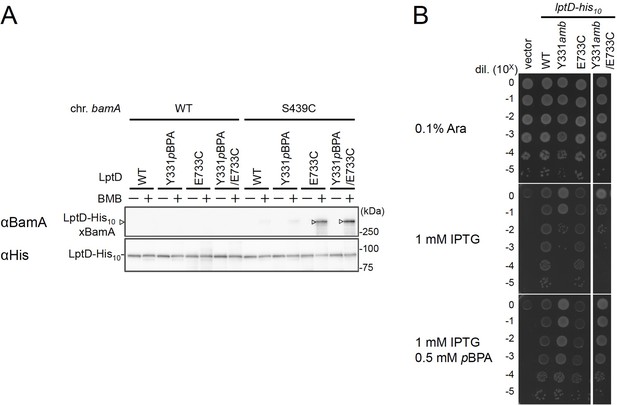
BMB crosslinking between LptD(Y331pBPA/E733C) and BamA(S439C).
(A) BMB crosslinking. Cells of RM3654 (bamA+, ΔbepA)/pEVOL-pBpF/pMW118‐bepA(E137Q) or RM3655 (bamA(S439C), ΔbepA)/pEVOL-pBpF/pMW118‐bepA(E137Q) carrying each pRM294-lptD-his10 derivative were grown at 30°C in L-medium containing 0.5 mM pBPA until early log phase and induced with 1 mM IPTG for 3 hr to express the indicated LptD variants. Cell cultures were treated with TCEP to reduce possible artificially formed disulfide bonds for E733C in LptD and S439C in BamA. The cultures were divided into two portions, each of which was treated with or without BMB. After quenching of BMB by addition of excess cysteine, total cellular proteins were acid-precipitated, solubilized with SDS buffer containing NEM, and subjected to pull-down with Ni-NTA agarose. The purified proteins were analyzed by 7.5% Laemmli SDS-PAGE and immunoblotting with the indicated antibodies. (B) Complementation activity of LptD derivatives. Cells of RM3588 (Para-lptD) carrying pEVOL-pBpF and pRM294 or each pRM294-lptD-his10 derivative were grown are analyzed as in (A, B). The result shown is a representative of two technical replicates. See Figure 4—figure supplement 2—source data 1 for gel and plate images for (A, B).
-
Figure 4—figure supplement 2—source data 1
A Zip file containing gel images (A) for the immunoblotting experiments using the anti-BamA and anti-His antibodies and plate images (B) for the complementation assays for the LptD(pBPA/Cys) derivatives.
- https://cdn.elifesciences.org/articles/70541/elife-70541-fig4-figsupp2-data1-v2.zip
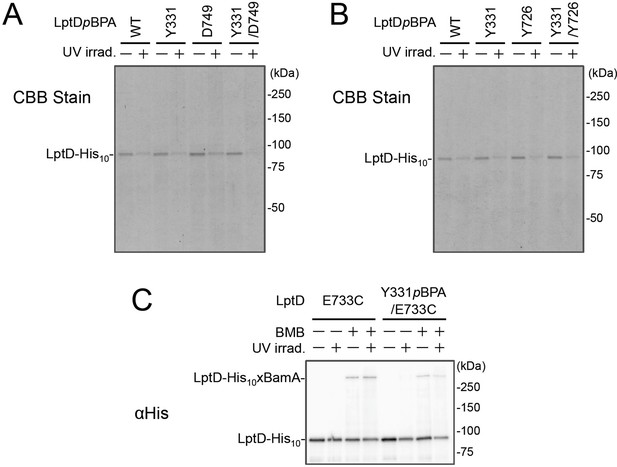
The amount of affinity purified LptD-His10 derivatives.
Affinity isolated proteins in Figure 4A–C were separated by 7.5% Laemmli SDS-PAGE and subjected to CBB staining (A and B for Figure 4A and B, respectively) or anti-His immunoblotting (C for Figure 4C). The result shown is a representative of two technical replicates. See Figure 4—figure supplement 3—source data 1 for gel images.
-
Figure 4—figure supplement 3—source data 1
A Zip file containing stained gel images.
- https://cdn.elifesciences.org/articles/70541/elife-70541-fig4-figsupp3-data1-v2.zip
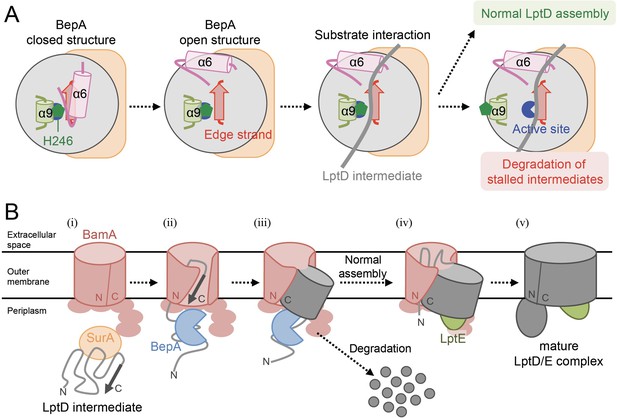
Model for the substrate recognition and discrimination by BepA.
(A) A schematic cartoon of the substrate recognition by BepA at its active site. See the text for details. (B) An overview of the proposed LptD assembly process and BepA-mediated discrimination of the assembling and stalled LptD species. See the text for details. Association of BepA with the assembly intermediate form of LptD on the BAM complex could transiently stabilize the LptD assembly intermediate and facilitate the association of LptE with LptD.
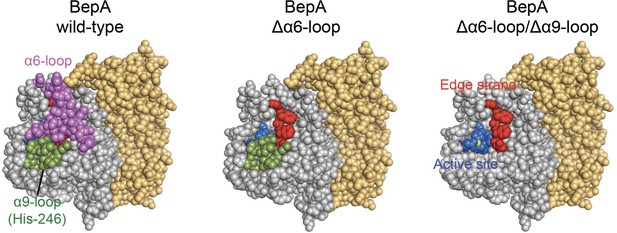
α6- and α9-loop regions shield the intramolecular active site and edge-strand of BepA.
The space-filling models of wild type BepA (left), BepA without the α6-loop (middle), and BepA without the α6- and α9-loops (right). The peptidase and the TPR domains of the BepA crystal structure (PDB code: 6AIT) are shown in gray and orange, respectively. The α6-loop, the α9-loop, the proteolytic active site (the HExxH motif and the third zinc ligand, Glu-201), and the edge-strand in the peptidase domain are shown in magenta, green, blue and red, respectively. The result shown is a representative of two technical replicates.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| strain, strain background (Escherichia coli) | E. coli strains | This study | N/A | Supplementary file 1 |
| strain, strain background (P1 bacteriophage) | P1vir | Laboratory stock | CGSC12133 | |
| recombinant DNA reagent | Plasmids | This study | N/A | Supplementary file 2 |
| sequence-based reagent | PCR primers | This study | N/A | described in the below |
| antibody | Penta-His HRP conjugate(mouse monoclonal) | QIAGEN | 34460 | (1:2000 or 1:3000 dilution) |
| antibody | Anti-BepA (rabbit polyclonal) | Narita et al., 2013 | N/A | (1:10000 dilution) |
| antibody | Anti-LptD (rabbit polyclonal) | Narita et al., 2013 | N/A | (1:50000 dilution) |
| antibody | Anti-BamA (rabbit polyclonal) | Gunasinghe et al., 2018 | N/A | (1:20000 dilution) |
| antibody | Anti-BamD (rabbit polyclonal) | Gunasinghe et al., 2018 | N/A | (1:10000 dilution) |
| antibody | Goat Anti-Rabbit IgG (H + L)-HRP Conjugate | Bio-Rad Laboratories | 1706515 RRID:AB_2617112 | (1:5000) |
| chemical compound, drug | H-p-Bz-Phe-OH | Bachem | F2800 | |
| chemical compound, drug | Methionine, L-[35S] Translation Grade | American Radiolabeled Chemicals | ARS 01014 | |
| chemical compound, drug | nProtein A Sepharose 4 Fast Flow | GE Healthcare | 17528004 | |
| chemical compound, drug | Ni-NTA Agarose | QIAGEN | 30250 | |
| commercial assay or kit | ECL Western Blotting Detection Reagents | GE Healthcare | RPN2106 | |
| commercial assay or kit | ECL Prime Western Blotting Detection Reagents | GE Healthcare | RPN2232 | |
| software, algorithm | Microsoft Excel | Microsoft | RRID:SCR_016137 | |
| software, algorithm | Bio-imaging Analyzer BAS-1800, BAS-5000 | Fujifilm/GE Healthcare | N/A | |
| software, algorithm | Image Qaunt LAS 4000 mini | Fujifilm/GE Healthcare | N/A | |
| software, algorithm | Multi Gauge | Fujifilm/GE Healthcare | RRID:SCR_014299 |
Additional files
-
Supplementary file 1
Strains used in this study.
- https://cdn.elifesciences.org/articles/70541/elife-70541-supp1-v2.docx
-
Supplementary file 2
Plasmids used in this study.
- https://cdn.elifesciences.org/articles/70541/elife-70541-supp2-v2.docx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/70541/elife-70541-transrepform-v2.docx





