Constitutive deficiency of the neurogenic hippocampal modulator AP2γ promotes anxiety-like behavior and cumulative memory deficits in mice from juvenile to adult periods
Figures
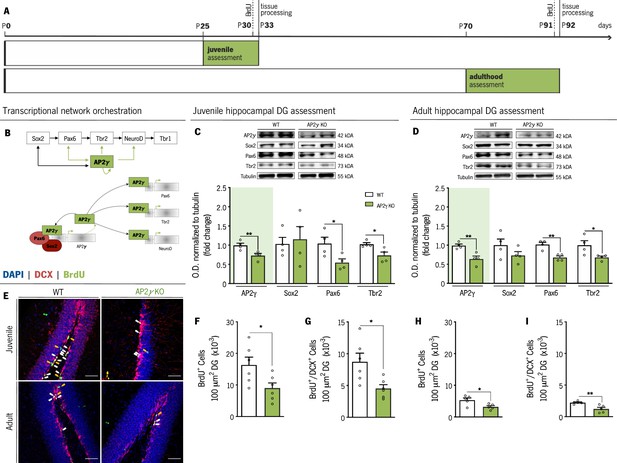
AP2γ constitutive and heterozygous deficiency reduces postnatal hippocampal neurogenesis both at juvenile and adult periods.
(A) Experimental timeline. (B) Transcriptional network of hippocampal neurogenesis under modulatory role of AP2γ. Western-blot analysis of AP2γ, Sox2, Pax6, and Tbr2 in juvenile (C) and adult (D) dentate gyrus (DG). (E) Hippocampal DG coronal sections stained for bromodeoxyuridine (BrdU) (green), doublecortin (DCX) (red), and DAPI (blue). BrdU+/DCX+ cells are indicated by white arrows and solely BrdU+ cell is identified with yellow arrows. (F–I) Cell counts of BrdU+ and BrdU+/DCX+ cells in the hippocampal DG of juvenile and adult mice. Data presented as mean ± SEM. Sample size: Western-blot analysis: nWT juvenile = 4; nAP2γ KO juvenile = 4; nWT adult = 4; nAP2γ KOadult = 4; Immunostainings: nWT juvenile = 6; nAP2γ KO juvenile = 6; nWT adult = 5; nAP2γ KOadult = 5 [Student’s t-test; ** p < 0.01; * p < 0.05; Statistical summary in Supplementary file 1]. Scale bars represent 50 μm. Abbreviations: WT, wild-type; AP2γ KO, AP2γ heterozygous knockout mice; O.D., optical density.
-
Figure 1—source data 1
Western-blot analysis at the juvenile and adult timepoints.
Western-blot analysis of AP2γ, Sox2, Pax6, and Tbr2 in juvenile (A) and adult (B) dentate gyrus (DG) protein extracts. (C and D) Representative Ponceau staining of membranes corresponding to the juvenile and adult timepoints. Surrounded by orange squares are demonstrated the quantified bands and denoted with a small orange star are identified the bands presented in the main Figure 1. Sample size: Western-blot analysis: nWT juvenile = 4; nAP2γ; KO juvenile = 4; nWT adult = 4; nAP2γ; KO adult = 4; Abbreviations: WT, wild-type; AP2γ KO, AP2γ heterozygous knockout mice.
- https://cdn.elifesciences.org/articles/70685/elife-70685-fig1-data1-v2.pdf
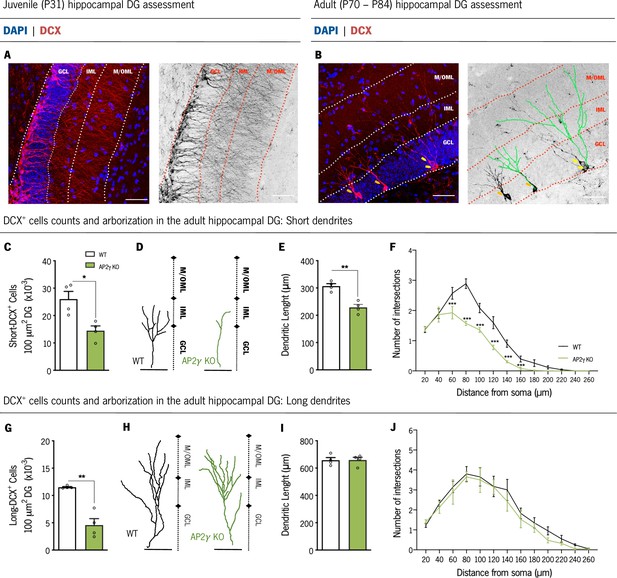
AP2γ constitutive and heterozygous deficiency reduces DCX+ cell population, and the dendritic length of short-DCX+ cells and its arborization complexity in adult mice.
(A and B) Dorsal hippocampal DG coronal sections stained for doublecortin (DCX) (red) and DAPI (blue) with the corresponding color conversion images. These representative images include the DG subregions, the granular cell layer (GCL), the inner and medial/outer molecular layers (IML and M/OML, respectively). Short DCX+ cells are indicated by orange arrows and long DCX+ cells by yellow arrows. (B) Dendritic tree of short (left) and long (right) DCX+ cells are traced in green. Cell counts of short (C) and long (G) DCX+ cells. Dendritic length of reconstructed short (D and E) and long DCX+ (H and I) cells, and respective sholl analysis (F and J). Data presented as mean ± SEM. Sample size: nWT adult = 4; nAP2γ KOadult = 4. [Student’s t-test and Repeated measures ANOVA; ***p < 0.001, ** p < 0.01, * p < 0.05; Statistical summary in Supplementary file 1]. Scale bars represent 50 μm. Abbreviations: WT, wild-type; AP2γ KO, AP2γ heterozygous knockout mice; P, Postnatal day.
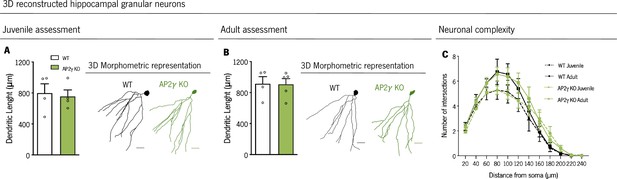
AP2γ constitutive and heterozygous deficiency does not impact on the morphology of hippocampal granular neurons, both at the juvenile and adult periods.
Dendritic length (A and B) and complexity (C) of three-dimensional (3D) neuronal reconstructed hippocampal granular neurons in juvenile (A) and adult (B) mice. Data presented as mean ± SEM. Sample size: 3D neuronal reconstruction: nWT juvenile = 4; nAP2γ KO juvenile = 4; nWT adult = 4; nAP2γ KO adult = 5. [Student’s t-test and Repeated measures ANOVA; Statistical summary in Supplementary file 1]. Scale bars represent 50 μm. Abbreviations: WT, wild-type; AP2γ KO, AP2γ heterozygous knockout mice.
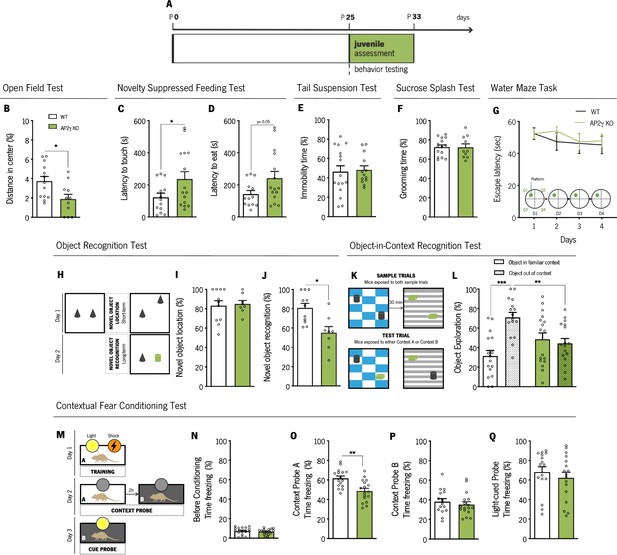
AP2γ constitutive and heterozygous deficiency increases anxiety-like behavior and promotes cognitive deficits in juvenile mice.
(A) Timeline of behavioral assessment. Anxiety-like behavior was assessed through the open-field (OF) (B) and the novelty suppressed feeding (NSF) (C and D) tests. Coping and anhedonic-like behavior by the tail-suspension (TST) (C) and the sucrose splash (SST) (D) tests. (E) To evaluate cognitive behavior, juvenile mice were subjected to the Morris water maze (MWM) (G), the object recognition (ORT) (H–J), the object-in-context recognition (OIC) (K and L) and the contextual fear conditioning (CFC) (M–Q). Data presented as mean ± SEM. Sample size: OF: nWT = 13; nAP2γ KO = 11; NSF: nWT = 13; nAP2γ KO = 15; TST: nWT = 16; nAP2γ KO = 13; SST: nWT = 15; nAP2γ KO = 10; MWM: nWT = 10; nAP2γ KO = 9; ORT: nWT = 11; nAP2γ KO = 8; OIC: nWT = 16; nAP2γ KO = 17; CFC: nWT = 16; nAP2γ KO = 17. [Student’s t-test, Repeated measures ANOVA and Two-way ANOVA; *** p < 0.001; ** p < 0.01; * p < 0.05; Statistical summary in Supplementary file 1]. Abbreviations: WT, wild-type; AP2γ KO, AP2γ heterozygous knockout mice.
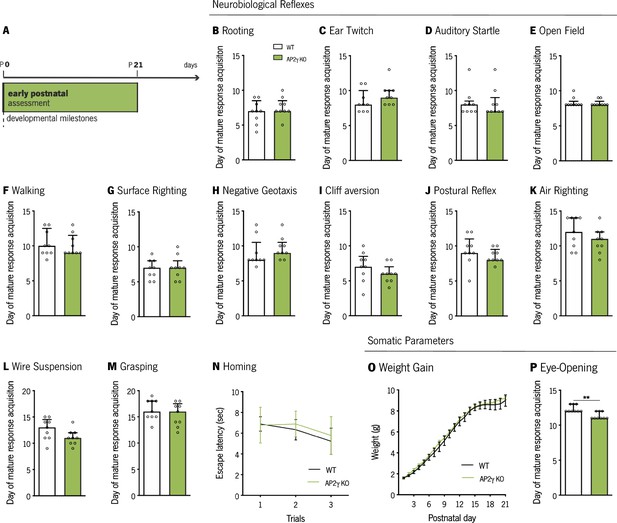
AP2γ constitutive and heterozygous deficiency does not impact on sensory-motor development.
(A) Timeline of early development assessment. (B–N) Set of established protocols to analyze the acquisition of mature responses, in neurobiological reflexes related to tactile (B and C) and auditory reflexes (D), motor function (E and F), vestibular system formation (G–K), strength (L and M), and olfactory maturation (N). Somatic parameters were also assessed namely: (O) bodyweight gain from postnatal day (P) one to P21 of WT and AP2γ KO mice, and (P) eye-opening day. For the weight gain pattern, data is presented as mean ± SEM; for the remaining tests, data was plotted as median ± Interquartile range. Sample Size: nWT = 9; nAP2γ KO = 9. [Mann-Whitney and Repeated measures ANOVA, ** p < 0.01, Statistical summary in Supplementary file 1]. Abbreviations: WT, wild-type; AP2γ KO, AP2γ heterozygous knockout mice.
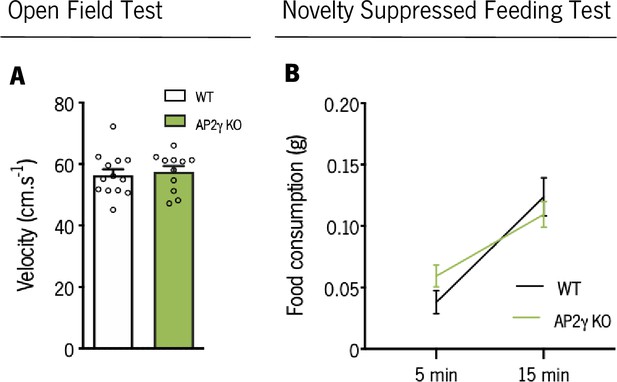
In juvenile mice, AP2γ constitutive and heterozygous deficiency does not impact on motor activity in the OF, nor on food consumption in the NSF test.
(A) Average velocity assessed through the open field (OF) test in juvenile mice. (B) Food consumption after novelty suppressed feeding test exposure. Data presented as mean ± SEM. Sample Size: OF: nWT = 13; nAP2γ KO = 11; NSF: nWT = 13; nAP2γ KO = 15 [Student’s t-test, Statistical summary in Supplementary file 1]. Abbreviations: WT, wild-type; AP2γ KO, AP2γ heterozygous knockout mice.
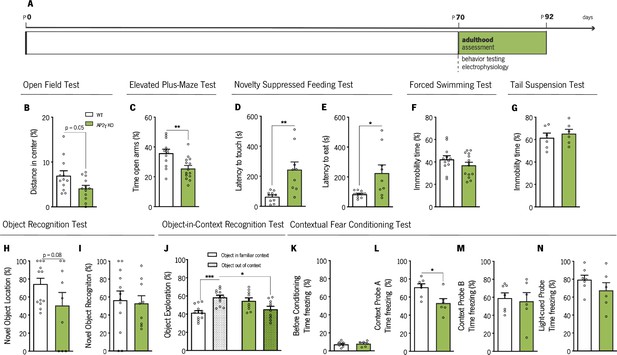
Behavioral assessment of adult mice.
(A) Experimental timeline. Anxiety-like behavior was assessed through open-field (OF) (B), elevated plus-maze (EPM) (C) and novelty suppressed feeding (NSF) (D and E) tests, while coping behavior was evaluated through forced-swimming test (FST) (G) and the tail-suspension test (TST) (G). Object recognition test (ORT) (H and I), object-in-context recognition (OIC) (J) and the contextual fear conditioning (CFC) (K–N) were performed to assess cognitive behavior. Data presented as mean ± SEM. Sample size: OF, EPM and FST: nWT = 12; nAP2γ KO = 14; NSF: nWT = 10; nAP2γ KO = 9;TST: nWT = 6; nAP2γ KO = 6; ORT: nWT = 12; nAP2γ KO = 9; OIC: nWT = 12; nAP2γ KO = 10; CFC: nWT = 7; nAP2γ KO = 6. [Student’s t-test and Two-way ANOVA; *** p < 0.001; ** p < 0.01; * p < 0.05; Statistical summary in Supplementary file 1]. Abbreviations: WT, wild-type; AP2γ KO, AP2γ heterozygous knockout mice.
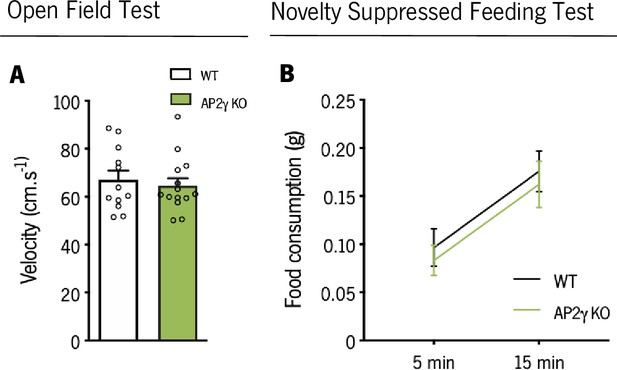
In adult mice, AP2γ constitutive and heterozygous deficiency does not impact on motor activity in the OF, nor on food consumption in NSF test.
(A) Average velocity assessed through the open field (OF) test in adult mice. (B) Food consumption after novelty suppressed feeding test exposure. Data presented as mean ± SEM. Sample size: OF: nWT = 12; nAP2γ KO = 14; NSF: nWT = 10; = 9; MWM: nWT = 10; nAP2γ KO = 10. [Student’s t-test and Repeated measures ANOVA, Statistical summary in Supplementary file 1]. Abbreviations: WT, wild-type; AP2γ KO, AP2γ heterozygous knockout mice.
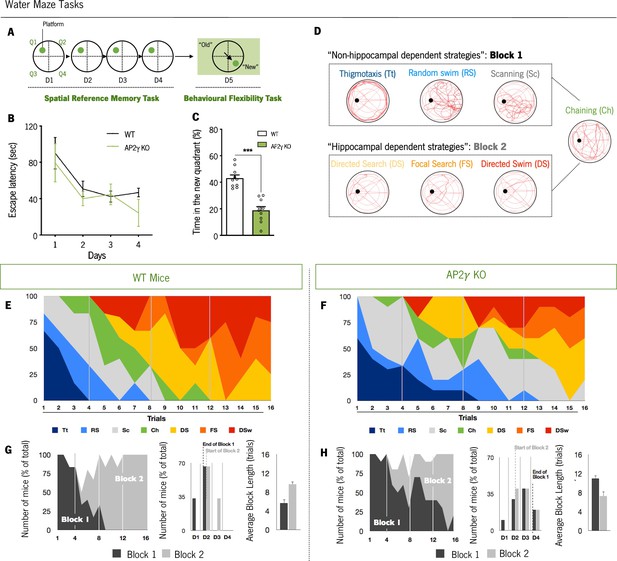
Cognitive performance of adult mice in the Morris water maze test.
(A and B) Spatial reference memory was assessed as the average escape latency to find a hidden and fixed platform in each test day. (C) In the last testing day, animals were subjected to a reversal-learning task to test behavioral flexibility. (D) Schematic representation of typical strategies to find the platform during spatial memory evaluation grouped according to its dependence of the hippocampus (Block 1: Non-hippocampal dependent strategies; Block 2: Hippocampal dependent strategies). Average of each strategy used for WT (E) and AP2γ KO (F) mice, by trial number. The prevalence of each block along with trials, the distribution of strategies-block boundaries, and overall block length are shown for (G) WT and (H) AP2γ KO mice. Data presented as mean ± SEM. nWT = 10; nAP2γ KO = 10. [Repeated measures ANOVA and Student’s t-test; ***p < 0.001; Statistical summary in Supplementary file 1]. Abbreviations: WT, wild-type; AP2γ KO, AP2γ heterozygous knockout mice.
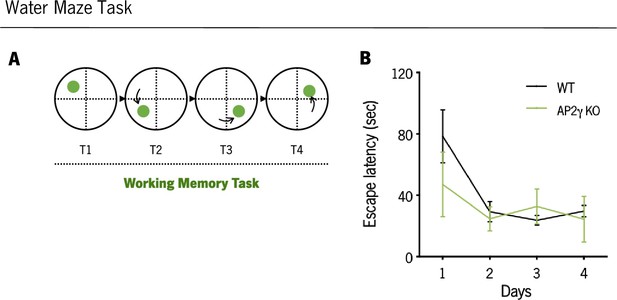
In adult mice, AP2γ constitutive and heterozygous deficiency does not impact the working memory acquisition.
(A and B) Working memory task evaluated in the Morris water maze (MWM) test. Data presented as mean ± SEM. Sample size: MWM: nWT = 10; nAP2γ KO = 10. [Repeated measures ANOVA, Statistical summary in Supplementary file 1]. Abbreviations: WT, wild-type; AP2γ KO, AP2γ heterozygous knockout mice.
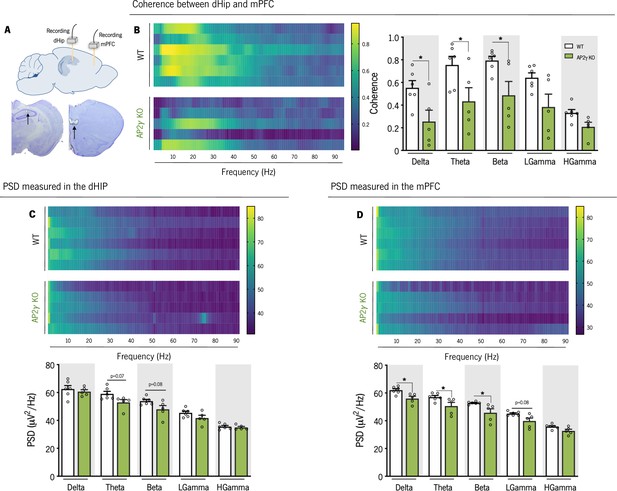
In adult mice, AP2γ constitutive and heterozygous deficiency induces deficits in spectral coherence between the dorsal hippocampus (dHip) and the medial prefrontal cortex (mPFC), impacting on neuronal activity.
(A) Identification of the local field potential (LFP) recording sites, with a depiction of the electrode positions (upper panel), and representative Cresyl violet-stained sections, with arrows indicating electrolytic lesions at the recording sites (lower panel). (B) Spectral coherence between the dHip and mPFC (left panel). Group comparison of the coherence values for each frequency (right panel). (C) Power spectral density (PSD) was measured in the dHip (C) and mPFC (D). Heatmaps of PSD activity (upper panel) and group comparison for each frequency (lower panel). Each horizontal line in the Y-axis of the presented spectrograms represents an individual mouse. Frequency bands range: delta (1–4 Hz), theta (4–12 Hz), beta (12–20 Hz), low gamma (20–40 Hz), and High gamma (40–90 Hz). Data presented as mean ± SEM. nWT = 6; nAP2γ KO = 5. [Repeated measures ANOVA; *p < 0.05; Statistical summary in Supplementary file 1]. Abbreviations: WT, wild-type; AP2γ KO, AP2γ heterozygous knockout mice.
-
Figure 6—source code 1
Local field potentials analysis between the dorsal hippocampus (dHip) and medial prefontal cortex (mPFC).
- https://cdn.elifesciences.org/articles/70685/elife-70685-fig6-code1-v2.zip
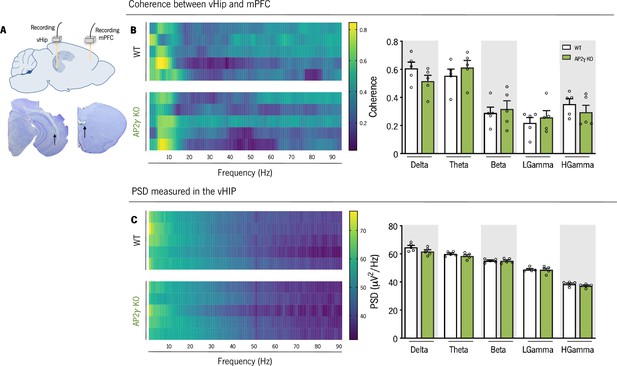
In adult mice, AP2γ constitutive and heterozygous deficiency does not impact on spectral coherence between the ventral hippocampus (vHip) and the medial prefrontal cortex (mPFC), nor the neuronal activity in each region.
(A) Local field potentials (LFP) recording sites, with a depiction of the electrode positions (upper panel), and representative Cresyl violet-stained section (lower panel). (B) Spectral coherence (left panel) and group comparison for each frequency (right panel), between the vHip and mPFC of adult WT and AP2γ KO mice. (C) Power spectral density (PSD) (upper panel), and group comparison of the PSD values in the vHip for each frequency (lower panel). In spectrograms, each horizontal line in the Y-axis represents an individual mouse. Frequency bands range: delta (1–4 Hz), theta (4–12 Hz), beta (12–20 Hz), low gamma (20–40 Hz), and High gamma (40–90 Hz). Data presented as mean ± SEM. nWT = 5; nAP2γ KO = 5. [Repeated measures ANOVA, Statistical summary in Supplementary file 1]. Abbreviations: WT, wild-type; AP2γ KO, AP2γ heterozygous knockout mice; vHip, ventral hippocampus; mPFC, medial prefrontal cortex.
-
Figure 6—figure supplement 1—source code 1
Local field potentials analysis between the ventral hippocampus (vHip) and medial prefontal cortex (mPFC).
- https://cdn.elifesciences.org/articles/70685/elife-70685-fig2-code2-v2.zip
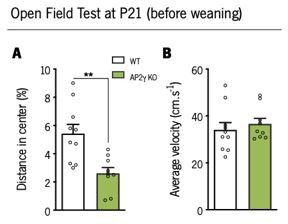
Before weaning, anxiety-like behavior was assessed through the open-field (OF) test.
AP2γ KO mice exhibited a decreased in distance traveled in center, when compared to WT , suggesting an anxious-like phenotype (A). No differences in locomotor activity was detected between groups as denoted by a similar average velocity during the test (B). Data presented as mean ± SEM. Sample Size: OF: nWT = 10; nAP2γ KO = 9. [Student’s t-test].

Cognitive performance of juvenile mice in the MWM test.
(A) Spatial reference memory was assessed as the average escape latency to find a hidden and fixed platform in each test day. Data presented as mean ± SEM. Sample size: MWM: nWT = 10; nAP2γKO = 9. [Repeated measures ANOVA].
Tables
Results from the milestones protocol tests included in the assessment of early postnatal neurodevelopment.
Sample size: nWT = 9; n AP2γ KO = 9. Abbreviations: WT, wild-type; AP2γ KO, AP2γ heterozygous mice; P, Postnatal day.
| Milestone test | WTDay (median) | AP2γ KODay (median) | Statistical test, significanceMann-Whitney test | Typical range | |
|---|---|---|---|---|---|
| Rooting | 7 | 7 | U = 31.50, p = 0.43 | P1 – P15 | |
| Ear twitch | 8 | 9 | U = 24.50, p = 0.16 | P6 – P14 | |
| Auditory startle | 8 | 7 | U = 37.50, p = 0.85 | P7 – P16 | |
| Open field | 8 | 8 | U = 39.50, p > 0.99 | P6 – P15 | |
| Walking | 10 | 9 | U = 38.00, p = 0.78 | P7 – P14 | |
| Surface righting | 7 | 7 | U = 38.50, p = 0.88 | P1 – P10 | |
| Negative geotaxis | 8 | 9 | U = 25.00, p = 0.17 | P3 – P15 | |
| Cliff aversion | 7 | 6 | U = 26.00; p = 0.22 | P1 – P14 | |
| Postural reflex | 9 | 8 | U = 26.00, p = 0.21 | P5 – P21 | |
| Air righting | 12 | 11 | U = 29.00, p = 0.32 | P7 – P16 | |
| Wire suspension | 13 | 11 | U = 21.50, p = 0.10 | P5 – P21 | |
| Grasping | 16 | 16 | U = 30.00, p = 0.37 | P13 - P17 | |
| Eye-opening | 12 | 11 | U = 9.00, p < 0.01 | P7 - P17 | |
| WT Mean ±SEM | AP2γ KO Mean ± SEM | Statistical test, significance Repeated measures ANOVA | |||
| Homing | Trial 1 | 6.89 s ± 0.44 | 6.89 s ± 1.32 | F (1,48) = 0.06, p = 0.80 | |
| Trial 2 | 6.33 s ± 1.12 | 6.89 s ± 1.12 | |||
| Trial 3 | 5.22 s ± 1.57 | 5.78 s ± 1.57 | |||
| Weight gain pattern | 8.99 g ± 0.53 | 9.22 g ± 0.16 | F (1,16) = 0.32, p = 0.58 | ||
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Genetic reagent (Mus musculus, male) | Tfap2c+/- | Dr. Hubert Schorle (Bonn University Medical School) | Male mice maintained in a 129/SV background | |
| Chemical compound, drug | BrdU, 50 mg/kg | Sigma-Aldrich | # 9,285 | Intraperitoneal injection |
| Antibody | alpha-tubulin (Mouse monoclonal) | Sigma | #5,168 | WB (1:5000) |
| Antibody | Anti-AP2γ (Goat polyclonal) | Abcam | #31,288 | WB (1:500) |
| Antibody | Anti-Pax6 (Rabbit polyclonal) | Millipore | #2,237RRID:AB_1587367 | WB (1:1000) |
| Antibody | Anti-Sox2 (Mouse monoclonal) | Abcam | #7,935 | WB (1:500) |
| Antibody | Anti-mouse (Goat monoclonal) | BioRad | #1706516RRID:AB_11125547 | WB (1:10000) |
| Antibody | Anti-rabbit (Goat monoclonal) | BioRad | #1706515RRID:AB_11125142 | WB (1:10000) |
| Antibody | Anti-goat (Donkey polyclonal) | Santa Cruz Biotechnologies | #sc-2020RRID:AB_631728 | WB (1:7500) |
| Commercial assay or kit | SuperSignal west Femto reagent | ThermoFisher | #34,096 | |
| Antibody | Anti-BrdU (Rat monoclonal) | Abcam | #6,326RRID:AB_305426 | IF (1:100) |
| Antibody | Anti-Doublecortin (Rabbit polyclonal) | Abcam | #18,723RRID:AB_732011 | IF (1:100) |
| Antibody | Anti-Doublecortin (Goat monoclonal) | Santa Cruz Biotechnologies | #sc-8066RRID:AB_2088494 | IF (1:500) |
| Antibody | Alexa Fluor 488 (Goat Anti-rat polyclonal) | Invitrogen | #32,731 | IF (1:1000) |
| Antibody | Alexa Fluor 568 (Goat Anti-rabbit polyclonal) | Invitrogen | #11,011RRID:AB_143157 | IF (1:1000) |
| Antibody | Alexa Fluor 594 (Donkey Anti-Goat polyclonal) | Invitrogen | #A32758RRID:AB_2534105 | IF (1:1000) |
| other | 4',6-diamidino-2-phenylindole (DAPI stain) | Sigma Aldrich | #8,417 | IF (1:200) |
| Commercial assay or kit | Anti-fade Fluorescence Mounting Medium | Abcam | #ab104135 | |
| Software, algorithm | Activity Monitor software | MedAssociates | ||
| Software, algorithm | Kinoscope software | Kokras et al., 2017 | ||
| Software, algorithm | Fiji software | Schindelin et al., 2012 | RRID: SCR_002285 | |
| Software, algorithm | Ethovision XT 11.5 | Noldus | RRID:SCR_000441 | |
| Software, algorithm | Signal Software | CED | ||
| Software, algorithm | Prism v.8 | GraphPad Software Inc | RRID:SCR_002798 | |
| Software, algorithm | MATLAB | MathWorks Inc | RRID:SCR_005547 |
Additional files
-
Supplementary file 1
Statistical summary of results.
- https://cdn.elifesciences.org/articles/70685/elife-70685-supp1-v2.docx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/70685/elife-70685-transrepform1-v2.pdf





