Proximal and distal spinal neurons innervating multiple synergist and antagonist motor pools
Figures
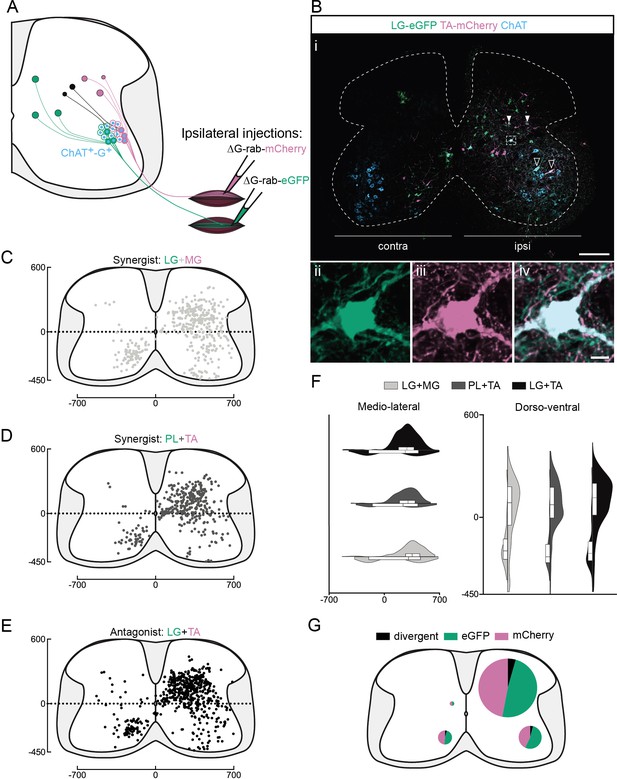
Organization of divergent premotor interneurons (INs) in the lumbar spinal cord.
(A) Experimental strategy to describe divergent premotor INs that project to two motor pools of synergist (injection in tibialis anterior [TA] and peroneus longus [PL] or lateral gastrocnemius [LG] and medial gastrocnemius [MG]) or antagonist (TA and LG) pair of muscles. (Bi) Representative example of a lumbar transverse section following an injection in the TA (ΔG-Rab-mCherry) and LG (ΔG-Rab-eGFP), showing ChAT (grey blue), GFP (Green Fluorescent Protein, green), and mCherry (pink). A divergent premotor IN is highlighted in the dashed box. Filled arrowheads show divergent premotor INs and contour arrowheads show infected motoneurons (MNs). The dashed line drawn outlines the grey matter contour. Higher magnification of a divergent premotor IN that has been infected by the ΔG-Rab-eGFP and ΔG-Rab-mCherry, showing (ii) eGFP, (iii) mCherry, and (iv) the overlay. More representative examples of lumbar sections following injections in LG and MG, TA and PL, and LG and TA are shown in Figure 1—figure supplements 1–3, respectively. Distribution of the lumbar divergent premotor INs following injections in (C) LG and MG (n = 2), (D) PL and TA (n = 2), and (E) LG and TA (n = 3). (F) Asymmetric violin plots showing the medio-lateral and dorso-ventral distributions of divergent premotor INs. The halves correspond, respectively, to the dorsal (top) and ventral (bottom) distributions and to the ipsilateral (right) and contralateral (left) distributions of divergent premotor INs in the lumbar cord. Violin areas were normalized on the number of divergent INs. (G) Distribution of the premotor INs within each quadrant of the lumbar cord, with pie sizes proportional to the percentage of premotor INs in each quadrant of the lumbar cord. Numbers along the axis indicate distances (in µm). Scale bars: (Bi) 200 µm; (Biv) 10 µm. Raw number of eGFP, mCherry, and double-labelled premotor neurons per samples per muscle pair injected, is shown in Figure 1—figure supplements 1–3.
-
Figure 1—source data 1
Source data for Figure 1C-G.
- https://cdn.elifesciences.org/articles/70858/elife-70858-fig1-data1-v2.xlsx
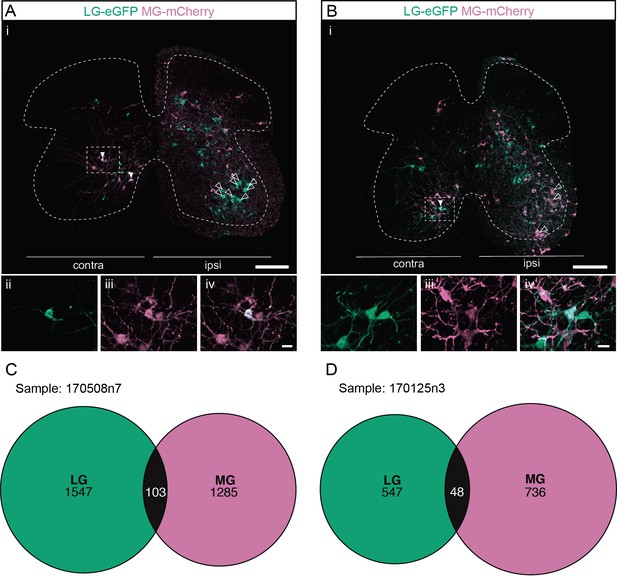
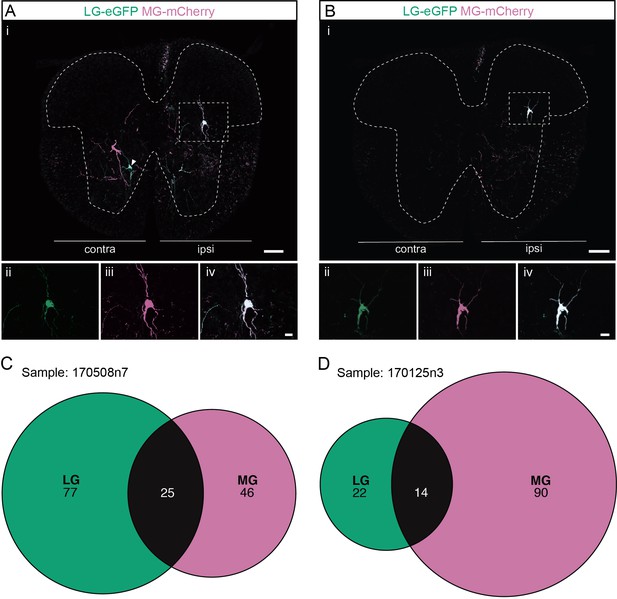
Divergent premotor interneurons (INs) in the lumbar spinal cord following injections in synergists lateral gastrocnemius (LG) and medial gastrocnemius (MG).
(A, B) Representative examples of lumbar transverse sections following injections in the LG (ΔG-Rab-eGFP) and MG (ΔG-Rab-mCherry) showing GFP (green) and mCherry (pink). A divergent premotor IN is highlighted in the dashed box. Filled arrowheads show divergent premotor INs and contour arrowheads show infected motoneurons (MNs). The dashed lines drawn outline the grey matter. Higher magnification of a divergent premotor IN that has been infected by the ΔG-Rab-eGFP and ΔG-Rab-mCherry, showing (ii) mCherry, (iii) eGFP, and (iv) the overlay. (C, D) Venn diagrams showing the distributions of lumbar infected premotor INs in the two samples injected in LG and MG. Scale bars: (Ai, Bi) 200 µm; (Aiv, Biv) 20 µm.
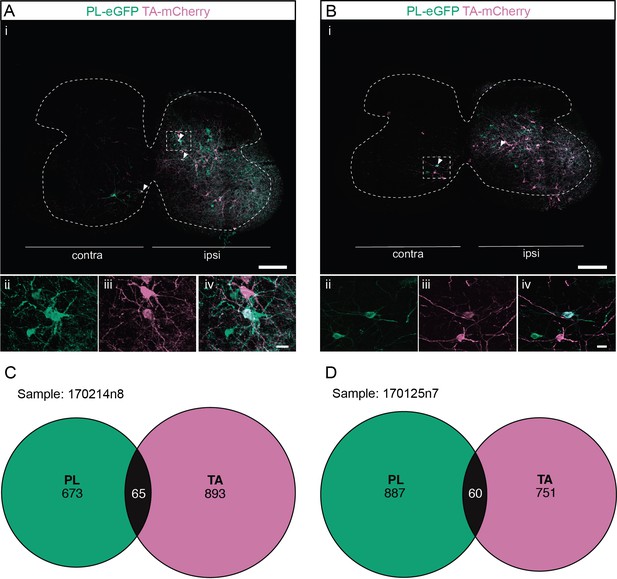
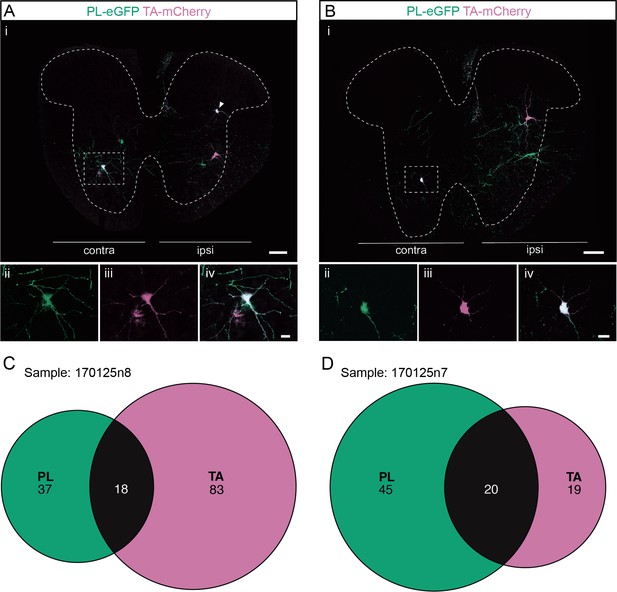
Divergent premotor interneurons (INs) in the lumbar spinal cord following injections in synergists peroneus longus (PL) and tibialis anterior (TA).
(A, B) Representative examples of lumbar transverse sections following injections in the PL (ΔG-Rab-eGFP) and TA (ΔG-Rab-mCherry) showing GFP (green) and mCherry (pink). A divergent premotor IN is highlighted in the dashed box. Filled arrowheads show divergent premotor INs. The dashed lines drawn outline the grey matter contour. Higher magnification of a divergent premotor IN that has been infected by the ΔG-Rab-eGFP and ΔG-Rab-mCherry, showing (ii) mCherry, (iii) eGFP, and (iv) the overlay. (C, D) Venn diagrams showing the distributions of lumbar infected premotor INs in the two samples injected in PL and TA. Scale bars: (Ai, Bi) 200 µm; (Aiv, Biv) 20 µm.
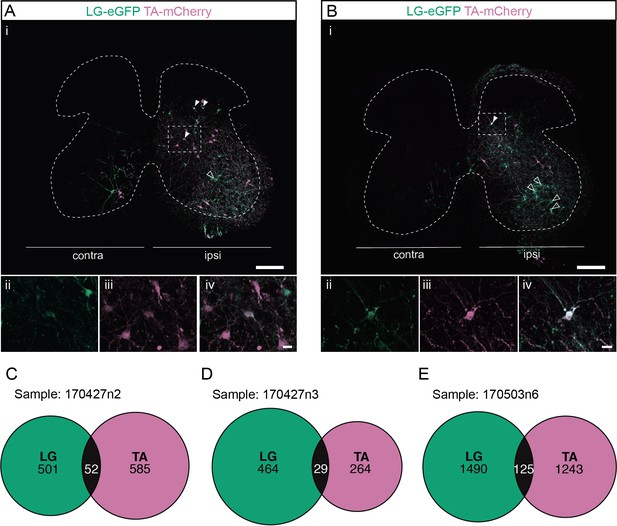
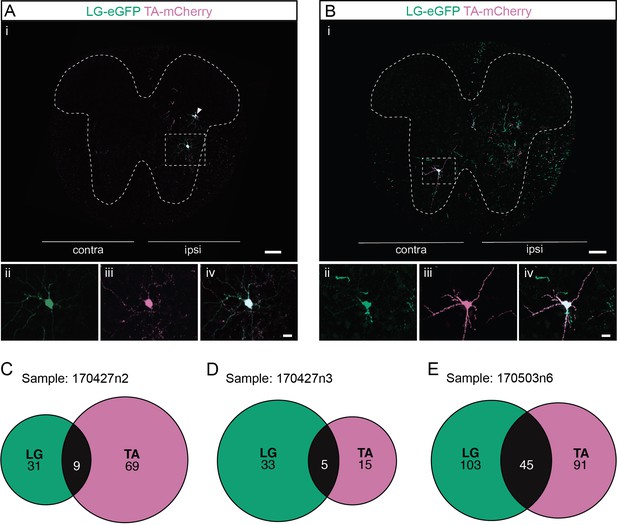
Divergent premotor interneurons (INs) in the lumbar spinal cord following injections in antagonists lateral gastrocnemius (LG) and tibialis anterior (TA).
(A, B) Representative examples of lumbar transverse sections following injections in the LG (ΔG-Rab-eGFP) and TA (ΔG-Rab-mCherry) showing GFP (green) and mCherry (pink). A divergent premotor IN is highlighted in the dashed box. Filled arrowheads show divergent premotor INs and contour arrowheads show infected motoneurons (MNs). The dashed lines drawn outline the grey matter. Higher magnification of a divergent premotor IN that has been infected by the ΔG-Rab-eGFP and ΔG-Rab-mCherry, showing (ii) mCherry, (iii) eGFP, and (iv) the overlay. (C–E) Venn diagrams showing the distributions of lumbar infected premotor INs in the three samples injected in LG and TA. Scale bars: (Ai, Bi) 200 µm; (Aiv, Biv) 20 µm.
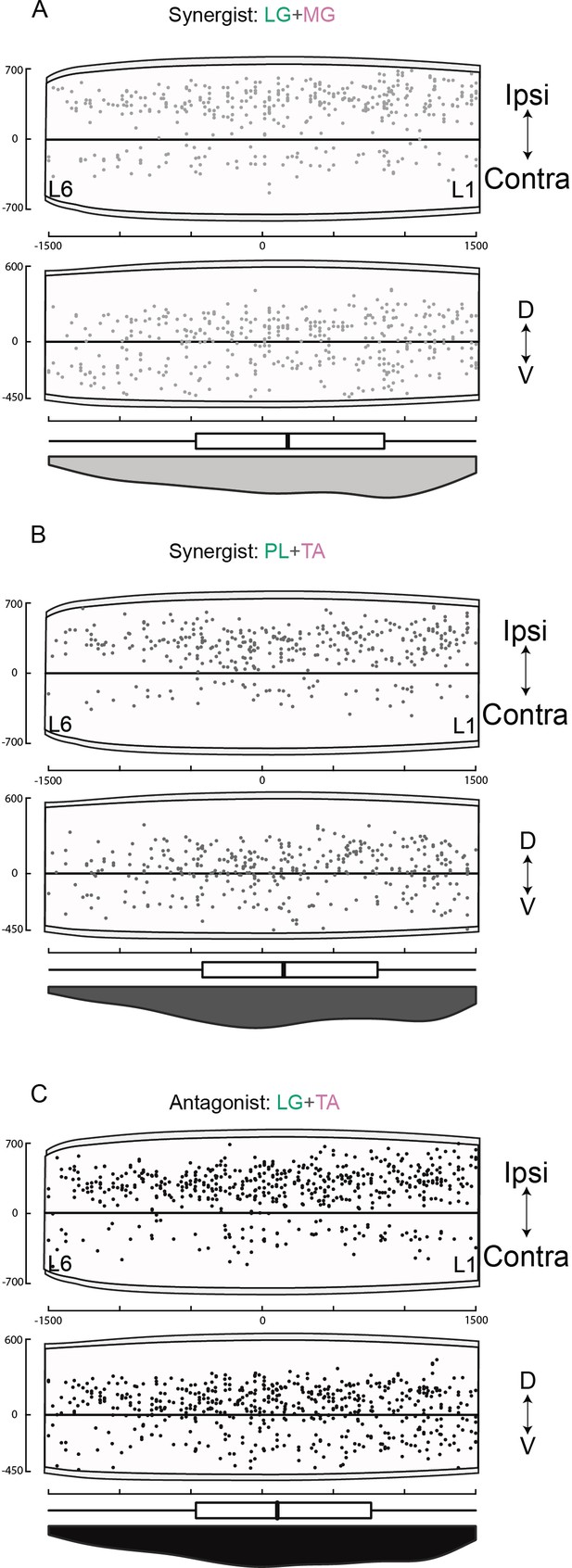
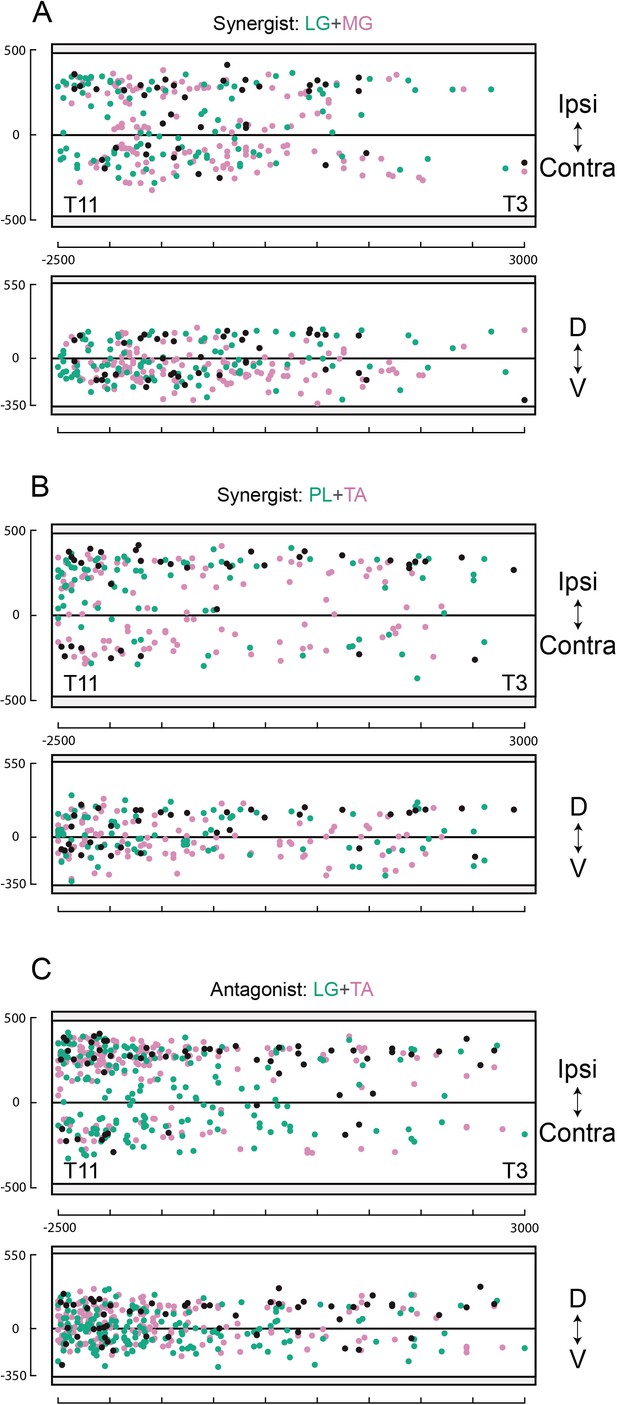
Rostro-caudal distributions of divergent lumbar premotor interneurons (INs).
Rostro-caudal distributions of divergent premtor INs along the sagittal and coronal axis of the lumbar cord following injections in (A) lateral gastrocnemius (LG) and medial gastrocnemius (MG) (n = 2), (B) peroneus longus (PL) and tibialis anterior (TA) (n = 2), and (C) LG and TA (n = 3). Numbers along the axis indicate distances (in µm).
-
Figure 1—figure supplement 4—source data 1
Source data for Figure 1—figure supplement 4A-C.
- https://cdn.elifesciences.org/articles/70858/elife-70858-fig1-figsupp4-data1-v2.xlsx
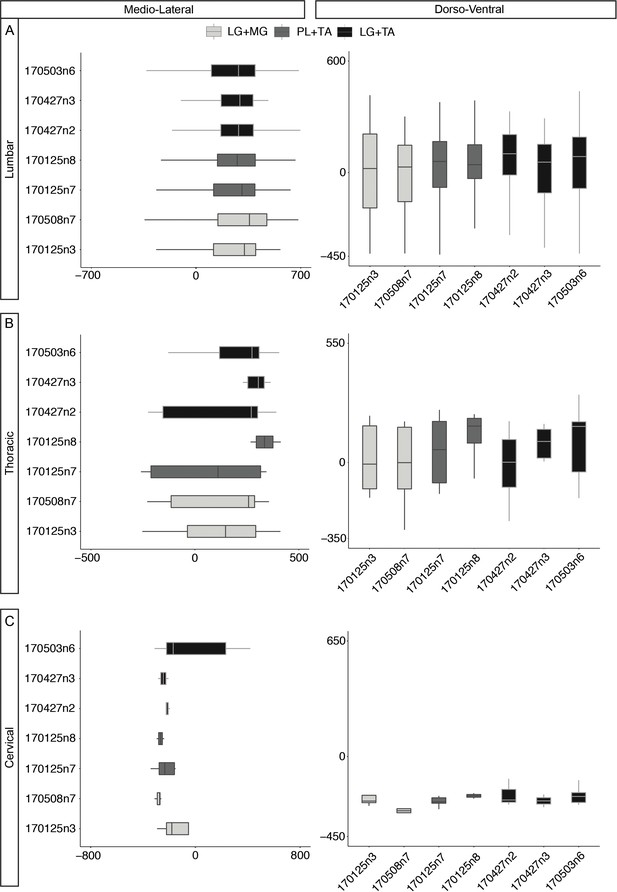
Medio-lateral and dorso-ventral distributions of divergent premotor neurons across individual experiments.
Boxplo showing the medio-lateral and the dorso-ventral distributions of divergent premotor neurons in the (A) lumbar, (B) thoracic, and (C) cervical regions across individual experiments. The distributions are consistent across experiment and pairs of muscles injected. Numbers along the axis indicate the sample codes and distances (in µm).
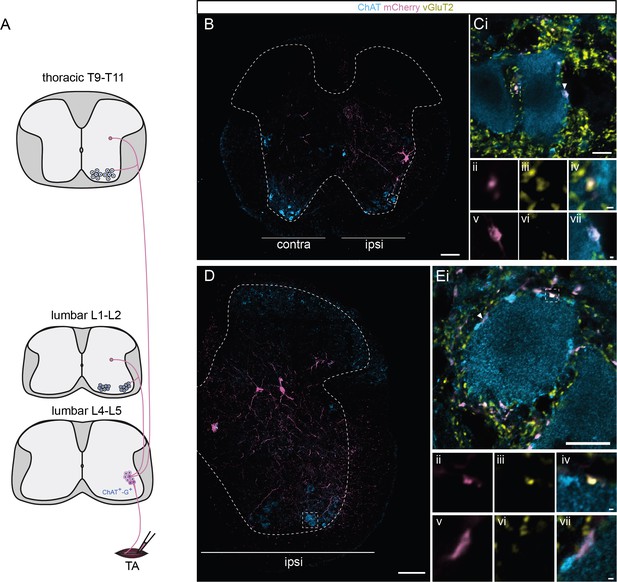
Excitatory boutons from infected premotor neurons and apposed to motoneurons (MNs) reveal divergence through different segments and regions of the spinal cord.
(A) Schematic showing experimental design to visualize projections of excitatory boutons to MNs. Representative images of transverse sections in the (B) thoracic region and (D) L1 (upper lumbar) segment, following an injection of ΔG-Rab-mCherry in the tibialis anterior (TA), showing ChAT (blue grey) and mCherry (pink). MNs with vGluT2+ (yellow); mCherry+ boutons in apposition are highlighted in the dashed boxes. The dashed lines drawn outline the grey matter. (C, E) Dashed boxes from B and D at higher magnification. Dashed boxes (enlarged in panels ii, iii, and iv) indicate a ChAT−, vGluT2+, mCherry+ bouton and arrowheads (enlarged in v, vi, and vii) show ChAT−, vGluT2−, mCherry+ boutons in apposition to (C) L1 and (E) thoracic MNs. Scale bars: (B, D) 100 µm; (Ci, Ei) 10 µm; (Civ–vii, Eiv–vii) 0.5 µm.
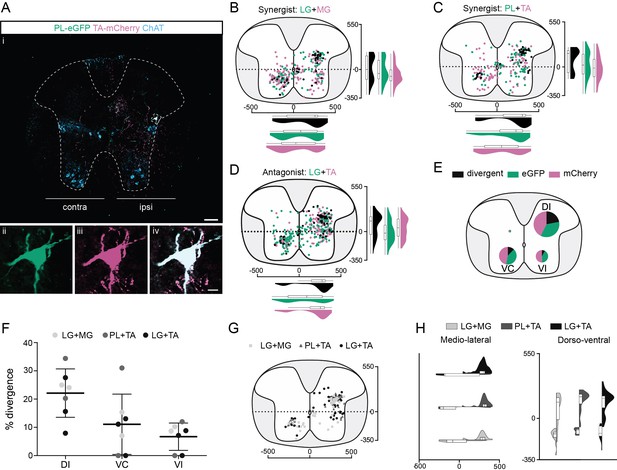
Organization of divergent premotor neurons in the thoracic segments.
(Ai) Representative example of a thoracic transverse section following an injection in the peroneus longus (PL) (ΔG-Rab-eGFP) and tibialis anterior (TA) (ΔG-Rab-mCherry), showing ChAT (grey blue), GFP (green), and mCherry (pink). A divergent premotor neuron is highlighted in the dashed box. The dashed line drawn outlines the grey matter contour. Higher magnification of a divergent premotor neuron that has been infected by both ΔG-Rab-eGFP and ΔG-Rab-mCherry, showing (ii) eGFP, (iii) mCherry, and (iv) the overlay. More representative examples of thoracic sections following injections in lateral gastrocnemius (LG) and medial gastrocnemius (MG), TA and PL, and LG and TA are shown in Figure 2—figure supplements 1–3, respectively. Distribution of the thoracic premotor neurons infected following injections in (B) LG and MG (n = 2), (C) PL and TA (n = 2), and (D) LG and TA (n = 3). Divergent premotor neurons infected from both injections are labelled in black. The violin plots show the dorso-ventral and medio-lateral distributions of divergent (black), GFP-positive (green), and mCherry-positive (pink) premotor neurons along the medio-lateral and dorso-ventral axis. Each violin area is normalized to 1. (E) Pies showing the distribution of infected premotor neurons in each quadrant; the size of the pies is proportional to the number of infected neurons. (F) Plot showing the divergence rate in each quadrant of the thoracic cord. DI: dorsal ipsilateral; VC: ventral contralateral; VI: ventral ipsilateral. (G) Overlap of distributions of divergent thoracic premotor neurons followings each pair of muscles injected. (H) Asymmetric violin plots showing the medio-lateral and dorso-ventral distributions of divergent premotor neurons. The halves correspond, respectively, to the dorsal (top) and ventral (bottom) distributions and to the ipsilateral (right) and contralateral (left) distributions of divergent premotor neurons in the thoracic cord. Violin areas were normalized on the number of divergent neurons. When not specified numbers along the axis indicate distances (in µm). Scale bars: (Ai) 100 µm; (Aiv) 10 µm. Raw number of eGFP, mCherry, and double-labelled premotor neurons per samples per muscle pair injected, is shown in Figure 2—figure supplements 1–3.
-
Figure 2—source data 1
Source data for Figure 2B–G.
- https://cdn.elifesciences.org/articles/70858/elife-70858-fig2-data1-v2.xlsx
Divergent premotor interneurons (INs) in the thoracic spinal cord following injections in synergists lateral gastrocnemius (LG) and medial gastrocnemius (MG).
(A, B) Representative examples of thoracic transverse sections following injections in the LG (ΔG-Rab-eGFP) and MG (ΔG-Rab-mCherry) showing GFP (green) and mCherry (pink). A divergent premotor IN is highlighted in the dashed box. The filled arrowhead shows an additional divergent premotor IN. The dashed lines drawn outline the grey matter. Higher magnification of a divergent premotor IN that has been infected by the ΔG-Rab-eGFP and ΔG-Rab-mCherry, showing (ii) mCherry, (iii) eGFP, and (iv) the overlay. (C, D) Venn diagrams showing the distributions of thoracic infected premotor INs in the two samples injected in LG and MG. Scale bars: (Ai, Bi) 100 µm; (Aiv, Biv) 20 µm.
Divergent premotor interneurons (INs) in the thoracic spinal cord following injections in synergists peroneus longus (PL) and tibialis anterior (TA).
(A, B) Representative examples of thoracic transverse sections following injections in the PL (ΔG-Rab-eGFP) and TA (ΔG-Rab-mCherry) showing GFP (green) and mCherry (pink). A divergent premotor IN is highlighted in the dashed box. The filled arrowhead shows an additional divergent premotor IN. The dashed lines drawn outline the grey matter contour. Higher magnification of a divergent premotor IN that has been infected by the ΔG-Rab-eGFP and ΔG-Rab-mCherry, showing (ii) mCherry, (iii) eGFP, and (iv) the overlay. (C, D) Venn diagrams showing the distributions of thoracic infected premotor INs in the two samples injected in PL and TA. Scale bars: (Ai, Bi) 100 µm; (Aiv, Biv) 20 µm.
Divergent premotor interneurons (INs) in the thoracic spinal cord following injections in antagonists lateral gastrocnemius (LG) and tibialis anterior (TA).
(A, B) Representative examples of thoracic transverse sections following injections in the LG (ΔG-Rab-eGFP) and TA (ΔG-Rab-mCherry) showing GFP (green) and mCherry (pink). A divergent premotor IN is highlighted in the dashed box. The filled arrowhead shows an additional divergent premotor IN. The dashed lines drawn ouline the grey matter contour. Higher magnification of a divergent premotor IN that has been infected by the ΔG-Rab-eGFP and ΔG-Rab-mCherry, showing (ii) mCherry, (iii) eGFP, and (iv) the overlay. (C–E) Venn diagrams showing the distributions of thoracic infected premotor INs in the three samples injected in LG and TA. Scale bars: (Ai, Bi) 100 µm; (Aiv, Biv) 20 µm.
Rostro-caudal distributions of divergent thoracic premotor neurons.
Rostro-caudal distributions of divergent premotor interneurons (INs) shown in the coronal (top) and sagittal (bottom) axes of the thoracic cord following injections in (A) lateral gastrocnemius (LG) and medial gastrocnemius (MG) (n = 2), (B) peroneus longus (PL) and tibialis anterior (TA) (n = 2), and (C) LG and TA (n = 3). Numbers along the axis indicate distances (in µm).
-
Figure 2—figure supplement 4—source data 1
Source data for Figure 2—figure supplement 4A–C.
- https://cdn.elifesciences.org/articles/70858/elife-70858-fig2-figsupp4-data1-v2.xlsx
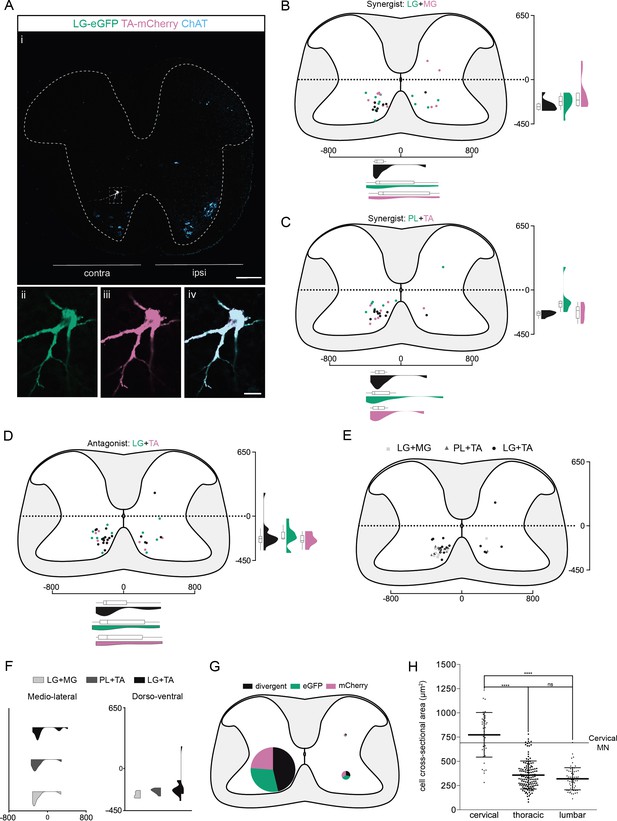
Organization of divergent premotor long descending propriospinal neurons (LDPNs) in the cervical spinal cord.
(Ai) Representative example of an upper cervical transverse section following an injection in the lateral gastrocnemius (LG) (ΔG-Rab-eGFP) and tibialis anterior (TA) (ΔG-Rab-mCherry), showing ChAT (grey blue), GFP (green), and mCherry (pink). A divergent premotor LDPN is highlighted in the dashed box. The dashed line drawn outlines the grey matter contour. Higher magnification of the divergent premotor LDPN, showing (ii) eGFP, (iii) mCherry, and (iv) the overlay. More representative examples of cervical sections following injections in LG and medial gastrocnemius (MG), TA and peroneus longus (PL), and LG and TA are shown in Figure 3—figure supplements 1–3, respectively. (B–D) Distribution of the cervical premotor LDPNs following injections in (C) LG and MG (n = 2), (D) PL and TA (n = 2), and (E) LG and TA (n = 3). Divergent premotor LDPNs infected from both injections are labelled in black. The violin plots show the dorso-ventral and medio-lateral distributions of divergent (black), GFP-positive (green), and mCherry-positive (pink) premotor LDPNs along the medio-lateral and dorso-ventral axis. Each violin area is normalized to 1. (E) Overlap of the distribution of cervical divergent premotor LDPNs followings each pair of muscles injected. (F) Asymmetric violin plots showing the medio-lateral and dorso-ventral distributions of premotor divergent LDPNs. The halves correspond, respectively, to the dorsal (top) and ventral (bottom) distributions and to the ipsilateral (right) and contralateral (left) distributions of divergent premotor LDPNs in the cervical cord. Violin areas were normalized on the number of divergent neurons. (G) Pies showing the distribution of infected premotor LDPNs in each quadrant; the size of the pies is proportional to the number of infected premotor LDPNs in each quadrant. (H) Plot showing the distribution of the sectional areas of divergent premotor neurons in each region of the spinal cord. The dashed line (labelled cervical motoneuron [MN]) corresponds to the mean sectional area of cervical MNs (n = 17 MNs). When not specified numbers along the axis indicate distances (in µm). Scale bars: (Ai) 200 µm; (Aiv) 20 µm. Raw number of eGFP, mCherry, and double-labelled premotor neurons per samples per muscle pair injected, are shown in Figure 3—figure supplements 1–3.
-
Figure 3—source data 1
Source data for Figure 3B–H.
- https://cdn.elifesciences.org/articles/70858/elife-70858-fig3-data1-v2.xlsx
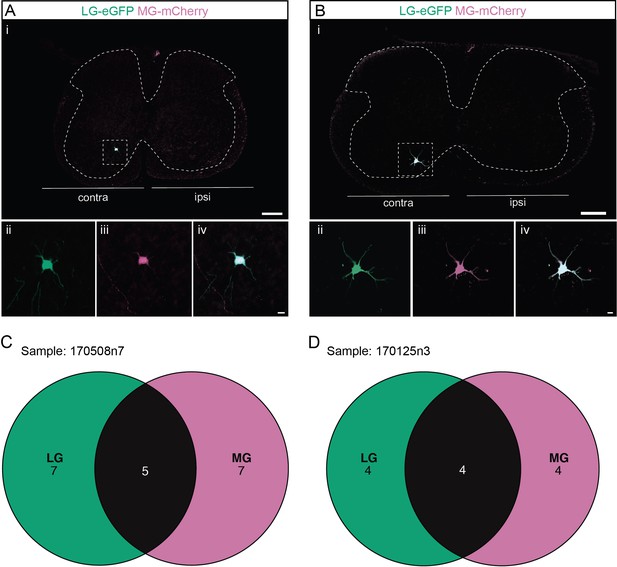
Divergent premotor interneurons (INs) in the cervical spinal cord following injections in synergists lateral gastrocnemius (LG) and medial gastrocnemius (MG).
(A, B) Representative examples of cervical transverse sections following injections in the LG (ΔG-Rab-eGFP) and MG (ΔG-Rab-mCherry) showing GFP (green) and mCherry (pink). A divergent premotor IN is highlighted in the dashed box. The dashed lines drawn outline the grey matter contour. Higher magnification of a divergent premotor IN that has been infected by the ΔG-Rab-eGFP and ΔG-Rab-mCherry, showing (ii) mCherry, (iii) eGFP, and (iv) the overlay. (C, D) Venn diagrams showing the distributions of cervical infected premotor INs in the two samples injected in LG and MG. Scale bars: (Ai, Bi) 200 µm; (Aiv, Biv) 20 µm.
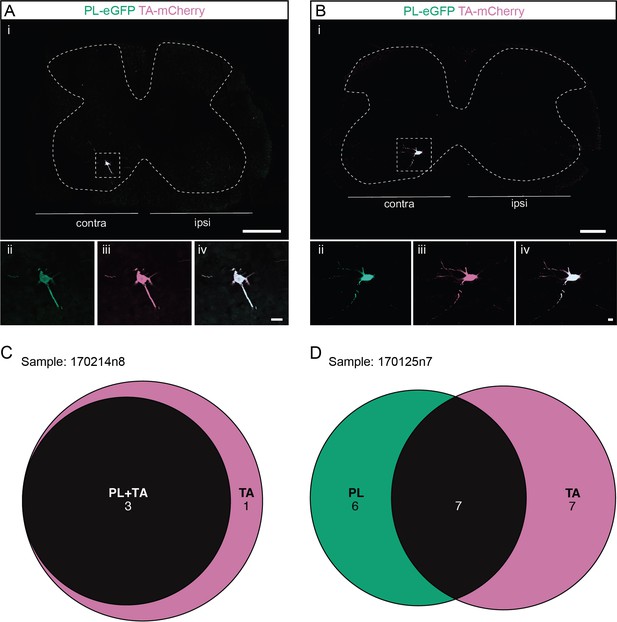
Divergent premotor interneurons (INs) in the cervical spinal cord following injections in synergists peroneus longus (PL) and tibialis anterior (TA).
(A, B) Representative examples of cervical transverse sections following injections in the PL (ΔG-Rab-eGFP) and TA (ΔG-Rab-mCherry) showing GFP (green) and mCherry (pink). A divergent premotor IN is highlighted in the dashed box. The dashed lines drawn outline the grey matter contour. Higher magnification of a divergent premotor IN that has been infected by the ΔG-Rab-eGFP and ΔG-Rab-mCherry, showing (ii) mCherry, (iii) eGFP, and (iv) the overlay. (C, D) Venn diagrams showing the distributions of cervical infected premotor INs in the two samples injected in PL and TA. Scale bars: (Ai, Bi) 200 µm; (Aiv, Biv) 20 µm.
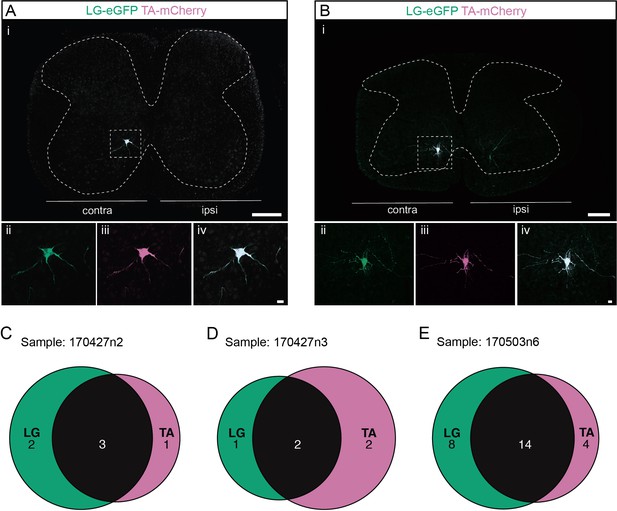
Divergent premotor interneurons (INs) in the cervical spinal cord following injections in antagonists lateral gastrocnemius (LG) and tibialis anterior (TA).
(A, B) Representative examples of cervical transverse sections following injections in the LG (ΔG-Rab-eGFP) and TA (ΔG-Rab-mCherry) showing GFP (green) and mCherry (pink). A divergent premotor IN is highlighted in the dashed box. The dashed lines drawn outline the grey matter contour. Higher magnification of a divergent premotor IN that has been infected by the ΔG-Rab-eGFP and ΔG-Rab-mCherry, showing (ii) mCherry, (iii) eGFP, and (iv) the overlay. (C–E) Venn diagrams showing the distributions of cervical infected premotor INs in the three samples injected in LG and TA. Scale bars: (Ai, Bi) 200 µm; (Aiv, Biv) 20 µm.
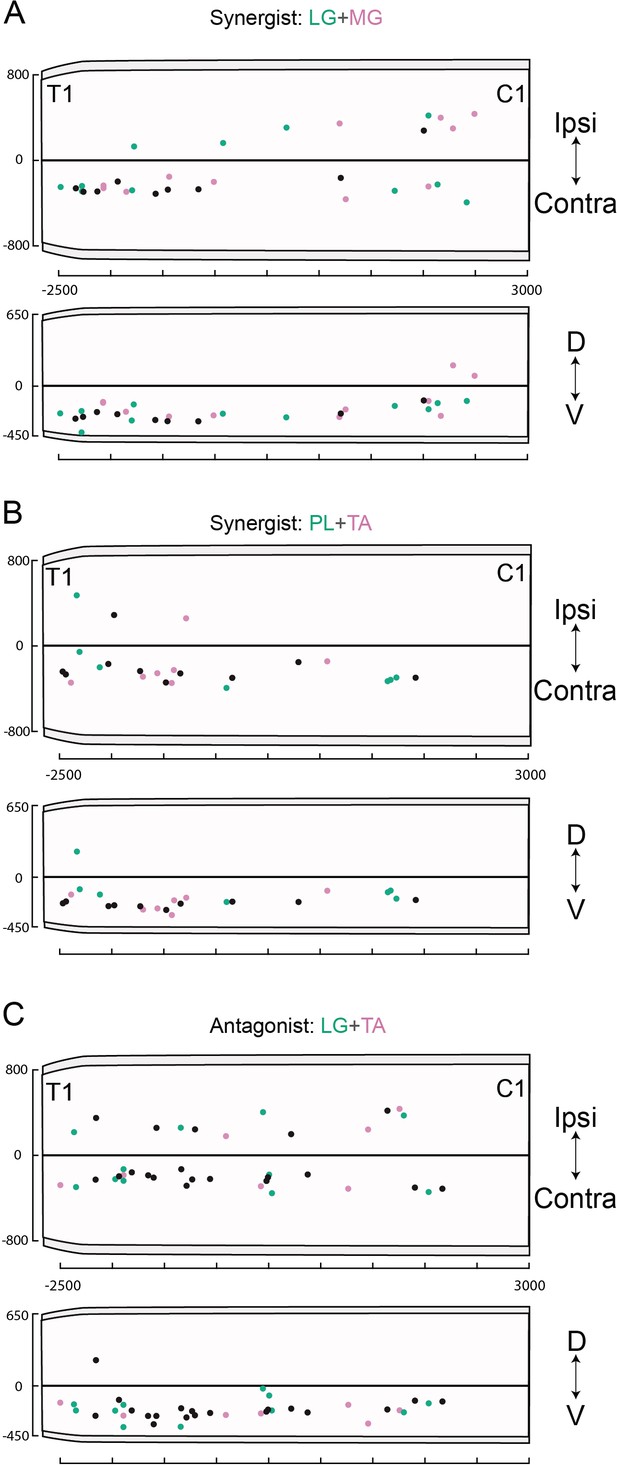
Rostro-caudal distributions of divergent cervical premotor neurons.
Rostro-caudal distributions of divergent premotor interneurons (INs) along the coronal (top) and sagittal (bottom) coronal axes of the cervical cord following injections in (A) lateral gastrocnemius (LG) and medial gastrocnemius (MG) (n = 2), (B) peroneus longus (PL) and tibialis anterior (TA) (n = 2), and (C) LG and TA (n = 3). Numbers along the axis indicate distances (in µm).
-
Figure 3—figure supplement 4—source data 1
Source data for Figure 3—figure supplement 4A–C.
- https://cdn.elifesciences.org/articles/70858/elife-70858-fig3-figsupp4-data1-v2.xlsx
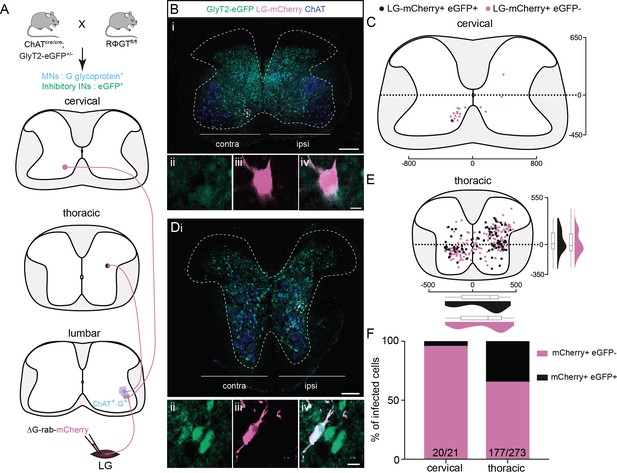
Non-glycinergic, non-cholinergic cervical premotor long descending propriospinal neurons (LDPNs), and mixed populations of inhibitory and non-inhibitory thoracic premotor neurons revealed by injections in GlyT2-eGFP; RΦGT mice.
(A) Experimental strategy to determine whether thoracic and cervical premotor neurons are inhibitory. (B, D) Representative example of (Bi) a cervical and (Di) a thoracic transverse section following an injection in the lateral gastrocnemius (LG) (ΔG-Rab-mCherry) using GlyT2-eGFP; RΦGT mice, showing ChAT (blue), GFP (green), and mCherry (pink). The dashed boxes highlight the infected premotor LDPNs. The dashed lines drawn outline the grey matter contours. Higher magnification of the dashed box areas, highlighting (Bii–iv) a GFP−, mCherry+ cervical premotor LDPN on the contralateral lamina VIII and (Dii–iv) a GFP+, mCherry+ thoracic premotor neuron in ipsilateral intermediate lamina. Distribution of the (C) cervical and (E) thoracic premotor neurons infected, following injections in the LG of GlyT2-eGFP; RΦGT mice (n = 3). The violin plots show the dorso-ventral and medio-lateral distributions of GFP+, mCherry+ (black) and GFP−, mCherry+ (pink) premotor neurons along the medio-lateral and dorso-ventral axis. Each violin area is normalized to 1. (F) Proportions of inhibitory premotor neurons in the thoracic and the cervical region of GlyT2-eGFP; RΦGT mice following injections in the LG (n = 3). When not specified numbers along the axis indicate distances (in µm). Scale bars: (Bi) 200 µm; (Di) 100 µm; (Biv, Div) 10 µm.
-
Figure 4—source data 1
Source data for Figure 4C, E,F.
- https://cdn.elifesciences.org/articles/70858/elife-70858-fig4-data1-v2.xlsx
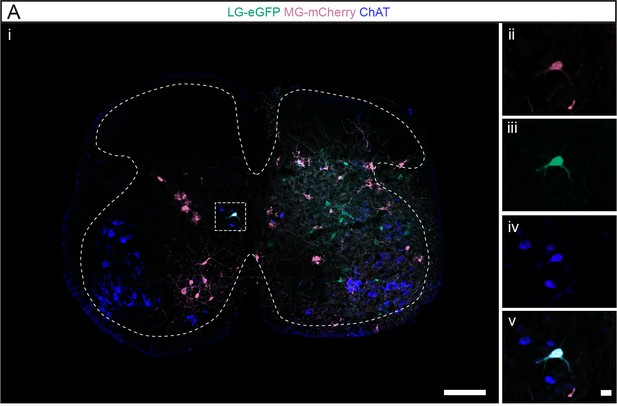
Lumbar V0c interneurons (INs) innervate multiple motor pools.
(A) Representative example of one of the few divergent V0c INs observed in the lumbar cord following injections in muscle pairs. The dashed lines drawn outline the grey matter. The higher magnifications show (ii) mCherry, (iii) eGFP, (iv) ChAT, and (v) the overlay. Scale bars: (Ai) 200 µm; (Av) 20 µm.
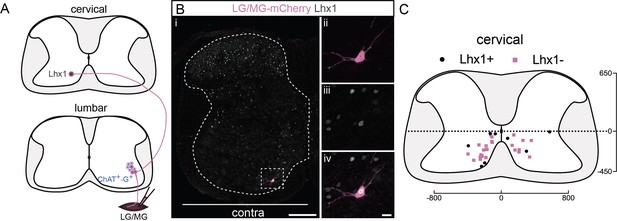
A subpopulation of cervical premotor long descending propriospinal neurons (LDPNs) expresses Lhx1.
(A) Experimental strategy to determine whether cervical premotor LDPNs express Lhx1. (Bi) Representative example of a transverse section from the cervical cord following an injection of ΔG-Rab-eGFP in the gastrocnemius (GS) muscles, showing a cervical premotor LDPN infected (pink) expressing Lhx1 (grey). The premotor LDPN expressing Lhx1 is highlighted in the dashed box. The dashed line drawn outlines the grey matter contour. Higher magnification of the premotor LDPN Lhx1+ that has been infected by the ΔG-Rab-mCherry, showing (ii) mCherry, (iii) Lhx1, and (iv) the overlay. (C) Distribution of the cervical premotor LDPNs following injections in GS whether they are Lhx1+ (black) or not (pink). Numbers along the axis indicate distances (in µm). Scale bars: (Bi) 200 µm; (Biv) 20 µm. The efficiency of Lhx1 staining along postnatal development is shown Figure 5—figure supplement 1.
-
Figure 5—source data 1
Source data for Figure 5C.
- https://cdn.elifesciences.org/articles/70858/elife-70858-fig5-data1-v2.xlsx
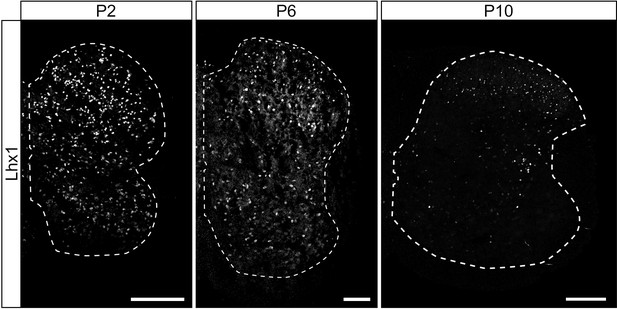
The number of neurons labelled with anti-Lhx1 antibody decreases in the spinal cord over postnatal development.
Lhx1 staining at P2, P6, and P10 showing the decrease in the number of Lhx1-positive cells in the spinal cord through early postnatal development. The dashed lines drawn outline the grey matter. Scale bars: 100 µm.
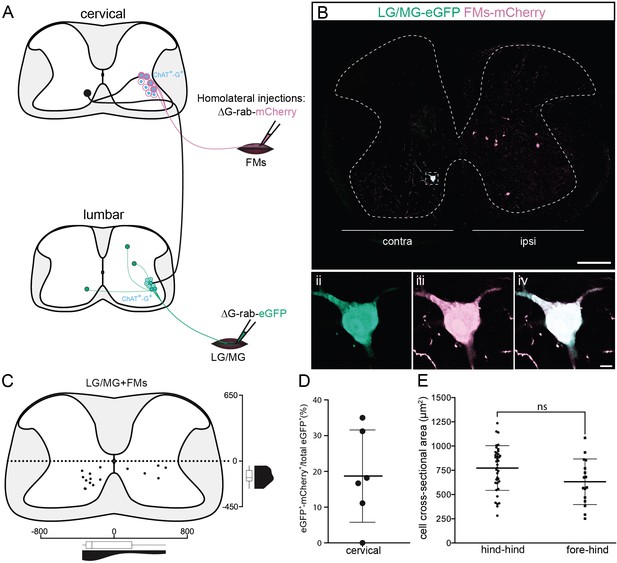
Cervical premotor long descending propriospinal neurons (LDPNs) innervate homolateral lumbar and cervical motoneurons (MNs).
(A) Experimental strategy to determine whether divergent cervical premotor LDPNs that innervate homolateral lumbar and cervical MNs do exist. (Bi) Representative example of a transverse section from the cervical cord following an injection in forearm muscles (FMs) (ΔG-Rab-mCherry) and GS (ΔG-Rab-eGFP) muscles, showing a premotor LDPN infected from the two contralateral motor pools. The dashed box highlights the divergent premotor LDPN. The dashed line drawn outlines the grey matter contour. Dashed box area at higher magnification, showing (ii) eGFP, (iii) mCherry, and (iv) the overlay. (C) Distribution of the premotor LDPNs infected from the homolateral injections in GS and FMs. The violin and box plots show the distribution of divergent premotor LDPNs innervating homolateral local FMs and distant GS motor pools along the medio-lateral and dorso-ventral axis. Each violin area is normalized to 1. (D) Proportion of cervical premotor LDPNs that also project to FM motor pools per animal. (E) Plot showing the sectional area of the cervical divergent premotor LDPNs that diverge to two pools of lumbar MNs (hind_hind) and to the pools of GS and FM MNs (fore_hind). When not specified numbers along the axis indicate distances (in µm). Scale bars: (Bi) 200 µm; (Biv) 10 µm.
-
Figure 6—source data 1
Source data for Figure 6C–E.
- https://cdn.elifesciences.org/articles/70858/elife-70858-fig6-data1-v2.xlsx
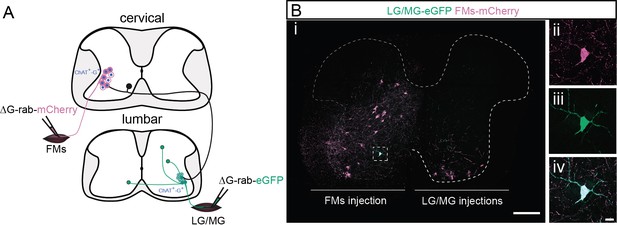
Cervical premotor long descending propriospinal neurons (LDPNs) innervate ipsilateral cervical and contralateral lumbar motoneurons (MNs).
(A) Experimental strategy to examine whether divergent cervical premotor LDPNs innervate ipsilateral cervical and contralateral lumbar motor pools. (Bi) Representative example of a transverse section from the cervical cord following injections in contralateral GS (ΔG-Rab-eGFP) and ipsilateral forearm muscle (FM) (ΔG-Rab-mCherry) muscles, showing a double-labelled premotor LDPN. The dashed box highlights the divergent premotor LDPN. The dashed line drawn outlines the grey matter. Dashed box area at higher magnification, showing (ii) mCherry, (iii) eGFP, and (iv) the overlay. Scale bars: (Bi) 200 µm; (Biv) 20 µm.
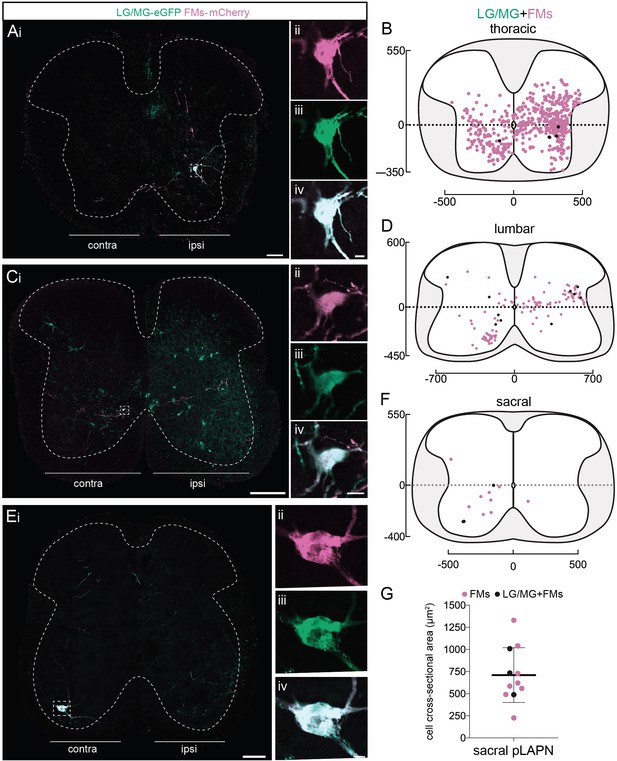
Premotor long ascending propriospinal neurons (LAPNs) are distributed in the thoracic, lumbar, and sacral spinal cord, and diverge to homolateral lumbar and cervical motoneurons (MNs).
(Ai, Ci, Ei) Representative examples of transverse sections from the (A) thoracic, (C) lumbar, and (E) sacral spinal cord following injections in homolateral GS (ΔG-Rab-eGFP) and forearm muscles (FMs) (ΔG-Rab-mCherry), showing ChAT (blue grey), GFP (green), and mCherry (pink). Dashed boxes highlight INs that were infected from the injections in homolateral GS and FMs. The dashed lines drawn indicate the grey matter. High magnification of the dashed boxes showing double infected premotor neurons in the (Aii–iv) thoracic, (Cii–iv) lumbar, and (Eii–iv) sacral cord. Distributions of the ascending single-labelled (pink) and divergent (black) premotor neurons in the (B) thoracic, (D) lumbar, and (F) sacral cord following injections in homolateral GS (ΔG-Rab-eGFP) and FMs (ΔG-Rab-mCherry). (G) Plot showing the size of the sacral premotor LAPNs infected from GS and FMs injections (black) or from the injection in FMs only (pink). When not specified numbers along the axis indicate distances (in µm). Scale bars: (Ai, Ei) 100 µm; (Ci) 200 µm; (Aiv, Civ, Eiv) 10 µm.
-
Figure 6—figure supplement 2—source data 1
Source data for Figure 6—figure supplement 2B, D, F, G.
- https://cdn.elifesciences.org/articles/70858/elife-70858-fig6-figsupp2-data1-v2.xlsx
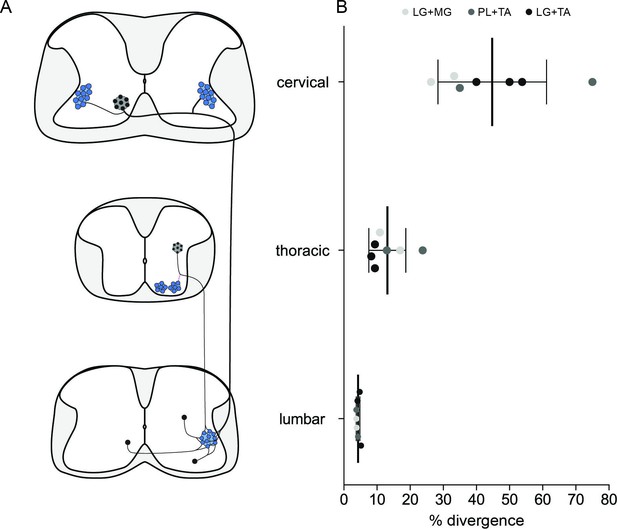
Divergence rates throughout the spinal cord and circuits.
(A) Schematic summarizing the projections determined. (B) Plot showing the increase of the apparent divergence rate with the distance between innervated motoneurons (MNs) and premotor neurons.
-
Figure 7—source data 1
Source data for Figure 7B.
- https://cdn.elifesciences.org/articles/70858/elife-70858-fig7-data1-v2.xlsx
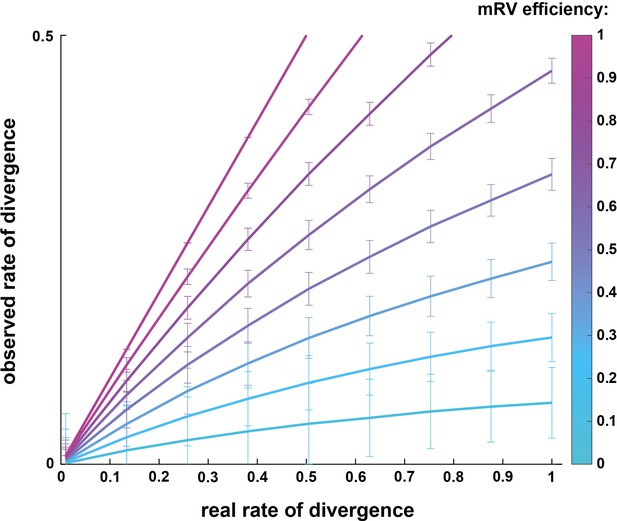
Simulation comparing observed vs real rates of divergence depending on trans-synaptic mRV efficiency.
Simulation of the spreading of mRV in premotor circuits following double injections, extracted from a binomial distribution. Plot showing the relation between observed rate of divergence depending on the real rate of divergence within premotor spinal circuits. This simulation was run with the simplifying assumption that the efficiencies of viral transfer are equal and independent from each other across spinal cord regions.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Rabies virus) | ΔG-Rab-eGFP | Gift from M. Tripodi lab, LMCB Cambridge | G-deleted Rabies virus | |
| Strain, strain background (Rabies virus) | ΔG-Rab-mCherry | Gift from M. Tripodi lab, LMCB Cambridge | G-deleted Rabies virus | |
| Strain, strain background (Mus musculus) | ChAT-IRES-Cre | Jackson Laboratory | IMSR Cat# JAX:006410; RRID:IMSR_JAX:006410 | Allele symbol: Chattm2(cre)Lowl; maintained on a C57BL6/J background |
| Strain, strain background (Mus musculus) | RΦGT | Jackson Laboratory | IMSR Cat# JAX:024708; RRID:IMSR_JAX:024708 | Allele symbol: Gt(ROSA)26 Sortm1 (CAG-RABVgp4,- TVA)Arenk; maintained on a C57BL6/J background |
| Strain, strain background (Mus musculus) | GlyT2-eGFP | Gift from H. Zeilhofer lab, University of Zurich | IMSR Cat# RBRC04708; RRID:IMSR_RBRC04708 | Allele symbol: Tg(Slc6a5-EGFP) 1Uze; maintained on a C57BL6/J background |
| Cell line (Homo sapiens, female) | HEK293t/17 | Gift from M. Tripodi lab, LMCB Cambridge | RRID:CVCL_1926 | ATCC, cat. no. CRL-1126 |
| Cell line (Mesocricetus auratus, male) | BHK-21 | Gift from M. Tripodi lab, LMCB Cambridge | RRID: CVCL_1915 | ATCC # CCL-10 |
| Cell line (Mesocricetus auratus, male) | BHK-G | Gift from M. Tripodi lab, LMCB Cambridge | RRID: CVCL_1915 | Modified from ATCC Cat# CCL-10; RRID: CVCL_1915 to express the rabies glycoprotein |
| Antibody | anti-ChAT (Goat polyclonal) | Millipore | Cat# AB144P; RRID:AB_2079751 | IF (1:100) |
| Antibody | anti-mCherry (Chicken polyclonal) | Abcam | Cat# ab205402; RRID:AB_2722769 | IF (1:2500) |
| Antibody | anti-GFP (Rabbit polyclonal) | Abcam | Cat# ab290; RRID:AB_303395 | IF (1:2500) |
| Antibody | anti-vGluT2 (Guinea pig polyclonal) | Millipore | Cat# AB2251-I; RRID:AB_2665454 | IF (1:2500) |
| Antibody | anti-Lhx1 (Rabbit polyclonal) | Gift from T. Jessell lab, Columbia University, New York | IF (1:5000) | |
| Antibody | anti-Rabbit IgG H&L Alexa Fluor 647 (Donkey polyclonal) | Abcam | Cat# ab150079; RRID:AB_2722623 | IF (1:1000) |
| Antibody | anti-Goat IgG H&L Alexa Fluor 405 (Donkey polyclonal preadsorbed) | Abcam | Abcam Cat# AB175665; RRID:AB_2636888 | IF (1:200) |
| Antibody | anti-Rabbit IgG H&L Alexa Fluor488 (Donkey polyclonal Highly Cross-Adsorbed) | Thermo Fisher Scientific | Cat# A-21206; RRID:AB_2535792 | IF (1:1000) |
| Antibody | anti-Chicken IgY (IgG) H&L Cy3- AffiniPure (Donkey polyclonal) | Jackson Immuno Research Labs | Cat# 703-165-155; RRID:AB_2340363 | IF (1:1000) |
| Chemical compound, drug | Mowiol 4–88 | Sigma-Aldrich | Cat# 81381–250 G | |
| Software, algorithm | ZEN Digital Imaging for Light Microscopy: Zen Blue 2.3 | Carl Zeiss light microscopy imaging systems | RRID:SCR_013672 | |
| Software, algorithm | Imaris 9.1 | Bitplane | RRID:SCR_007370 | |
| software, algorithm | R 3.6.2 | R Project for Statistical Computing | RRID:SCR_001905 | |
| Software, algorithm | Prism 7.0 | GraphPad | RRID:SCR_002798 | |
| Software, algorithm | Adobe illustrator version CC2019 | Adobe | RRID:SCR_010279 |
Additional files
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/70858/elife-70858-transrepform1-v2.docx
-
Supplementary file 1
Numbers of MNs and premotor neurons, and medio-lateral and dorso-ventral distributions of divergent premotor neurons across individual experiments.
Distribution of divergent premotor neurons per region of the spinal cord across individual experiments, expressed as median± first/third quartile.
- https://cdn.elifesciences.org/articles/70858/elife-70858-supp1-v2.xlsx
-
Supplementary file 2
Details of muscles infected following forearm injections.
(+) means that a fluorescent signal was found in muscle fibres, (−) means that no fluorescence was observed following muscle dissections.
- https://cdn.elifesciences.org/articles/70858/elife-70858-supp2-v2.xlsx
-
Supplementary file 3
List of the mice used for each experiment, including genotype and figure in which they are shown.
- https://cdn.elifesciences.org/articles/70858/elife-70858-supp3-v2.xlsx





