Cre/lox regulated conditional rescue and inactivation with zebrafish UFlip alleles generated by CRISPR-Cas9 targeted integration
Figures
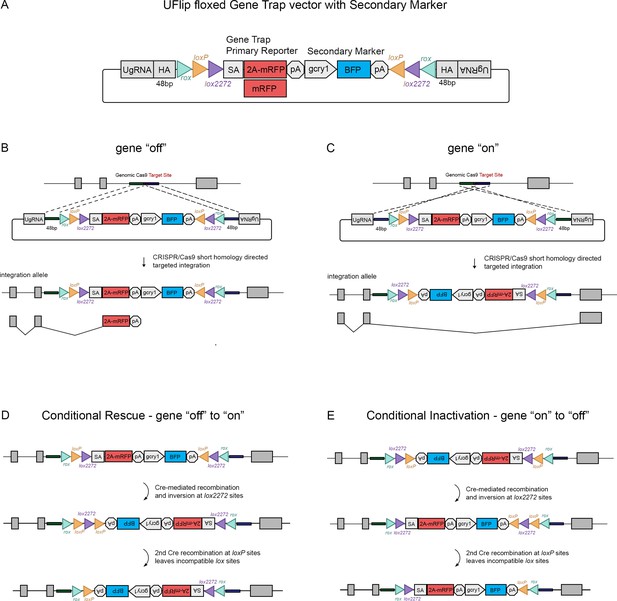
The UFlip floxed gene trap vector for isolation of conditional gene alleles generated by GeneWeld CRISPR-Cas9 targeted integration.
(A) Diagram of the UFlip. The vector contains a floxed rox loxP lox2272 gene trap plus secondary marker loxP lox2272 rox cassette. The cassette is flanked by cloning sites for homology arms (HA) complementary to a genomic CRISPR target site, and universal gRNA sites (UgRNA) for in vivo liberation of the targeting cassette. (B) Gene ‘off’ alleles are generated by integration of the UFlip cassette into an intron in the active orientation, leading to transcription termination and splicing of the primary transcript in the mRFP gene trap. (C) Gene ‘on” alleles are generated by integration of the UFlip cassette into an intron in the passive orientation. This is driven by cloning the genomic 5’ homology arm downstream of the UFlip cassette, and cloning the genomic 3’ homology arm upstream of the UFlip cassette. Integration at the genomic CRISPR-Cas9 target site occurs in the opposite orientation. During transcription RNA polymerase reads through the integrated UFlip cassette, which is spliced out with the intron during processing of the primary transcript. (D) Cre-mediated recombination at an ‘off’ allele locks the cassette in the ‘on’ orientation. The first recombination occurs stochastically at either lox2272 or loxP sites. The diagram shows the intermediate that forms if the first recombination occurs at the lox2272 sites. (E) Cre-mediated recombination at an ‘on’ allele locks the cassette in the ‘off’ orientation. The first recombination occurs stochastically at either lox2272 or loxP sites. The diagram shows the intermediate that forms if the first recombination occurs at the lox2272 sites. BFP, blue fluorescent protein; gcry1, gamma crystallin 1 promoter; myl7, cardiac myosin light chain 7 promoter; 2 A, porcine teschvirus-1 2A peptide; mRFP, monomeric red fluorescent protein; pA, transcription termination and polyadenylation signal; SA, splice acceptor.
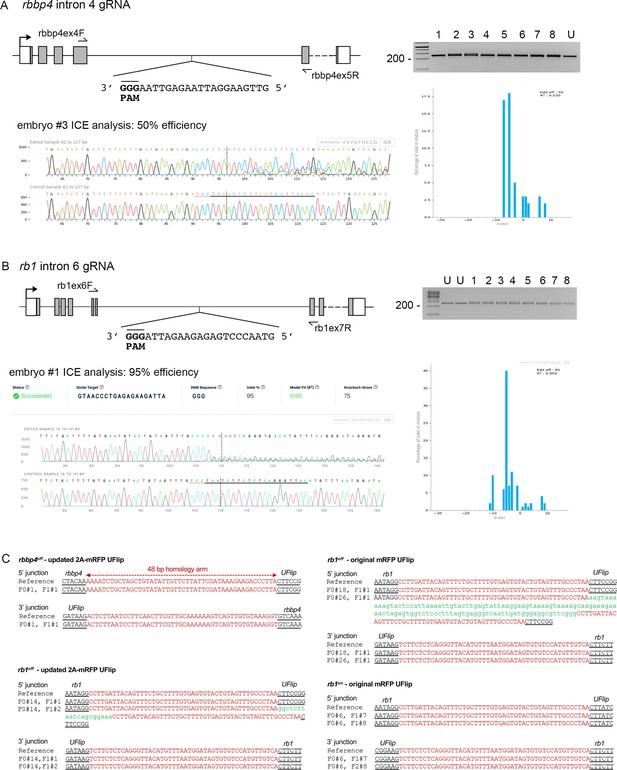
rbbp4 and rb1 intronic gRNA efficiency and F1 UFlip allele junction analysis.
(A) rbbp4 gene model with sequence of the intron 4 reverse strand gRNA. Gel image of PCR amplicons surrounding the target site from 8 Cas9 plus gRNA injected and 1 uninjected (U) embryo. Amplicons from embryo #3 and the uninjected embryo were sequenced and analyzed with Synthego’s ICE software, and indicate 50% indel efficiency at the target site. Plot shows the range and percentage of indels present in the sequences. PAM sequence shown in bold and underlined. (B) rb1 gene model with sequence of the intron 6 reverse strand gRNA. Gel image of PCR amplicons surrounding the target site from eight embryos injected with Cas9 and the gRNA (1-8), and two uninjected embryos (U). Amplicons from embryo #1 and an uninjected embryo were sequenced and analyzed with Synthego’s ICE software, and indicate 95% indel efficiency at the target site. Plots show the range and percentage of indels present in the sequences. PAM sequences shown in bold and underlined. (C) 5’ and 3’ genomic-UFlip integration junctions were PCR amplified from F1 transgenic zebrafish fin clip genomic DNA. The PCR products were sequenced and aligned to the reference sequence expected for a precise integration at the genomic target site. Capitalized red nucleotides represent 48 bp homology arms. Lowercase green nucleotides represent random inserted sequences.
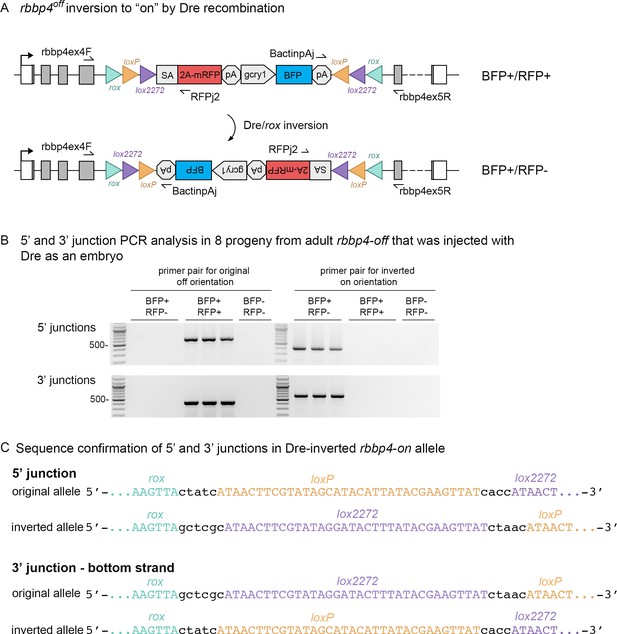
Dre mRNA injection into rbbp4off/+ embryos leads to inversion of the UFlip cassette and efficient germline recovery of an inverted rbbp4on allele.
(A) Diagram illustrating Dre-mediated inversion of the rbbp4off allele to the on orientation. Repeated inversion of the cassette will continue as long as Dre is present. The final allele is predicted to be in the inverted ‘on’ orientation at a frequency of 50%. (B) PCR junction analysis of 8 embryos from an F1 adult that had been injected with Dre mRNA at the one-cell stage. Three embryos positive for expression of the lens BFP secondary marker show the expected 5’ and 3’ junction PCR amplicons for the inverted rbbp4on allele. (C) Sequence analysis confirms Dre-mediated inversion of the cassette from the ‘off’ to ‘on’ orientation in BRP+/RFP - embryos.
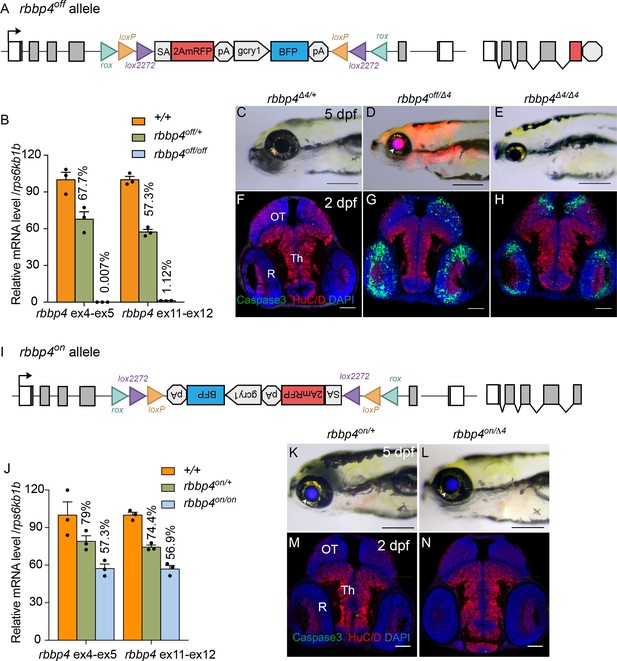
Molecular and phenotypic characterization of rbbp4off and rbbp4on alleles.
(A) Diagram of the rbbp4off allele. (B) Plot of RT-qPCR results from wild type +/+ (n=3), heterozygous rbbp4off/+(n=3), and homozygous rbbp4off/off (n=3) larvae showing the relative level of rbbp4 mRNA transcript using reference gene rps6kb1b. Primer pairs were located in exons 4 and 5, or downstream exons 11 and 12. (C – E) Gross phenotype of rbbp4Δ4/+ (C), rbbp4off/Δ4 (D), and rbbp4Δ4/Δ4 (E) 5 dpf larvae. Arrowhead in (D) points to overlap of rbbp4off 2A-mRFP primary reporter and gcry1:BFP secondary reporter expression in the lens, which appears purple. (F – H) Caspase-3 and HuC/D labeling of sectioned head tissue from 2 dpf rbbp4Δ4/+ (F) rbbp4off/Δ4 (G) and rbbp4Δ4/Δ4 (H) embryos. (I) Diagram of the rbbp4on allele. (J) Plot of RT-qPCR results from wild type +/+ (n=3), heterozygous rbbp4on/+ (n=3), and homozygous rbbp4on/on (n=3) larvae showing the relative level of rbbp4 mRNA transcript using reference gene rps6kb1b. Primer pairs were located in exons 4 and 5, or downstream exons 11 and 12. (K, L) Gross phenotype of rbbp4on/+ (K) and rbbp4on/Δ4 (L) 5 dpf larvae. The rbbp4on allele secondary marker gcry1:BFP expression is visible in the lens. Caspase-3 and HuC/D labeling of sectioned head tissue from 2 dpf rbbp4Δ4/+ (M) and rbbp4off/Δ4 (N) embryos. OT, optic tectum; R, retina; Th, thalamic region. Error bars represent mean ± s.e.m. Scale bars: 200 μm (C–E, K, L), 50 μm (F–H, M,N).
-
Figure 4—source data 1
Source data for rbbp4 RT-quantitative PCR analysis in wildtype, heterozygous and homozygous embryos from rbbp4off/+ and rbbp4on/+ incrosses.
- https://cdn.elifesciences.org/articles/71478/elife-71478-fig4-data1-v2.xlsx
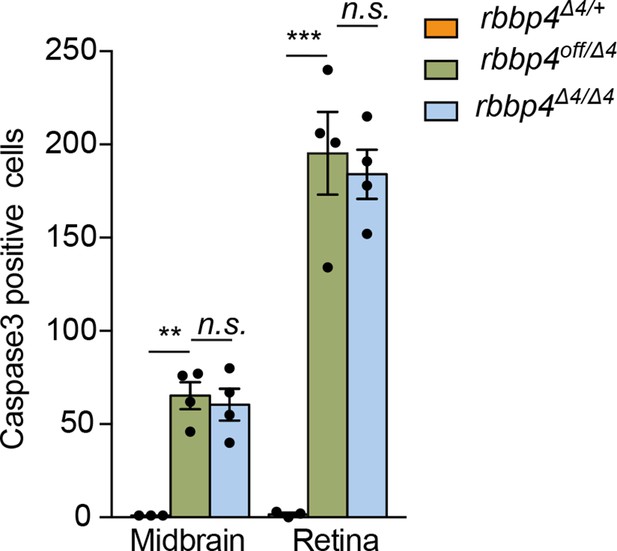
Quantification of activated caspase-3a labeled cells in rbbp4Δ4/+, rbbp4off/Δ4, and rbbp4Δ4/Δ 2 dpf embryo midbrain and retina.
Plot of quantification of caspase-3a labeled cells in rbbp4Δ4/+ (n=3), rbbp4off/Δ4 (n=4), and rbbp4Δ4/Δ4 (n=4). rbbp4off/Δ4 vs. rbbp4Δ4/+ midbrain (** p<0.01) and retina (** p<0.01). rbbp4off/Δ4 vs. rbbp4Δ4/Δ4 midbrain (n.s. p=0.6865) and retina (n.s. p=0.6778). Error bars represent mean ± s.e.m. with two-tailed t-test.
-
Figure 4—figure supplement 1—source data 1
Source data for quantification of activated caspase-3a labeled cells in rbbp4Δ4/+, rbbp4off/Δ4, and rbbp4Δ4/Δ 2 dpf embryo midbrain and retina.
- https://cdn.elifesciences.org/articles/71478/elife-71478-fig4-figsupp1-data1-v2.xlsx
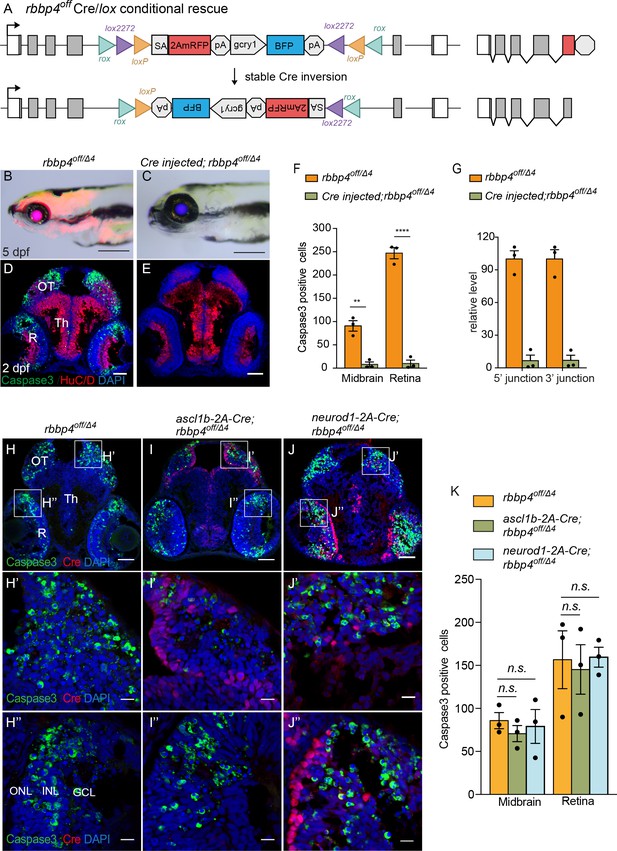
Ubiquitous and cell-type specific Cre-mediated conditional rescue with rbbp4-off.
(A) Diagram of expected Cre mediated inversion of rbbp4off to on orientation. (B) Gross morphological phenotype of microcephaly and microphthalmia in 5 dpf transheterozygous rbbp4off/Δlarva. (C) Cre injected 5 dpf transheterozygous rbbp4off/Δ4larva shows rescue of gross phenotype and loss of mRFP expression. (D) Activated caspase-3a labeling throughout midbrain and retina section from 2 dpf transheterozygous rbbp4off/Δ4embryo. (E) Absence of activated caspase-3a labeling in midbrain and retina of 2 dpf transheterozygous rbbp4off/Δ4embryo after Cre injection. (F) Quantification of caspase-3a labeling in control rbbp4off/Δ4(n=3) and Cre injected rbbp4off/Δ4 (n=3) midbrain (** p<0.01) and retina (**** p<0.0001). (G) Genomic DNA qPCR quantification of rbbp4off original orientation 5’ and 3 junctions in control rbbp4off/Δ4 (n=3) and Cre injected rbbp4off/Δ4 (n=3). Cre injection reduced the level of rbbp4off original orientation 5’ (>93%) and 3’ junctions (>93%). (H – J”) Activated caspase-3A and Cre labeling in sectioned head tissue from 2 dpf rbbp4off/Δ4 (H-H"), ascl1b-2A-Cre; rbbp4off/Δ4 (I-I"), and neurod1-2A-Cre; rbbp4off/Δ4 (J-J") embryos. (K) Quantification of caspase-3a labeling in rbbp4off/Δ4(n=3), ascl1b-2A-Cre; rbbp4off/Δ4 (n=3) and neurod1-2A-Cre; rbbp4off/Δ4 (n=3).rbbp4off/Δ4vs. ascl1b-2A-Cre; rbbp4off/Δ4 midbrain (n.s. p=0.3248) and retina (n.s. p=0.8153), and neurod1-2A-Cre; rbbp4off/Δ4 midbrain (n.s. p=0.7794) and retina (n.s. p=0.9365). OT, optic tectum; R, retina; Th, thalamic region. Error bars represent mean ± s.e.m. with two-tailed t-test. Scale bars: 200 μm (B, C), 50 μm (D, E, H – J). 10 μm (H’ – J”).
-
Figure 5—source data 1
Source data for quantification of activated caspase-3a labeling and rbbp4off inversion after Cre injection, activated caspase-3a labeling using ascl1b-2A-Cre and neurod1-2A-Cre.
- https://cdn.elifesciences.org/articles/71478/elife-71478-fig5-data1-v2.xlsx
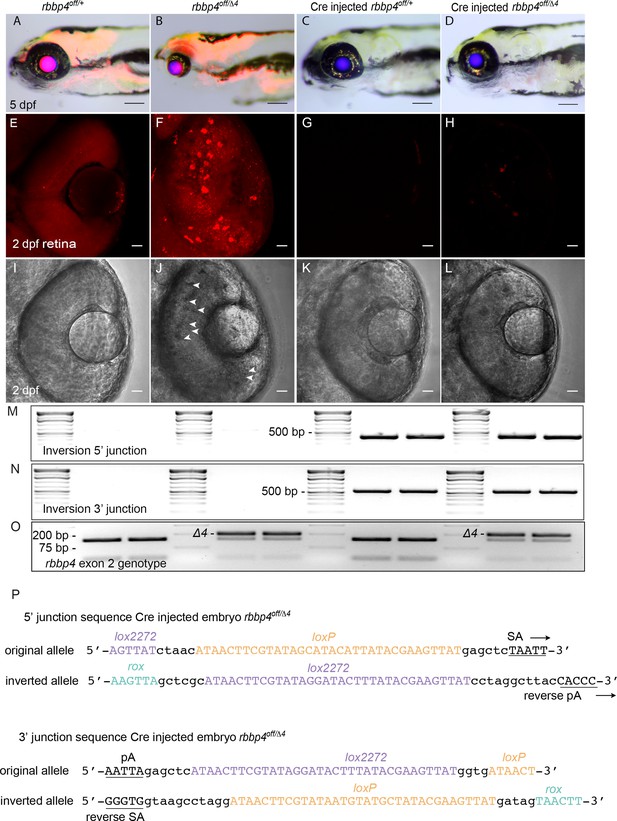
Live imaging and molecular analysis of rbbp4off conditional rescue by Cre injection.
(A – D) Gross morphology and mRFP expression in uninjected rbbp4off/+ (A) and rbbp4off/Δ4 (B), and Cre injected rbbp4off/+ (C) and rbbp4off/Δ4 (D) 5 dpf larva. Confocal live images of mRPF expression and transmitted light in 2 dpf retina from uninjected rbbp4off/+ (E, I), uninjected rbbp4off/Δ4 (F, J), Cre injected rbbp4off/+ (G, K) and rbbp4off/Δ4 (H, L). (M, N) 5’ and 3’ junctions of the inverted allele detected by genomic DNA PCR. (O) PCR amplicon genotyping of the rbbp4Δ4 exon 2 4 bp deletion allele. (P) Sequence of the 5’ and 3 junction amplicons confirms rbbp4off stable inversion to the on orientation by Cre. Scale bar: 200 μm (A - D). 20 μm (E - L).
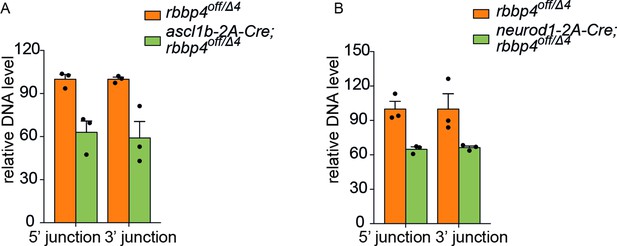
qPCR quantification of rbbp4off allele inversion by ascl1b-2A-Cre and neurod1-2A-Cre.
(A) Genomic DNA qPCR quantification of rbbp4off original orientation 5’ and 3 junctions in control rbbp4off/Δ4 (n=3) and in ascl1b-2A-Cre; rbbp4off/Δ4(n=3). (B) Genomic DNA qPCR quantification of rbbp4off original orientation 5’ and 3 junctions in control rbbp4off/Δ4 (n=3) and in neurod1-2A-Cre; rbbp4off/Δ4Δ4(n=3). Error bars represent mean ± s.e.m.
-
Figure 5—figure supplement 2—source data 1
Source data for qPCR quantification of rbbp4off inversion by ascl1b-2A-Cre and neurod1-2A-Cre.
- https://cdn.elifesciences.org/articles/71478/elife-71478-fig5-figsupp2-data1-v2.xlsx
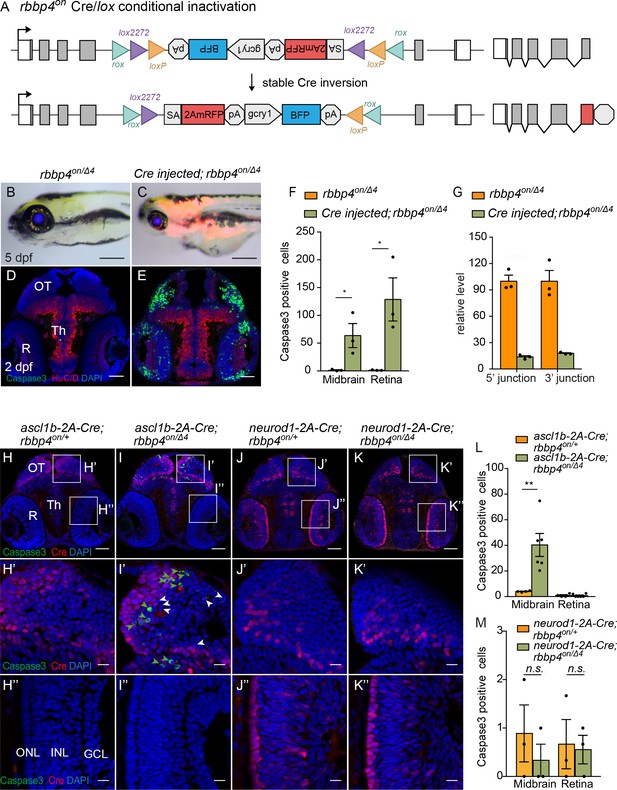
Ubiquitous and cell-type specific Cre-mediated conditional inactivation with rbbp4-on.
(A) Diagram of expected Cre-mediated inversion of rbbp4on to “off” orientation. (B) Normal morphological phenotype in 5 dpf transheterozygous rbbp4on/Δ4larva. (C) Induction of microcephaly and microphthalmia and mRFP expression in Cre injected 5 dpf transheterozygous rbbp4on/Δ4larva. (D) Absence of activated caspase-3a labeling in sectioned tissue from 2 dpf uninjected transheterozygous rbbp4on/Δ4embryo. (E) Activated caspase-3a labeling in the midbrain and retina of 2 dpf transheterozygous rbbp4on/Δ4embryo after Cre injection. (F) Quantification of caspase-3a labeling in control rbbp4on/Δ4(n=3) and Cre-injected rbbp4on/Δ4(n=3) midbrain (* p<0.05) and retina (* p<0.05). (G) Genomic DNA qPCR quantification of rbbp4on original orientation 5’ and 3 junctions in control rbbp4on (n=3) and Cre injected rbbp4on/Δ4 (n=3). (H – K”) Activated caspase-3a and Cre labeling in sectioned head tissue from 2 dpf ascl1b-2A-Cre; rbbp4D4/+ (H-H"), ascl1b-2A-Cre; rbbp4on/Δ4 (I-I"), neurod1-2A-Cre; rbbp4Δ4/+ (J-J"), and neurod1-2A-Cre; rbbp4on/Δ4 (K-K"), embryos. Green arrowheads, activated caspase-3a-positive cells. White arrowheads, hypercondensed and fragmented nuclei. (L) Quantification of caspase-3a labeling in ascl1b-2A-Cre; rbbp4on/+ (n=4) and ascl1b-2A-Cre; rbbp4on/Δ4 (n=6) midbrain (** p<0.01) and retina (n.s. p=0.8543). (M) Quantification of caspase-3a labeling in neurod1-2A-Cre; rbbp4on/+ (n=3) and neurod1-2A-Cre; rbbp4on/Δ4 (n=3) midbrain (n.s. p=0.3739) and retina (n.s. p=0.6433). OT, optic tectum; R, retina; Th, thalamic region. Error bars represent mean ± s.e.m. with two-tailed t-test. Scale bars: 200 μm (B, C), 50 μm (D, E, H – K). 10 μm (H’ – K”).
-
Figure 6—source data 1
Source data for quantification of activated caspase-3a labeling and rbbp4on inversion after Cre injection, activated caspase-3a labeling by ascl1b-2A-Cre and neurod1-2A-Cre.
- https://cdn.elifesciences.org/articles/71478/elife-71478-fig6-data1-v2.xlsx
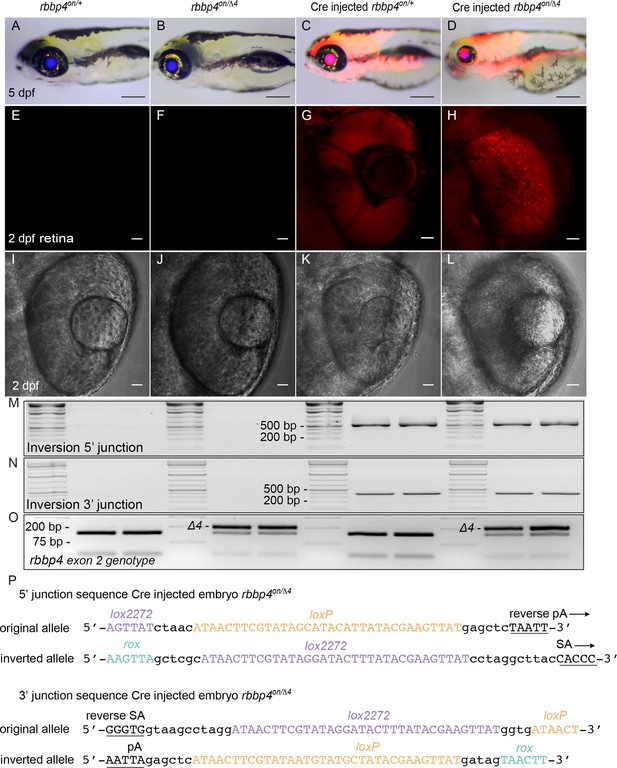
Live imaging and molecular analysis of rbbp4on conditional inactivation by Cre injection.
(A – D) Gross morphology and mRFP expression in uninjected rbbp4on/+ (A) and rbbp4on/Δ4 (B), and Cre injected rbbp4on/+ (C) and rbbp4on/Δ4 (D) 5 dpf larva. Confocal live images of mRPF expression and transmitted light in 2 dpf retina from uninjected rbbp4on/+ (E, I), uninjected rbbp4on/Δ4 (F, J), Cre injected rbbp4on/+ (G, K) and rbbp4on/Δ4 (H, L). (M, N) 5’ and 3’ junctions of the inverted allele detected by genomic DNA PCR. (O) PCR amplicon genotyping of the rbbp4Δ4 exon 2 4 bp deletion allele. (P) Sequence of the 5’ and 3 junction amplicons confirms rbbp4on stable inversion to the ‘off’ orientation by Cre.
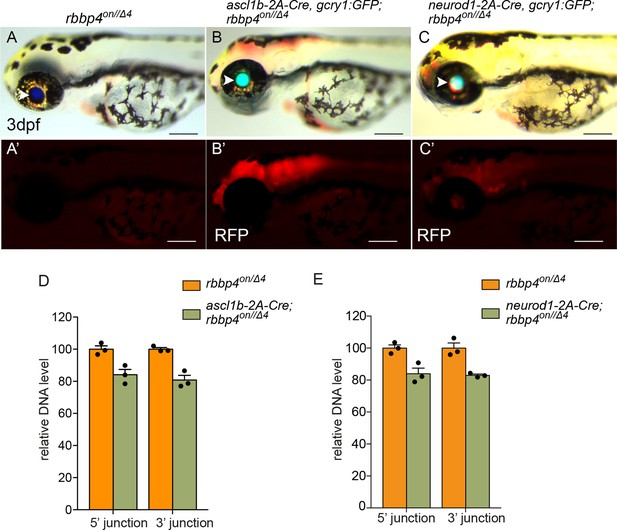
Induction of primary reporter RFP expression and qPCR quantification of rbbp4on allele inversion by ascl1b-2A-Cre and neurod1-2A-Cre.
Bright field and mRFP fluorescence images of rbbp4on/Δ4 (A, A’), ascl1b-2A-Cre; rbbp4on/Δ4 (B, B’), and neurod1-2A-Cre; rbbp4on/Δ4 (C, C’). White arrowheads point to blue lens expression from the rbbp4on allele and lens GFP expression from the 2A-Cre drivers. (D) Genomic DNA qPCR quantification of rbbp4on original orientation 5’ and 3 junctions in control rbbp4on (n=3) and ascl1b-2A-Cre; rbbp4on/Δ4 (n=3). (E) Genomic DNA qPCR quantification of rbbp4on original orientation 5’ and 3 junctions in control rbbp4on (n=3) and neurod1-2A-Cre; rbbp4on/Δ4 (n=3). Error bars represent mean ± s.e.m. Scale bars: 200 μm.
-
Figure 6—figure supplement 2—source data 1
Source data for qPCR quantification of rbbp4on inversion by ascl1b-2A-Cre and neurod1-2A-Cre.
- https://cdn.elifesciences.org/articles/71478/elife-71478-fig6-figsupp2-data1-v2.xlsx
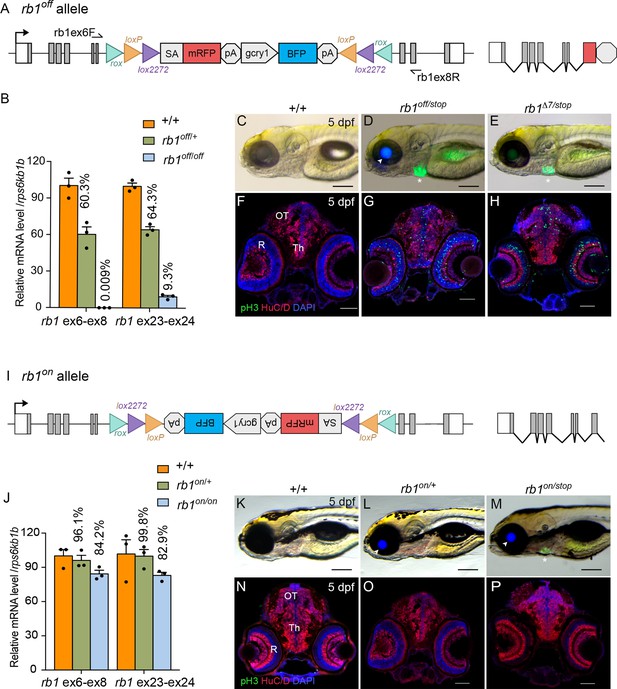
Molecular and phenotypic characterization of rb1off and rb1on alleles.
(A) Diagram of the rb1off allele. (B) Plot of RT-qPCR results from wild type +/+ (n=3), heterozygous rb1off/+ (n=3), and homozygous rb1off/off (n=3) larvae showing the relative level of rb1 mRNA transcript using reference gene rps6kb1b. Primer pairs were located in exons 6 and 8, or downstream exons 23 and 24. (C – E) Gross phenotype of wildtype +/+ (C), rb1off/stop (D), and rb1Δ7/stop (E) 5 dpf larvae. Arrowhead in D points to rb1off allele gcry1:BFP secondary reporter expression in lens. Asterisk marks the rb1stop allele mly7:GFP secondary reporter expression in heart. (F – H) pH3 and HuC/D labeling of sectioned head tissue from 5 dpf +/+ (F), rb1off/stop (G), and rb1Δ7/stop (H). (I) Diagram of the rb1on allele. (J) Plot of RT-qPCR results from wild type +/+ (n=3), heterozygous rb1on/+ (n=3), and homozygous rb1on/on (n=3) larvae showing the relative level of rb1 mRNA transcript using reference gene rps6kb1b. Primer pairs were located in exons 6 and 8, or downstream exons 23 and 24. (K, L) Gross phenotype of wildtype +/+ (K), heterozygous rb1on/+ (L) and transheterozygous rb1on/stop (M) 5 dpf larvae. Arrowhead in L, M points to rb1off allele gcry1:BFP secondary reporter expression in lens. Asterisk in M marks the rb1stop allele mly7:GFP secondary reporter expression in heart. pH3 and HuC/D labeling of sectioned head tissue from 5 dpf +/+ (N), heterozygous rb1on/+ (O) and transheterozygous rb1on/stop (P).OT, optic tectum; R, retina; Th, thalamic region. Error bars represent mean ± s.e.m. Scale bars: 200 μm (C–E, K–M), 50 μm (F–H, N–P).
-
Figure 7—source data 1
Source data for rb1 RT-quantitative PCR analysis in wildtype, heterozygous and homozygous embryos from rb1off/+ and rbbp4on/+ incrosses.
- https://cdn.elifesciences.org/articles/71478/elife-71478-fig7-data1-v2.xlsx
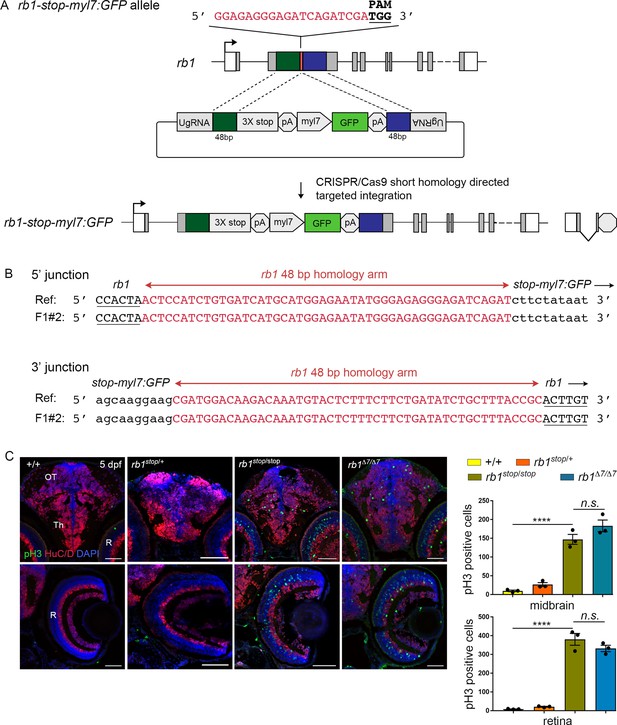
The rb1-stop integration allele recapitulates the rb1Δ7 indel loss-of-function phenotype.
(A) Diagram of rb1 gene model with exon 2 gRNA, the stop-PRISM-myl7:GFP targeting vector, and the resulting rb1-stop-myl7:GFP allele after GeneWeld CRISPR-Cas9 targeted integration. (B) F1 adult rb1-stop 5’ and 3’ junction analysis shows precise integration of the stop-PRISM cassette at the exon 2 target site. (C) Immunolocalization and quantification of pH3-labeled cells in control +/+ (n=3), rb1stop/+ (n=3), rb1stop/stop (n=3), and rb1Δ7/Δ7 (n=3) 5 dpf sectioned head tissue in the midbrain optic tectum and thalamic region (top row) and retina (bottom row) in both rb1stop/stop and rb1Δ7/Δ7 homozygotes. rb1stop/stop vs. +/+midbrain (**** p<0.0001) and retina (**** p<0.0001), rb1stop/stop vs. rb1Δ7/Δ7 midbrain (n.s. p=0.2951) and retina (n.s. p=0.1534). Th, thalamic region; OT, optic tectum; R, retina. Error bars represent mean ± s.e.m. with two-tailed t-test. Scale bars: 50 μm.
-
Figure 7—figure supplement 1—source data 1
Source data for quantification of pH3 positive cells in midbrain and retina of wildtype +/+, heterozygous rb1stop/stop, homozygous rb1stop/stop, and homozygous rb1Δ7/Δ7 5 dpf larva.
- https://cdn.elifesciences.org/articles/71478/elife-71478-fig7-figsupp1-data1-v2.xlsx
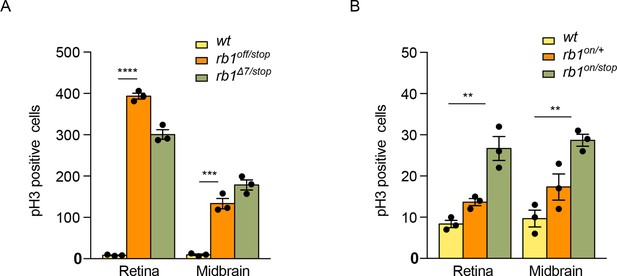
Quantification of pH3 labeled cells in rb1off/stop, rb1Δ7/stop, and rb1off/stop mutant larvae.
(A) Quantification of pH3 labeled cells in 5 dpf wild type +/+ (n=3), transheterozygous rb1off/stop (n=3), and transheterozygous rb1Δ7/stop (n=3) larvae. rb1off/stop larvae show a significant difference in pH3 levels from wildtype in the midbrain (*** p<0.001) and retina (**** p<0.0001). Associated with Figure 5 panels F, G, H. (B) Quantification of pH3-labeled cells in 5 dpf wild type +/+ (n=3), heterozygous rb1on/+(n=3), and transheterozygous rb1on/stop (n=3) larvae. rb1on/stop larvae show a significant difference in pH3 levels from wildtype in the midbrain (** p<0.01) and retina (** p<0.01). Error bars represent mean ± s.e.m. with two-tailed t-test. Associated with Figure 5 panels N, O, P.
-
Figure 7—figure supplement 2—source data 1
Source data for quantification of pH3 positive cells in midbrain and retina of wildtype +/+, heterozygous rb1on/+, and transheterozygous rb1on/stop 5 dpf larva.
- https://cdn.elifesciences.org/articles/71478/elife-71478-fig7-figsupp2-data1-v2.xlsx
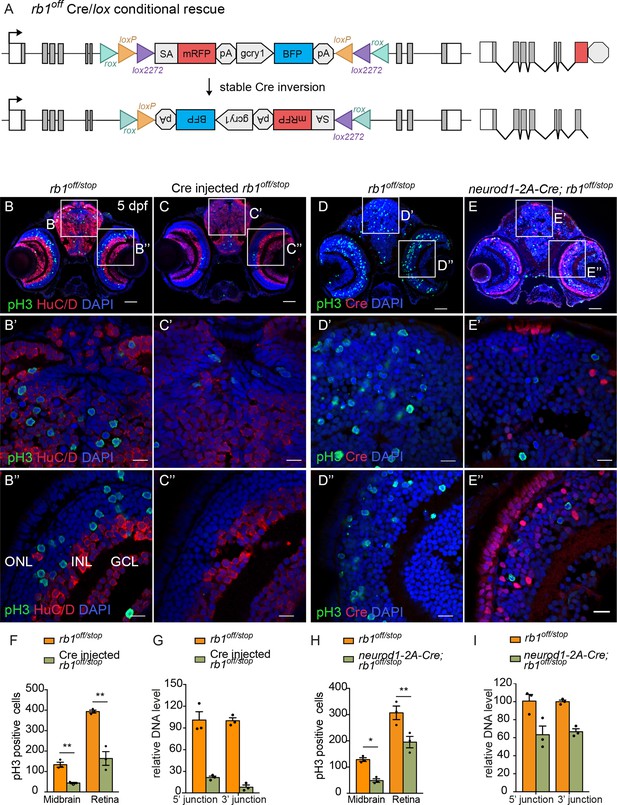
Ubiquitous and proneural neurod1-specific Cre-mediated conditional rescue with rb1off.
(A) Diagram of expected Cre-mediated inversion of rb1off to on orientation. (B, C) pH3 and HuC/D labeling of larval sectioned head tissue from 5 dpf transheterozygous rb1off/stop (B – B”) and Cre injected rb1off/stop (C – C”). (D, E) pH3 and Cre labeling of larval sectioned head tissue from 5 dpf transheterozygous rb1off/stop (D – D”) and neurod1-2A-Cre; rb1off/stop (E – E”). (F) Quantification of pH3-positive cells in control rb1off/stop (n=3) and Cre injected rb1off/stop (n=3) midbrain (** p<0.01) and retina (** p<0.01). (G) Genomic DNA qPCR quantification of rb1off original orientation DNA 5’ and 3’ junctions in control rb1off/stop (n=3) and Cre injected rb1off/stop (n=3). (H) Quantification of pH3-positive cells in rb1off/stop (n=3) and neurod1-2A-Cre; rb1off/stop (n=3) midbrain (** p<0.01) and retina (* p<0.05). (I) Genomic DNA qPCR quantification of rb1off original orientation DNA 5’ and 3’ junctions in control rb1off (n=3) and neurod1-2A-Cre; rb1off/stop (n=3). Error bars represent mean ± s.e.m. with two-tailed t-test. Scale bars: 50 μm (B - E), 10 μm (B’ – E”).
-
Figure 8—source data 1
Source data for quantification of pH3 labeling and rb1off inversion after Cre injection and neurod1-2A-Cre.
- https://cdn.elifesciences.org/articles/71478/elife-71478-fig8-data1-v2.xlsx
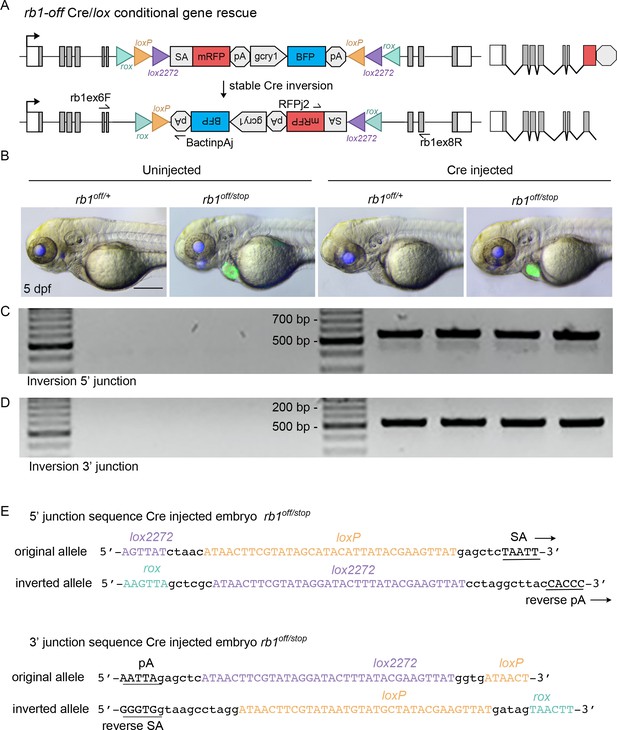
Molecular analysis of rb1off inversion and conditional rescue by Cre injection.
(A) Diagram of expected Cre-mediated inversion of rb1off to on orientation with primers for junction analysis. (B) Gross morphology in 5 dpf larva uninjected rb1off/+ and rb1off/stop, and Cre injected rb1off/+ and rb1off/stop. (C, D) 5’ and 3’ junctions of the inverted allele detected by genomic DNA PCR. (E) Sequence of the 5’ and 3 junction amplicons confirms rb1off stable inversion to the on orientation by Cre. Scale bar 200 μm (B).
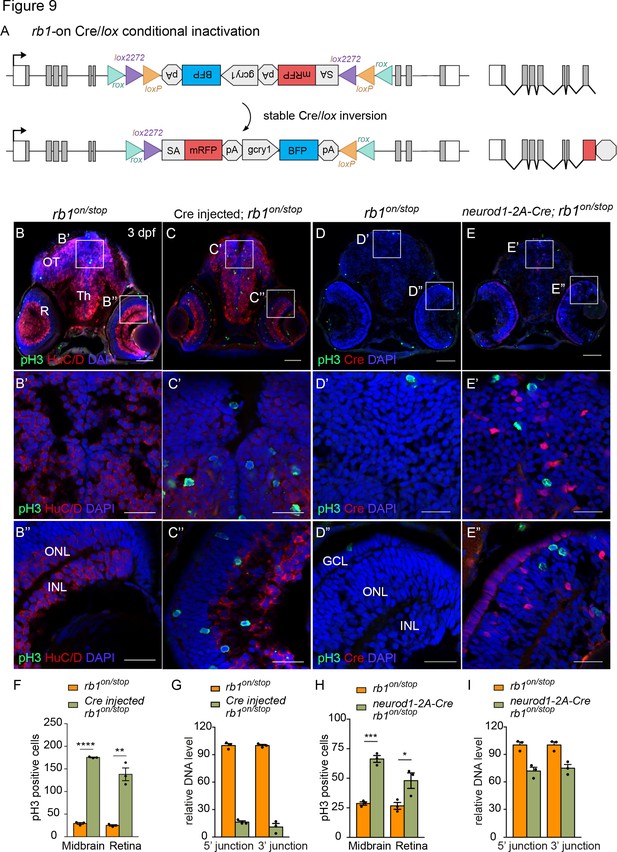
Ubiquitous and proneural neurod1-specific Cre-mediated conditional inactivation with rb1-on.
(A) Diagram of expected Cre mediated inversion of rb1on to ‘off’ orientation. (B, C) pH3 and HuC/D labeling of larval sectioned head tissue from 5 dpf transheterozygous rb1on/stop (B – B”) and Cre injected rb1on/stop (C – C”). (D, E) pH3 and Cre labeling of larval sectioned head tissue from 5 dpf transheterozygous rb1on/stop (D – D”) and neurod1-2A-Cre; rb1on/stop (E – E”). (F) Quantification of pH3-positive cells in control rb1on/stop (n=3) and Cre rb1on/stop (n=3) injected midbrain (**** p<0.0001) and retina (** p<0.01). (G) Genomic DNA qPCR quantification of rb1onoriginal orientation DNA 5’ and 3’ junctions in control rb1on/stop (n=3) and Cre injected rb1on/stop (n=3). (H) Quantification of pH3-positive cells in control rb1on/stop (n=3) and neurod1-2A-Cre; rb1on/stop (n=3) midbrain (*** p<0.001) and retina (* p<0.05). (I) Genomic DNA qPCR quantification of rb1on original orientation DNA 5’ and 3’ junctions in control rb1on/stop (n=3) control and neurod1-2A-Cre; rb1on/stop (n=3). Error bars represent mean ± s.e.m. with two-tailed t-test. Scale bars: 50 μm (B - E), 20 μm (B’ – E”).
-
Figure 9—source data 1
Source data for quantification of pH3 labeling and rb1on inversion after Cre injection and neurod1-2A-Cre.
- https://cdn.elifesciences.org/articles/71478/elife-71478-fig9-data1-v2.xls
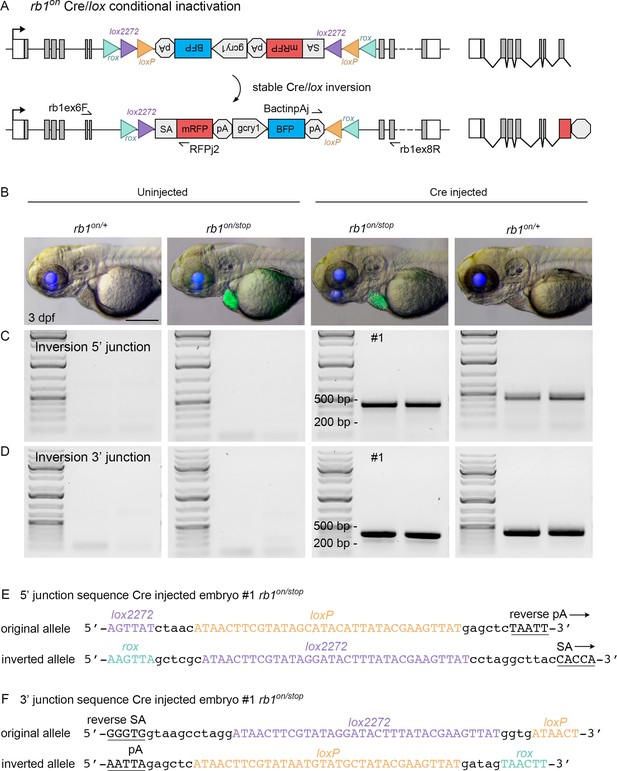
Molecular analysis of rb1-on inversion and conditional inactivation by Cre injection.
(A) Diagram of expected Cre-mediated inversion of rb1on to “off” orientation with primers for junction analysis. (B) Gross morphology in 5 dpf larva uninjected rbon/+ and rb1on/stop, and Cre injected rb1on/+ and rb1on/stop. (C, D) 5’ and 3’ junctions of the inverted allele detected by genomic DNA PCR. (E) Sequence of the 5’ and 3 junction amplicons confirms rb1on stable inversion to the “off” orientation by Cre. Scale bar 200 μm (B).
Tables
Genome intronic CRISPR gRNA sites and UFlip targeting vector homology arm sequences.
| Gene | Genomic sgRNA with PAM | 5' Homology arm | 3' Homology arm |
|---|---|---|---|
| rbbp4 | GTTGAAGGATTAAGAGTTAAGGG | AAAATCTGCTAGCTGTATATTGTTCTTATTTGAT GAAGAAGACCCTTA | ACTCTTAATCCTTCAACTTCGTTGCAAAAAAGTCA GTTGTGTAAAGGT |
| rb1 | ATTAGAAGAGAGTCCCAATGGGG | CCTTGATTACAGTTTCTGCTTTTGTGAG TGTACTGTAGTTTGCCCTAA | TCTTCTCTCAGGGTTACATGTTTAATGGATAGTGTGT CCATGTTGTCA |
Recovery of rbbp4 and rb1 UFlip floxed conditional alleles by GeneWeld CRISPR-Cas9 targeted integration.
| UFlip allele | Targeted Intron | Homology arm length bp/bp | Injected F0 embryo secondary marker expression | Germline transmission of secondary marker | Germline transmission of precise integration allele |
|---|---|---|---|---|---|
| rbbp4off is61 | 4 | 48/48 | 19% (26/134) | 29% (2/7) | 14% (1/7) |
| rb1off is58 | 6 | 48/48 | 47% (34/73) | 9% (3/33) | 6% (2/33) |
| rb1off is63 | 6 | 48/48 | 48% (60/125) | 25% (2/8) | 12.5% (1/8) |
| rb1on is57 | 6 | 48/48 | 43% (61/101) | 20% (5/25) | 4% (1/25) |
-
Table 2—source data 1
rbbp4 and rb1 UFlip embryo injection secondary marker F0 screening data and germline transmission data.
- https://cdn.elifesciences.org/articles/71478/elife-71478-table2-data1-v2.xlsx
Germline recovery of rbbp4on allele by Dre-mediated inversion of rbbp4off.
| Adult rbbp4off/+ | BFP-/RFP- embryos | BFP+/RFP + embryos (Off) original allele | BFP+/RFP- embryos (On) inverted allele | % transmission of On inverted allele/total BFP+ |
|---|---|---|---|---|
| F1 Female #1 | 48 | 37 | 0 | 0 |
| F1 Female #2 | 76 | 62 | 11 | 15% (11/73) |
| F1 Female #3 | 25 | 16 | 5 | 24% (5/21) |
| F1 Female #4 | 20 | 13 | 2 | 13% (2/15) |
| F1 Female #5 | 19 | 12 | 0 | 0 |
| F2 Female #1 | 18 | 24 | 2 | 7.7% (2/26) |
| F2 Female #2 | 50 | 25 | 19 | 43.2% (19/44) |
| F2 Female #3 | 36 | 40 | 4 | 9.1% (4/44) |
| F2 Female #4 | 22 | 14 | 0 | 0 |
| F2 Male #1 | 139 | 87 | 14 | 13.9% (14/101) |
| F2 Male #2 | 37 | 28 | 0 | 0 |
-
Experimental group 1: F1 embryos from the F0 founder rbbp4off/+ adult crossed to WIK were injected with 15 pg Dre mRNA. Inversion was confirmed by PCR on genomic DNA isolated from -injected embryos . 5 sibling injected embryos were raised to adulthood and were outcrossed to WIK. F2 embryos were screened for Primary (RFP+) and Secondary (BFP+) marker expression. Embryos inheriting the original rbbp4off allele show expression of both markers. Embryos inheriting an allele that was inverted from “off” to “on” by Dre recombination show expression of the secondary marker (BFP+) but lose expression of the primary marker due to the inverted orientation of the cassette. Experimental group 2: The same experiment was performed with an adult F1 rbbp4off/+ female crossed to WIK. F2 Dre mRNA injected embryos were raised to adulthood, and 7 adults screened for transmission of the inverted rbbp4on allele to the F3 generation.
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Gene (Danio rerio) | ascl1b | ensemble | ENSDARG00000009702 | |
| Gene (Danio rerio) | neurod1 | ensemble | ENSDARG00000019566 | |
| Gene (Danio rerio) | rb1 | ensemble | ENSDARG00000006782 | |
| Gene (Danio rerio) | rbbp4 | ensemble | ENSDARG00000029058 | |
| Strain, strain background (Danio rerio) | WIK | Zebrafish International Resource Center | ZIRC:ZL84 | Wildtype strain of zebrafish |
| Genetic reagent (Danio rerio) | Tg(ascl1b-2A-Cre) | McGrail lab | Tg(ascl1b-2A-Cre; gcry1:EGFP)is75 | Maintained in the lab of M. McGrail (Almeida et al., 2021) |
| Genetic reagent (Danio rerio) | Tg(neurod1-2A-Cre) | McGrail lab | Tg(neurod1-2A-Cre; gcry1:EGFP)is77 | Maintained in the lab of M. McGrail (Almeida et al., 2021) |
| Genetic reagent (Danio rerio) | rb1Δ7 | McGrail lab | rb1Δ7is54 | Maintained in the lab of M. McGrail (Solin et al., 2015) |
| Genetic reagent (Danio rerio) | rbbp4Δ4 | McGrail lab | rbbp4Δ4is60 | Maintained in the lab of M. McGrail (Schultz et al., 2018) |
| Genetic reagent (Danio rerio) | Tg(rb1-UFlip-on) | This paper | Tg(rb1-i6-UFlip-rox-lox2272-loxP-inverted<RFP;gcry1:BFP >lox2272-loxP-rox-on)is57 | Available from M. McGrail lab |
| Genetic reagent (Danio rerio) | Tg(rb1-UFlip-off) | This paper | Tg(rb1-i6-UFlip-rox-loxP-lox2272<RFP;gcry1:BFP >loxP-lox2272 -rox-off)is58 | Available from M. McGrail lab |
| Genetic reagent (Danio rerio) | Tg(rb1-UFlip-off) | This paper | Tg(rb1-i6-UFlip-rox-loxP-lox2272<2A-RFP;gcry1:BFP >loxP-lox2272 -rox-off)is63 | Available from M. McGrail lab |
| Genetic reagent (Danio rerio) | Tg(rbbp4-UFlip-off) | This paper | Tg(rbbp4-i4-UFlip-rox-lox2272-loxP-<2A-RFP;gcry1:BFP >lox2272-loxP-rox-off)is61 | Available from M. McGrail lab |
| Genetic reagent (Danio rerio) | Tg(rbbp4-UFlip-on) | This paper | Tg(rbbp4-i4-UFlip-rox-lox2272-loxP-<2A-RFP;gcry1:BFP >lox2272-loxP-rox-on)is62 | Available from M. McGrail lab |
| Genetic reagent (Danio rerio) | Tg(rb1-stop-PRISM) | This paper | Tg(rb1-3XSTOP; myl7:GFP)is59 | Available from M. McGrail lab |
| Recombinant DNA reagent | pT3TS-nCas9n | Wenbiao Chen | Addgene:46,757 | Plasmid for in vitro synthesis of Cas9 mRNA |
| Recombinant DNA reagent | pT3TS-Cre | Karl Clark | Plasmid for in vitro synthesis of Cre mRNA, available from K. Clark lab (Clark et al., 2011) | |
| Recombinant DNA reagent | pT3TS-Dre | Karl Clark | Dre cDNA (Anastassiadis et al., 2009) expression vector for in vitro mRNA synthesis, available from K. Clark lab | |
| Recombinant DNA reagent | p-CS2-KalTA4 | Martin Distel | Available from M. Distel lab (Distel et al., 2009) | |
| Recombinant DNA reagent | pUFlip-floxed-mRFP, gcry1:BFP | This paper | pUFlip(UgRNA-rox-loxP-lox2272<RFP; gcry1:BFP >loxP-lox2272-rox-UgRNA) | decommissioned |
| Recombinant DNA reagent | pUFlip-floxed-mRFP, myl7:BFP | This paper | pUFlip(UgRNA-rox-loxP-lox2272<RFP; myl7:BFP >loxP-lox2272-rox-UgRNA) | decommissioned |
| Recombinant DNA reagent | pUFlip-floxed-2A-mRFP, gcry1:BFP | This paper | pUFlip(UgRNA-rox-loxP-lox2272<2A-RFP; gcry1:BFP >loxP-lox2272-rox-UgRNA) | available from M. McGrail lab; Deposited at Addgene |
| Recombinant DNA reagent | pUFlip-floxed-2A-KalTA4, gcry1:BFP | This paper | pUFlip(UgRNA-rox-loxP-lox2272<2A-KalTA4; gcry1:BFP >loxP-lox2272-rox-UgRNA) | available from M. McGrail lab; Deposited at Addgene |
| Recombinant DNA reagent | pSTOP-PRISM-3Xstop-myl7:GFP | This paper | pPRISM(UgRNA-3XSTOP; myl7:GFP-UgRNA) | available from J. Essner lab; Deposited at Addgene |
| Sequence-based reagent | This paper | PCR primers and oligos | See Table 1 | |
| Commercial assay or kit | NEBuilder HiFi DNA Assembly Cloning kit | New England Biolabs | Catalog # E5520S | |
| Commercial assay or kit | PureYield Plasmid Miniprep System | Promega | Catalog # A1223 | |
| Commercial assay or kit | mMessage mMACHINE T3 Transcription Kit | Ambion | Catalog # AM1348 | |
| Commercial assay or kit | RNA Clean and Concentrator Kit (RCC) | Zymo | Catalog # R1013 | |
| Commercial assay or kit | pCR4 TOPO TA Cloning Kit | ThermoFisher/ Invitrogen | ThermoFisher:K457502 | |
| Commercial assay or kit | Superscript III | Invitrogen | Catalog # 18080093 | |
| Commercial reagent | SYBR Green | BioRad | Catalog # 1725271 | |
| Commercial reagent | Tissue-Tek O.C.T. Compound | Fisher | Catalog # 4,583 | |
| Software, algorithm | ICE | Synthego | Inference of CRISPR Edits (ICE) https://www.synthego.com/products/bioinformatics/crispr-analysis | Indel analysis of Sanger sequenced DNA |
| Software | Graphpad PRISM | Statistical analyses | ||
| Antibody | Rabbit polyclonal anti -Caspase-3a | BD Pharmingen | Catalog # 559,565 | (1:500) |
| Antibody | Mouse monoclonal anti-Cre Recombinase | Chemicon | Catalog # MAB3120 | (1:500) |
| Antibody | Mouse monoclonal anti-HuC/D | Invitrogen | Catalog # A21271 | (1:500) |
| Antibody | Mouse monoclonal anti-phospho-Histone H3 (Ser10) | Millipore | Catalog # 05–806 | (1:500) |
| Antibody | Rabbit polyclonal anti-phospho-Histone H3 | Millipore | Catalog # 06–570? | (1:1000) |
| Antibody | Rabbit polyclonal anti-Rbbp4 | Bethyl Laboratories | Catalog # A301-206A | (1:200) |
| Chemical compound, drug | DAPI | Invitrogen | Catalog # D1306 | (0.5 ug/ml) |
Primer oligonucleotide sequences.
| Primer name | Sequence | Purpose |
|---|---|---|
| rbbp4e × 4 F | ACCAAACACCCCTCCAAACCAG | intron sequencing, sgRNA analysis and genome/vector 5' junction analysis |
| rbbp4e × 5 R | AGTGCACTCTCCAGAGGGGT | intron sequencing, sgRNA analysis and genome/vector 3' junction analysis |
| rb1e × 6 F | CATGTTCCTCCTGGCCAAG | genome/vector 5' junction analysis |
| rb1e × 7 R | CACAAGGTCATCTTCCATCTG | genome/vector 3' junction analysis |
| rb1e × 2 F | GAGGAGCTCCAGTCCACTAAC | genotyping and genome/vector 5' junction analysis |
| rb1e × 2 R | CCCAAAACACAAGTGCGGTAA | genotyping and genome/vector 3' junction analysis |
| R-5'-junc-Stop-pPRISM | CGGTGGCTGAGACTTAATTACT | stop-PRISM genome/vector 5' junction analysis |
| F-3'-junc-(all)-pPRISM | TTCAGATCAATTAACCCTCACC | stop-PRISM genome/vector 3' junction analysis |
| RFPj | ATGACGTCCTCGGAGGAGGC | UFlip genome/vector junction analysis |
| RFPj2 | CCTTGGTCACCTTCAGCTTG | UFlip genome/vector junction analysis |
| BactinpAj | GCAAACGGCCTTAACTTTCC | UFlip genome/vector junction analysis |
| 2AR | CATAGGACCGGGGTTTTCTT | UFlip and 2A-Cre genome/vector junction analysis |
| rb1e × 6 F | CAGCTGGACCATGTTCCTCC | QPCR |
| rb1e × 8 R | CCCTGATTACGGCGTGATGT | QPCR |
| rbbp4e × 4 F | TAGTGACGTGCTGGTCTTTG | QPCR |
| rppb4e × 5 R | CAGGACAGACCATAACCTTCTT | QPCR |
| rbbp4e × 11_1 F | CTCTGTGTCTGAGGACAACATC | QPCR |
| rbbp4e × 12_1 R | TATCCCTGAACCTCAGTGTCT | QPCR |
| rps6kb1b_1 F | TCCTGATGACTCCACACTGA | QPCR |
| rps6kb1b_1 R | GGCGAGGTGAACGGATTT | QPCR |
| BFP_F | CTGCCTCATCTACAACGTCAA | genotyping |
| BFP_R | CTTAGCGGGTTTCTTGGATCTAT | genotyping |
| neujF | TCCAACTGAACCCCAGAACT | genotyping |
| ascjF | GTCAACATGGGCTTCCAGAC | genotyping |
| rbbp4e × 2 F | GCGTGATGACAGATCTCATATTGTTTTCCC | genotyping |
| rbbp4e × 2 R | CTGGTGACATCTGGCAACCACT | genotyping |
Additional files
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/71478/elife-71478-transrepform1-v2.docx
-
Source data 1
Gel image files.
- https://cdn.elifesciences.org/articles/71478/elife-71478-data1-v2.zip





