USP28 deletion and small-molecule inhibition destabilizes c-MYC and elicits regression of squamous cell lung carcinoma
Figures
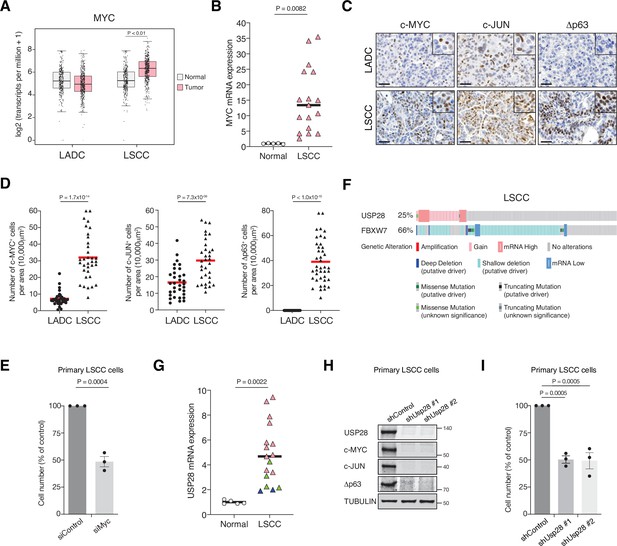
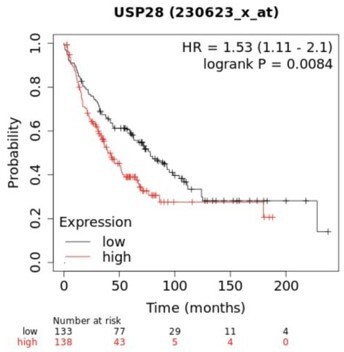
MYC, JUN, and Δp63 are highly expressed in lung squamous cell carcinoma (LSCC) tumours.
(A) Expression of MYC in human lung adenocarcinoma (LADC, n = 483), lung squamous cell carcinoma (LSCC, n = 486), and normal non‐transformed tissue (normal LSCC = 338, normal LADC = 347). In box plots, the centre line reflects the median. Data from TCGA and GTEx were analysed using GEPIA software. (B) Relative mRNA expression of MYC in normal lung tissue (n = 5) and LSCC (n = 17) patient samples from the Cordoba Biobank measured by RT-PCR. The p value was calculated using the Student’s two-tailed t test. Plots indicate mean. (C) Representative LADC and LSCC tumours stained with c-MYC, c-JUN, and Δp63 antibodies. Scale bars, 30 μm. (D) Quantification of c-MYC+ (LADC n = 33, LSCC n = 34), c-JUN+ (LADC n = 33, LSCC n = 33), and Δp63+ cells (LADC n = 41, LSCC n = 41) in LADC and LSCC tumours. Plots indicate mean. Student’s two-tailed t test was used to calculate p values. (E) Graph showing the difference in cell proliferation between control and MYC-depleted KF LSCC cells (n = 3). Graph indicates mean ± SEM. Student’s two-tailed t test was used to calculate p values. (F) Genetic alterations in ubiquitin-specific protease 28 (USP28) and FBXW7 genes in human LSCC. Each column represents a tumour sample (n = 178). Data from TCGA were analysed using cBioportal software. (G) Relative mRNA expression of USP28 in normal lung tissue (n = 5) and LSCC (n = 17) patient samples from the Cordoba Biobank measured by RT-PCR. The p value was calculated using the Student’s two-tailed t test. Plots indicate mean. See also Figure 1—figure supplement 1B. (H) shRNA-mediated knockdown of Usp28 decreases c-MYC, c-JUN, and Δp63 protein levels in primary KF LSCC cells. (I) Graph showing the difference in cell proliferation between control and Usp28-depleted KF LSCC cells (n = 3). Graph indicates mean ± SEM. One-way analysis of variance (ANOVA) with Dunnett’s multiple comparisons test was used to calculate p values. Source data for B, D, E, G, and I.
-
Figure 1—source data 1
c-MYC, c-JUN, Dp63 and USP28 are highly expressed in LSCC tumours.
- https://cdn.elifesciences.org/articles/71596/elife-71596-fig1-data1-v2.xlsx
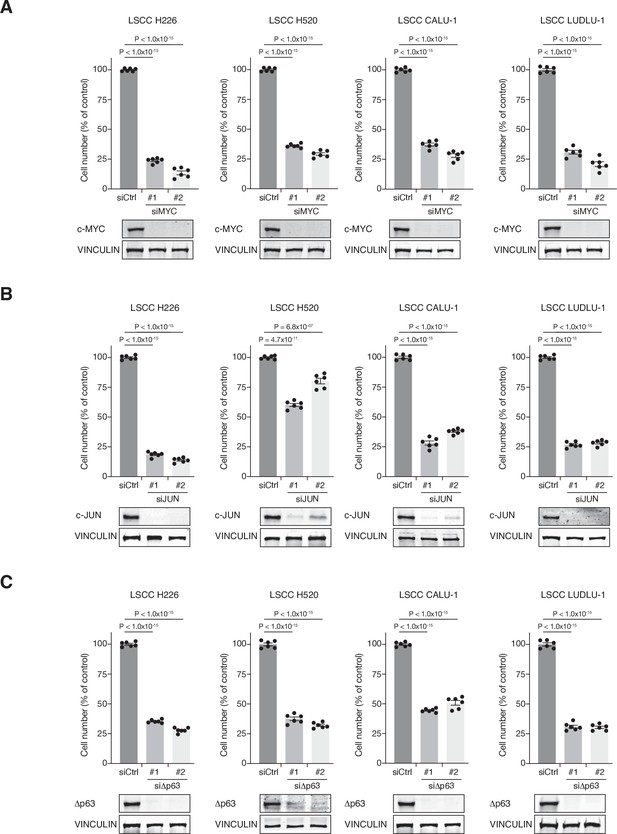
c-MYC, c-JUN and dp63 knockdown affects LSCC cell line growth.
(A) Graphs showing the difference in cell proliferation between control and siMYC-transfected human lung squamous cell carcinoma (LSCC) cell lines (NCI-H226, NCI-H520, CALU-1, and LUDLU-1). Graphs indicate mean ± SEM. p Values calculated using one-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test. (B) Graphs showing the difference in cell proliferation between control and siJUN-transfected human LSCC cell lines (NCI-H226, NCI-H520, CALU-1, and LUDLU-1). Graphs indicate mean ± SEM. p Values calculated using one-way ANOVA with Tukey’s multiple comparisons test. (C) Graphs showing the difference in cell proliferation between control and siΔp63-transfected human LSCC cell lines (NCI-H226, NCI-H520, CALU-1, and LUDLU-1). Graphs indicate mean ± SEM. p Values calculated using one-way ANOVA with Tukey’s multiple comparisons test. Source data for A, B, and C.
-
Figure 1—figure supplement 1—source data 1
c-MYC, c-JUN and Dp63 knockdown affect proliferation of human LSCC.
- https://cdn.elifesciences.org/articles/71596/elife-71596-fig1-figsupp1-data1-v2.xlsx
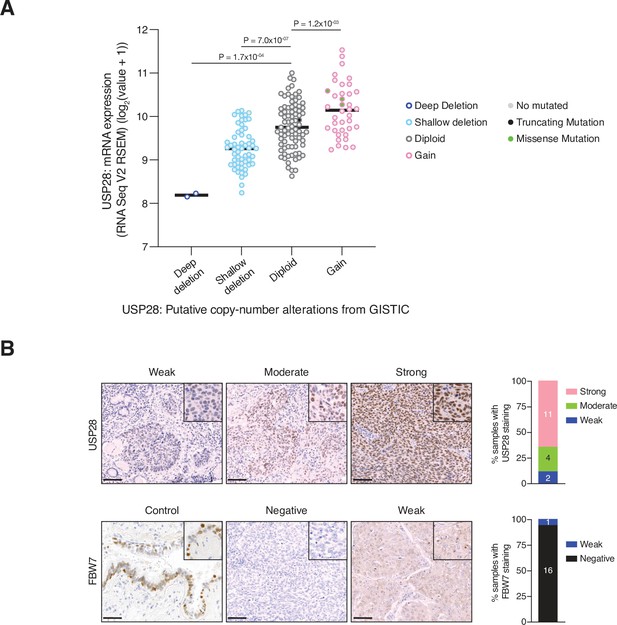
USP28 expression in LSCC tumours.
(A) Dot plot showing association between the log2 mRNA expression (Y-axis) and copy-number alterations (X-axis) for ubiquitin-specific protease 28 (USP28) gene. Data from TCGA were analysed using cBioportal software. One-way analysis of variance (ANOVA) with Bonferroni’s multiple comparisons test was used to calculate p values (n = 2 deep deletion, n = 57 shallow deletion, n = 81 diploid, n = 38 gain). (B) Representative human lung squamous cell carcinoma (LSCC) tumours stained with USP28 and FBW7 antibodies. Scale bars, 100 µm (left panel). Quantification of USP28 and FBW7 protein staining in LSCC tumours (n = 17) (right panel). Source data for A.
-
Figure 1—figure supplement 2—source data 1
USP28 copy-number vs mRNA expression in human LSCC patients.
- https://cdn.elifesciences.org/articles/71596/elife-71596-fig1-figsupp2-data1-v2.xlsx
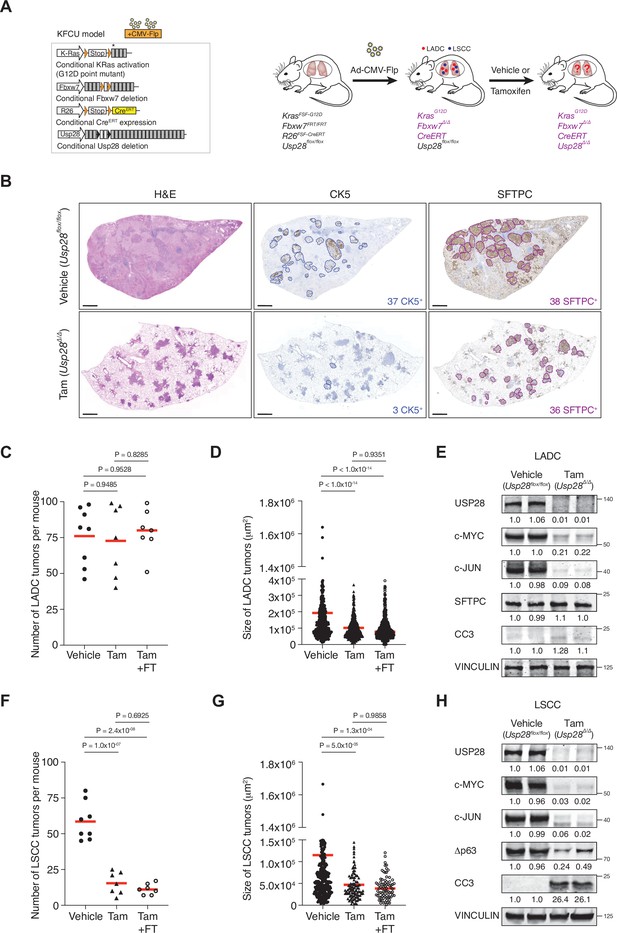
Ubiquitin-specific protease 28 (USP28) is an effective therapeutic target for lung squamous cell carcinoma (LSCC) tumours.
(A) Schematic representation of the KFCU (KrasFSF-G12D; Fbxw7FRT/FRT; Rosa26FSF-CreERT; Usp28flox/flox) model and experimental approach used to deplete conditional Usp28 alleles in established lung tumours. (B) Lung histology of animals treated as in A, showing both LSCC (CK5+) and lung adenocarcinoma (LADC) (SFTPC+) tumours in mice receiving vehicle but few LSCC lesions in mice receiving tamoxifen. Scale bars, 1000 μm. (C) Quantification of LADC tumours in vehicle-, tamoxifen-, and tamoxifen+ FT206-treated KFCU mice. Plots indicate mean. One-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test was used to calculate p values (n = 8 vehicle, n = 7 tamoxifen, n = 7 tamoxifen+ FT206). (D) Quantification of LADC tumour size in vehicle-, tamoxifen-, and tamoxifen+ FT206-treated KFCU mice. Plots indicate mean. One-way ANOVA with Tukey’s multiple comparisons test was used to calculate p values (n = 466 vehicle, n = 434 tamoxifen, n = 503 tamoxifen+ FT206). (E) Immunoblot analysis of LADC tumours probed for USP28, c-MYC, c-JUN, SFTPC, cleaved caspase-3 (CC3). VINCULIN is shown as loading control. (F) Quantification of LSCC tumours in vehicle-, tamoxifen-, and tamoxifen+ FT206-treated KFCU mice. Plots indicate mean. One-way ANOVA with Tukey’s multiple comparisons test was used to calculate p values (n = 8 vehicle, n = 7 tamoxifen, n = 7 tamoxifen+ FT206). (G) Quantification of LSCC tumour size in vehicle-, tamoxifen-, and tamoxifen+ FT206-treated KFCU mice. Plots indicate mean. One-way ANOVA with Tukey’s multiple comparisons test was used to calculate p values (n = 326 vehicle, n = 103 tamoxifen, n = 79 tamoxifen+ FT206). (H) Usp28 deletion induces apoptotic cell death (CC3) and decreases c-MYC, c-JUN, and Δp63 protein levels in LSCC lesions. Source data for C, D, F, and G.
-
Figure 2—source data 1
Quantification of LADC and LSCC tumours in the KFCU model.
- https://cdn.elifesciences.org/articles/71596/elife-71596-fig2-data1-v2.xlsx
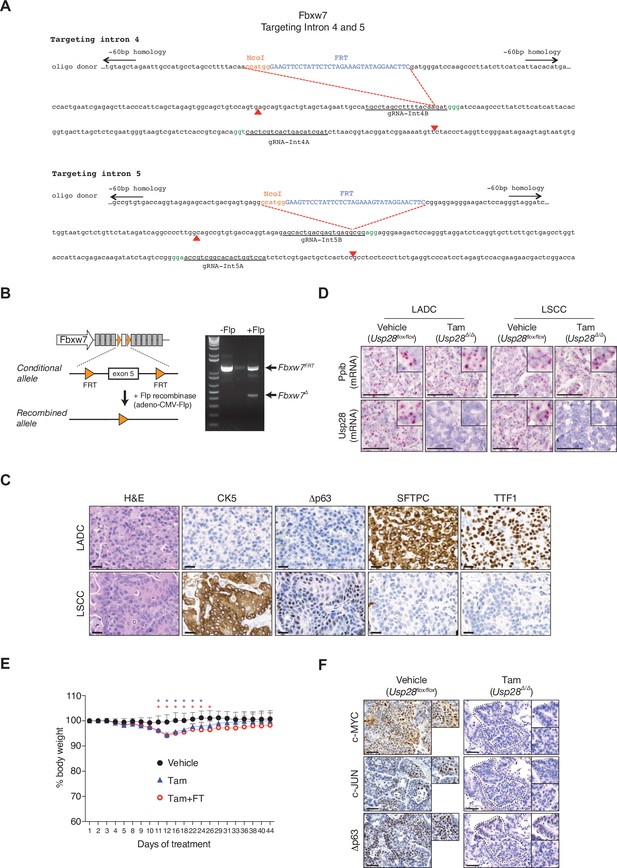
Gene 1 targeting strategy to generate a Fbxw7 FRT/FRT allele that can be deleted by Flp recombinase.
(A) Gene targeting strategy to generate conditional Fbxw7FRT/FRT animals. Two FRT sites were inserted into the intron 4 and 5 of Fbxw7 through the CRISPR-Cas9 technology. (B) Schematic representation of the conditional allele (left panel). In vitro recombination assay demonstrated efficient ablation of the exon 5 upon Flp recombinase adenovirus infection (right panel). (C) KFCU (KrasFSF-G12D; Fbxw7FRT/FRT; Rosa26FSF-CreERT; Usp28flox/flox) mice infected with adeno-CMV-Flp virus develop lung adenocarcinoma (LADC) (SFTPC+ and TTF1+) and lung squamous cell carcinoma (LSCC) (CK5+ and Δp63+) tumours. (D) In situ hybridization of ubiquitin-specific protease 28 (Usp28) and Pppib mRNA expression in vehicle- and tamoxifen-treated KFCU mice. Scale bars, 50 µm. (E) Monitoring tolerability in mice treated with vehicle, tamoxifen (Tam), or tamoxifen+ FT206. Body weights of animals during the course of treatment. Two-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test was used to calculate p values (n = 8 vehicle, n = 7 tamoxifen, n = 7 tamoxifen+ FT206). (F) KFCU tumours stained with c-MYC, c-JUN, and Δp63 antibodies. KFCU mice treated with vehicle (left panel) or tamoxifen (right panel). Inserts showing c-MYC+, c-JUN+, and Δp63+ LSCC tumours in mice receiving vehicle but partial positive or negative LSCC lesions in mice receiving tamoxifen. Scale bars, 50 μm. Source data for E.
-
Figure 2—figure supplement 1—source data 1
Body weights of animals treated with Vehicle, Tamoxifen (Tam) or Tamoxifen+FT206.
- https://cdn.elifesciences.org/articles/71596/elife-71596-fig2-figsupp1-data1-v2.xlsx
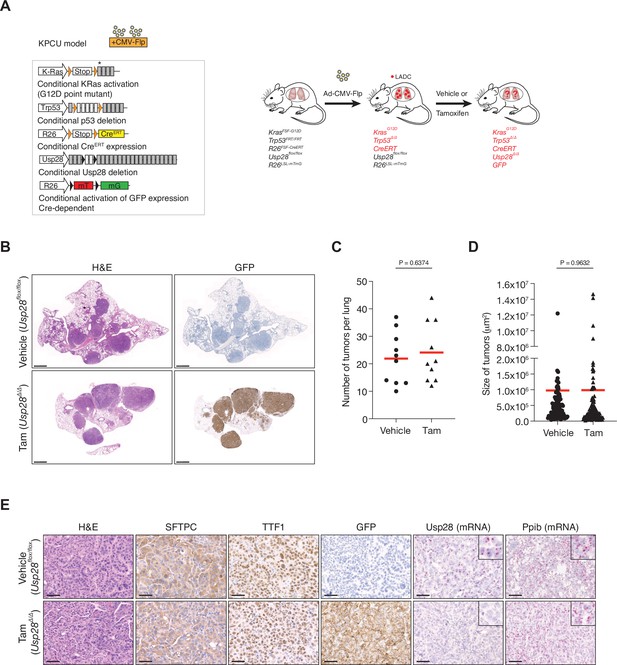
Ubiquitin-specific protease 28 (USP28) is not a therapeutic target for advanced KRasG12D; Trp53 mutant tumours.
(A) Schematic representation of the KPCU (KRasFSF-G12D; Trp53FRT/FRT; Rosa26FSF-CreERT; Usp28flox/flox; Rosa26LSL-mTmG) model and experimental approach used. At 10 weeks post-infection, KPCU mice were treated with vehicle or tamoxifen. (B) Representative images of H&E (left) and GFP (right) stains from mice of the indicated treatments. Scale bar, 1000 µm. (C) Quantification of mouse lung adenocarcinoma (LADC) tumours in the KPCU model. Plots indicate mean. Student’s two-tailed t test was used to calculate p values (n = 10 vehicle, n = 10 tamoxifen). (D) Quantification of LADC tumour size in vehicle- and tamoxifen-treated KPCU mice. Plots indicate mean. Student’s two-tailed t test was used to calculate p values (n = 110 vehicle, n = 130 tamoxifen). (E) Representative images illustrating histological analysis of lung lesions in KPCU mice, treated with vehicle or tamoxifen. H&E, SFTPC, TTF1, GFP immunohistochemistry staining and in situ hybridization of USP28 and PPIB mRNA expression. Scale bars, 50 µm. Source data for C and D.
-
Figure 3—source data 1
Quantification of LADC tumours in the KPCU model.
- https://cdn.elifesciences.org/articles/71596/elife-71596-fig3-data1-v2.xlsx
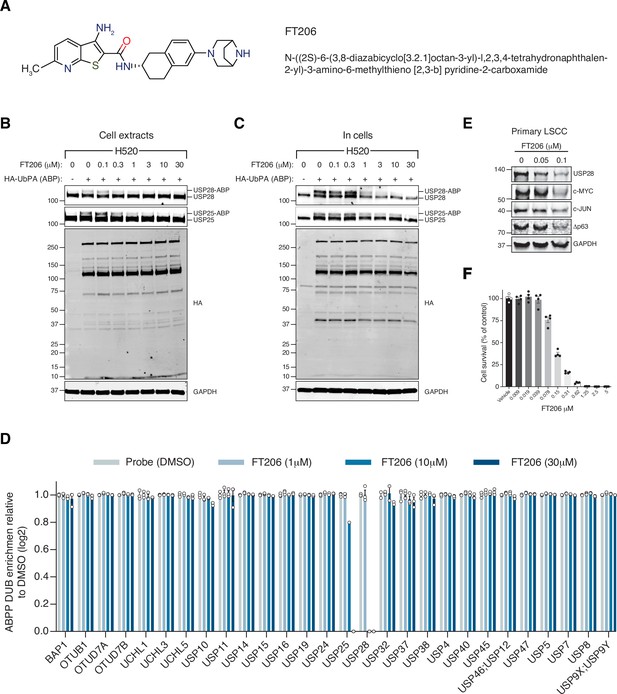
Ubiquitin-specific protease 28 (USP28) inhibitor selectivity and cellular target engagement.
(A) Structure of small-molecular inhibitor FT3951206/CRT0511973 (FT206). (B) Cellular DUB profiling in NCI-H520 lung squamous cell carcinoma (LSCC) cell extracts incubated with the indicated concentrations of FT206 prior to labelling with HA-UbPA, SDS-PAGE, and analysis by Western blotting. Inhibitor potency was reflected by competition with USP28/25-ABP (activity-based probe) adduct formation. (C) Cellular DUB profiling in NCI-H520 LSCC cells incubated with the indicated concentrations of FT206, lysed extracts labelled with HA-UbPA, and analysed as in B. (D) Activity-based probe profiling (ABPP) demonstrating the cellular DUB selectivity profile of cpd FT206 by quantitative mass spectrometry analysis at different inhibitor concentrations. Graph indicates mean ± SEM. (E) USP28 inhibition using FT206 (50 and 100 nM) reduces c-MYC, c-JUN, and Δp63 protein levels in primary KF LSCC cells. (F) USP28 inhibition using FT206 decreases cell proliferation in KF LSCC cells (n = 4). Graph indicates mean ± SEM. Source data for F.
-
Figure 4—source data 1
Activity-based Probe Profiling (ABPP) showing the cellular DUB selectivity profile of FT206 assessed by quantitative mass spectrometry.
- https://cdn.elifesciences.org/articles/71596/elife-71596-fig4-data1-v2.xlsx
-
Figure 4—source data 2
FT206 decreases cell proliferation in LSCC cells.
- https://cdn.elifesciences.org/articles/71596/elife-71596-fig4-data2-v2.pptx
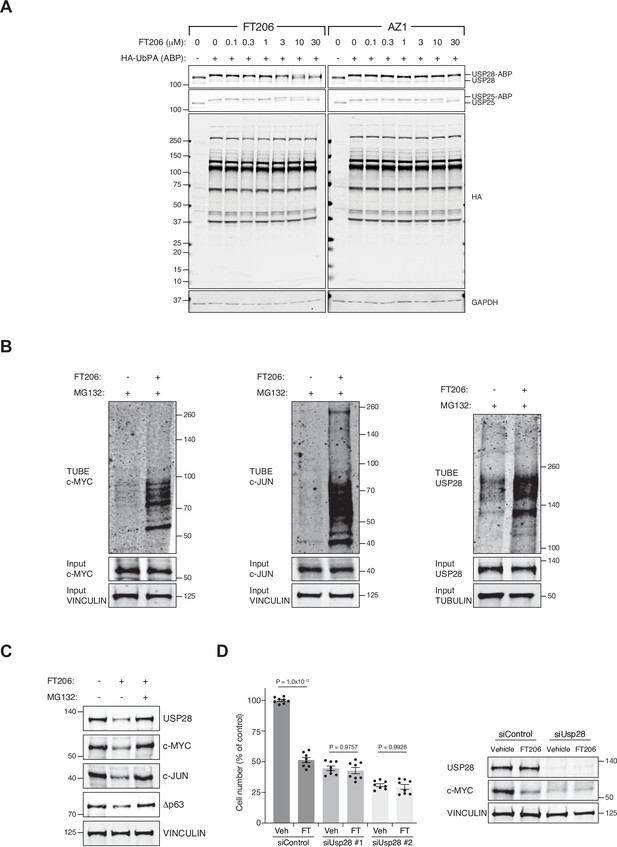
USP28 inhibitor targets USP28/25 and ubiquitylation levels of c-MYC, c-JUN and USP28.
(A) Comparison of ubiquitin-specific protease 28/25 (USP28/25) inhibitor potency by activity-based profiling. Human lung squamous cell carcinoma (LSCC) H520 crude cell extracts were incubated either with AZ1 or FT206 inhibitors at indicated concentrations, followed by HA-UbPA activity-based probe (ABP) labelling. Samples were analysed by SDS-PAGE and immunoblotted using USP28, USP25, HA, and GAPDH antibodies. Inhibitor potency was reflected by competition with USP28/25-ABP adduct formation. (B) TUBE pulldown of endogenous ubiquitylated c-MYC, c-JUN, and USP28 in LSCC cells upon co-treatment with MG132 and FT206. (C) Immunoblot of endogenous USP28, c-JUN, c-MYC, and Δp63 in LSCC cells upon co-treatment with MG132 and FT206. VINCULIN served as loading control. (D) Graphs showing the difference in cell proliferation between control, FT206-treated, and USP28-depleted LSCC cells. Graph indicates mean ± SEM. One-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test was used to calculate p values. VINCULIN is shown as loading control. Source data for D.
-
Figure 4—figure supplement 1—source data 1
Cell proliferation in control, FT206-treated and USP28-depleted LSCC cells.
- https://cdn.elifesciences.org/articles/71596/elife-71596-fig4-figsupp1-data1-v2.xlsx
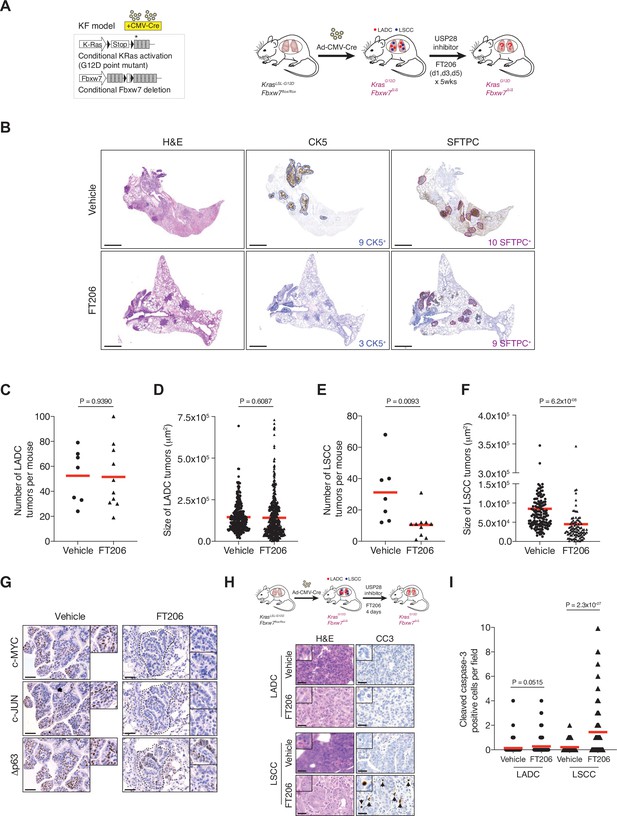
Pharmacological ubiquitin-specific protease 28 (USP28) inhibition reduces c-MYC, c-JUN, and Δp63 protein levels in mouse lung squamous cell carcinoma (LSCC) tumours and induces tumour cell death.
(A) Scheme depicting experimental design for in vivo test of FT206 (75 mg/kg), three times a week for 5 weeks. (B) Lung histology of animals treated as in A, showing both LSCC (CK5+) and lung adenocarcinoma (LADC) (SFTPC+) tumours in KRasLSL-G12D; Fbxw7f/f (KF) mice receiving vehicle but few LSCC lesions in mice receiving FT206. Scale bars, 1000 μm. (C) Quantification of LADC tumours per animal in vehicle- and FT206-treated KF mice. Plots indicate mean. p Values calculated using Student’s two-tailed t test (n = 7 vehicle, n = 10 FT206). (D) Quantification of LADC tumour size in vehicle- and FT206-treated KF mice. Plots indicate mean. Student’s two-tailed t test was used to calculate p values (n = 304 vehicle, n = 481 FT206). (E) Quantification of LSCC tumours per animal in vehicle- and FT206-treated KF mice. Plots indicate mean. p Values calculated using Student’s two-tailed t test (n = 7 vehicle, n = 10 FT206). (F) Quantification of LSCC tumour size in vehicle- and FT206-treated KF mice. Plots indicate mean. Student’s two-tailed t test was used to calculate p values (n = 156 vehicle, n = 96 FT206). (G) LSCC tumours stained with c-MYC, c-JUN, and Δp63 antibodies. KF animals treated with vehicle (left panel) or FT206 (right panel). Inserts showing c-MYC+, c-JUN+, Δp63+ LSCC tumours in mice receiving vehicle (left panel) but partial positive or negative LSCC lesions in mice receiving FT206 (right panel). Scale bars, 50 μm. (H) Scheme depicting experimental design for in vivo test of FT206 (75 mg/kg) for 4 days consecutively (upper panel). Cleaved caspase-3 (CC3) stain shows apoptotic cells (bottom panel). Scale bars, 50 μm. (I) Quantification of CC3-positive cells per field (20×) in LADC (n = 114 vehicle, 203 FT206) and LSCC (n = 94 vehicle, 167 FT206) tumours from KF mice treated as in H. Plots indicate mean. Student’s two-tailed t test was used to calculate p values. Source data for C, D, E, F, and I.
-
Figure 5—source data 1
Quantification of LADC and LSCC tumours in the KF model.
- https://cdn.elifesciences.org/articles/71596/elife-71596-fig5-data1-v2.xlsx
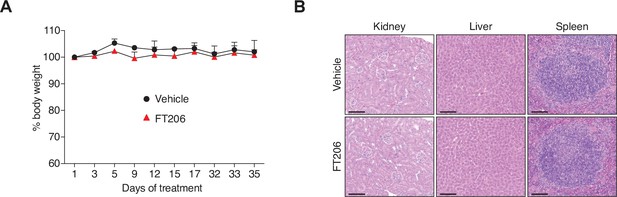
USP28 inhibitor FT206 tolerability in mice.
(A) Monitoring tolerability in mice treated with FT206 (75 mg/kg), three times a week for 5 weeks. Body weights of animals during the course of treatment (n = 3 vehicle, n = 3 FT206). (B) Kidney, liver, and spleen sections stained with H&E. Mice treated as in A. Bars, 100 μm. Source data for A.
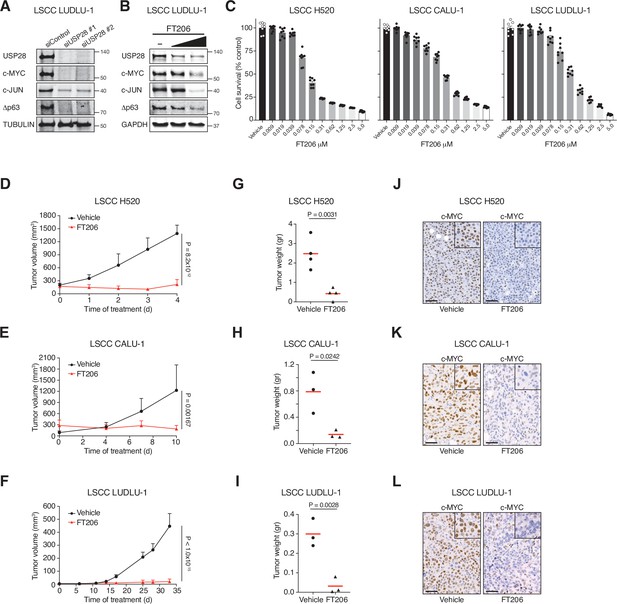
Pharmacological inhibition of ubiquitin-specific protease 28 (USP28) prevents human lung squamous cell carcinoma (LSCC) tumour progression and reduces c-MYC protein levels in xenograft models.
(A) Small interfering RNA (siRNA)-mediated knockdown of USP28 decreases c-MYC, c-JUN, and Δp63 protein levels in human LUDLU-1 LSCC cells. (B) USP28 inhibition using FT206 (0.2 and 0.4 μM) reduces c-MYC, c-JUN, and Δp63 protein levels in human LUDLU-1 LSCC cells. (C) USP28 inhibition using FT206 decreases cell proliferation in human LSCC (NCI-H520, CALU-1, and LUDLU-1) cell lines (n = 8). Graphs indicate mean ± SEM. (D, E, F) In vivo tumour graft growth curves of human LSCC (NCI-H520, CALU-1, and LUDLU-1) cell lines subcutaneously injected in flanks of immunocompromised mice. Animals with palpable tumours were treated with vehicle or FT206 (75 mg/kg) via oral gavage. Plots indicate mean ± SD of the tumour volumes. p Values calculated from two-way analysis of variance (ANOVA) with Bonferroni’s multiple comparisons test (NCI-H520 n = 4 vehicle and 4 FT206; CALU-1 n = 3 vehicle and 3 FT206; LUDLU-1 n = 3 vehicle and 3 FT206). (G, H, I) Mice treated as in D, E, and F, respectively. Plots showing the weight of xenograft tumours at the end point. Student’s two-tailed t test was used to calculate p values (NCI-H520 n = 4 vehicle and 4 FT206; CALU-1 n = 3 vehicle and 3 FT206; LUDLU-1 n = 3 vehicle and 3 FT206). (J, K, L) c-MYC immunohistochemistry stainings of NCI-H520, CALU-1, and LUDLU-1 xenografts in mice treated as in D, E, and F, respectively. Scale bars, 50 μm. Source data for C, D, E, F, G, H, and I.
-
Figure 6—source data 1
USP28 inhibition impairs tumour growth in human LSCC xenografts.
- https://cdn.elifesciences.org/articles/71596/elife-71596-fig6-data1-v2.xlsx
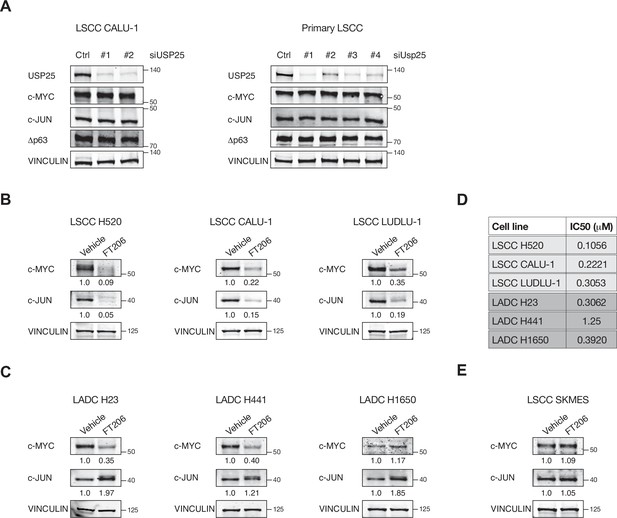
USP25 deletion does not affect c-MYC,c-JUN and Δp63 protein levels.
(A) Immunoblot of endogenous ubiquitin-specific protease 25 (USP25), c-JUN, c-MYC, and Δp63 in USP25-depleted lung squamous cell carcinoma (LSCC) cells. VINCULIN served as loading control. (B) Immunoblot of endogenous c-MYC and c-JUN in LSCC cells upon FT206 treatment (IC50 doses display in panel D). VINCULIN served as loading control. (C) Immunoblot of endogenous c-MYC and c-JUN in lung adenocarcinoma (LADC) cells upon FT206 treatment (IC50 doses display in panel D). VINCULIN served as loading control. (D) IC50 values (doses that inhibits 50% of the cell viability) were calculated after exposure of human LADC and LSCC cells to different concentrations of FT206 compound. (E) Immunoblot of endogenous c-MYC and c-JUN in USP28 mutant LSCC cells upon FT206 treatment. The LSCC cell line SKMES contains a nonsense mutation in Usp28 (c.193G > T). VINCULIN served as loading control.
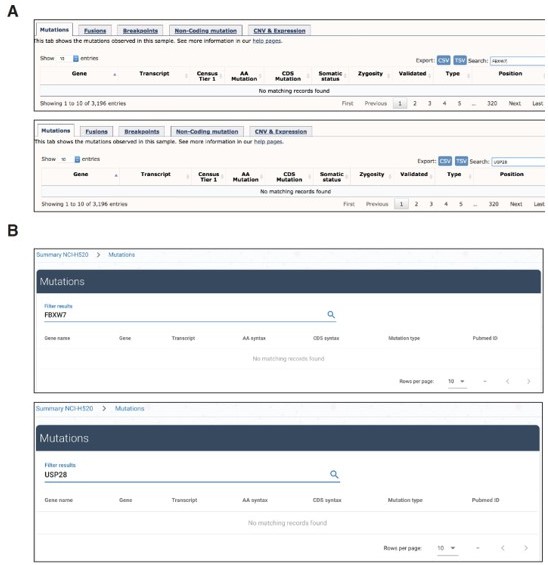
Analysis in COSMIC (panel A) or canSAR (panel B) databases showing no evidence of mutations in USP28 nor FBXW7 genes.
Tables
Primers for quantitative polymerase chain reaction (qPCR).
| Name | Primer (5′–3′) | |
|---|---|---|
| Forward | Reverse | |
| ACTIN | GAAAATCTGGCACCACACCT | TAGCACAGCCTGGATAGCAA |
| USP28 | ACTCAGACTATTGAACAGATGTACTGC | CTGCATGCAAGCGATAAGG |
| MYC | TCTCCTTGCAGCTGCTTAG | GTCGTAGTCGAGGTCATAG |
List of reagents.
| Reagent | Source | Identifier |
|---|---|---|
| Antibodies | ||
| Rabbit anti-CK5 | Abcam | Abcam Cat# ab52635, RRID:AB_869890 |
| Rabbit anti-c-MYC | Abcam | Abcam Cat# ab32072, RRID:AB_731658 |
| Goat anti-GFP | Abcam | Abcam Cat# ab6673, RRID: AB_305643 |
| Rabbit anti-Ki67 | Abcam | Abcam Cat# ab16667, RRID: AB_302459 |
| Rabbit anti-TTF1 | Abcam | Abcam Cat# ab76013, RRID:AB_1310784 |
| Rabbit anti-USP28 | Abcam | Abcam Cat# ab126604, RRID:AB_11127442 |
| Rabbit anti-USP25 | Abcam | Abcam Cat# ab187156 |
| Rabbit anti-ACTIN | Abcam | Abcam Cat# ab8227, RRID:AB_2305186 |
| Rabbit anti-USP28 | Atlas | Atlas Antibodies Cat# HPA006779, RRID:AB_1080517 |
| Rabbit anti-Δp63 | BioLegend | BioLegend Cat# 619001, RRID:AB_2256361 |
| Mouse anti-c-JUN | BD Biosciences | BD Biosciences Cat# 610326, RRID:AB_397716 |
| Rabbit anti-FBW7 | Bethyl | Bethyl Cat# A301-721A, RRID:AB_1210898 |
| Rabbit anti-USP7 | Enzo | Enzo Life Sciences Cat# BML-PW0540, RRID:AB_224147 |
| Mouse anti-GAPDH | Invitrogen | Thermo Fisher Scientific Cat# MA5-15738, RRID:AB_10977387 |
| Rabbit anti-SFTPC | Millipore | Millipore Cat# AB3786, RRID:AB_91588 |
| Rabbit anti-caspase-3 active | R&D Systems | R&D Systems Cat# AF835, RRID:AB_2243952 |
| Rat anti-HA | Roche | Roche Cat# 11666606001, RRID:AB_514506 |
| Mouse anti-TUBULIN | Sigma | Sigma-Aldrich Cat# T5168, RRID:AB_477579 |
| Mouse anti-VINCULIN | Sigma | Sigma-Aldrich Cat# V9131, RRID:AB_477629 |
| Virus strains | ||
| Adeno-CMV-Cre | UI viral vector core | VVC-U of Iowa-5-HT |
| Adeno-CMV-Flp | UI viral vector core | VVC-U of Iowa-530HT |
| Chemicals, peptides, and recombinant proteins | ||
| Doxycycline hyclate | Sigma | D9891 |
| Tamoxifen | Sigma | T5648 |
Additional files
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/71596/elife-71596-transrepform1-v2.pdf
-
Source data 1
Complete immunblots and gel figures.
- https://cdn.elifesciences.org/articles/71596/elife-71596-supp1-v2.zip