Classification and genetic targeting of cell types in the primary taste and premotor center of the adult Drosophila brain
Figures
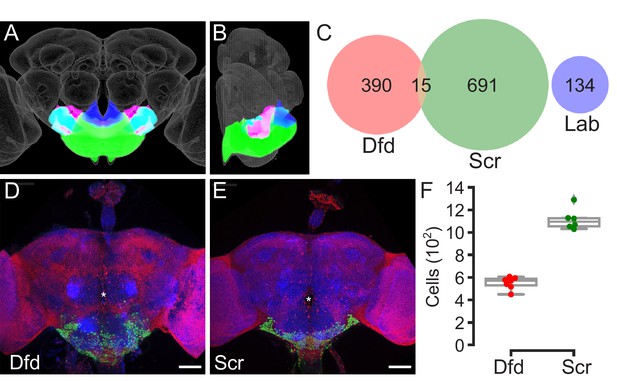
Estimating the number of cells in the subesophageal zone (SEZ).
(A, B) Anterior (A) and medial (B) views of the central brain of the Drosophila melanogaster adult showing the location of the gnathal ganglia (GNG, green), saddle (SAD, fuchsia), antennal mechanosensory and motor center (AMMC, cyan), and prow (royal blue) in relation to the JRC 2018 unisex brain template (grey). Together, the GNG, SAD, AMMC, and PRW compose the SEZ. (C) Venn diagram of single cells with detectable Dfd (red), Scr (green), and/or lab (blue) as assessed with a single-cell transcriptome atlas. (D, E) Example overview images of the samples used to count the number of cells expressing Dfd-LexA or Scr-LexA. LexAop-nls-GCAMP6s (green) driven by Dfd-LexA (D) or Scr-LexA (E) in the adult central brain. All nuclei are labeled with His2Av-mRFP (red) and neuropil is labeled with nc82 (blue). Asterisks denote the location of the esophageal foramen. Scale bars, 50 μm. (F) Box plots displaying counts of cell bodies labeled by both His2Av-RFP and LexAop-nls-GCaMP6s when driven with Dfd-LexA (n = 7) or Scr-LexA (n = 6). Whiskers denote spread of samples within 1.5 interquartile range from the mean.
-
Figure 1—source data 1
Dfd-LexA and Scr-LexA cell counts for panel F.
- https://cdn.elifesciences.org/articles/71679/elife-71679-fig1-data1-v2.csv
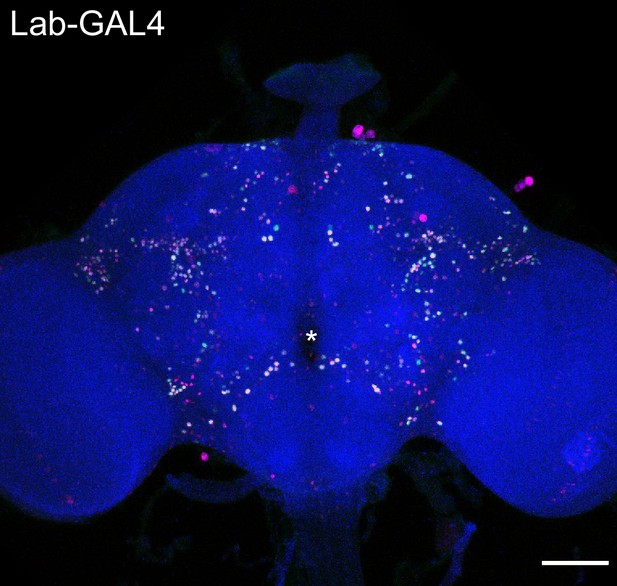
Expression of labial-GAL4 in the adult central brain.
Labial-GAL4 driving UAS-nls-GCaMP6s (green) and UAS-His2Av-mRFP (fuchsia) in an adult female brain. Neuropil is labeled with nc82 (blue). Asterisk denotes the location of the esophageal foramen. Note that cell bodies throughout the central brain are labeled. Scale bar 50 μm.
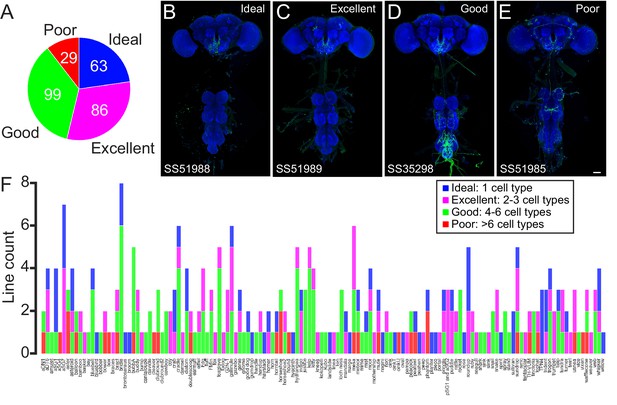
Quality of the lines in the subesophageal zone (SEZ) Split-GAL4 Collection.
(A) The proportion of (royal blue), excellent (fuchsia), good (green), and poor (red) split-GAL4 lines included in the collection. (B–E) Examples of lines from each quality class. An example central nervous system is shown for each line. Expression pattern of the UAS reporter is shown in green, while neuropil is labeled with nc82 in blue. Scale bar is 50 μm. Each split-GAL4 line labels the same cell type, sundrop, but is of ideal (B), excellent (C), good (D), or poor (E) quality. (F) The number of ideal (royal blue), excellent (fuchsia), good (green), and poor (red) split-GAL4 lines included in the collection arranged by targeted neuron type.
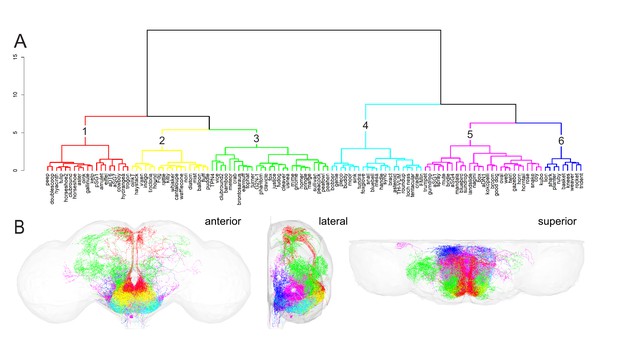
Hierarchical clustering of subesophageal zone (SEZ) neuronal cell types.
(A) Clustering of SEZ neuron types with NBLAST reveals six distinct morphological groups: group 1: red; group 2: yellow; group 3: green; group 4: cyan; group 5: fuchsia; group 6: royal blue. Group number is indicated by the black number above each cluster. The vertical axis represents the distance or dissimilarity between the clusters. (B) Morphology of all neuron types in each cluster plotted according to the color code in (A). Central brain neuropil (gray) is plotted for reference. Anterior (left), lateral (middle), and superior (right) views are shown.
-
Figure 3—source code 1
R code used for NBLAST clustering and visualization of cell type clusters.
- https://cdn.elifesciences.org/articles/71679/elife-71679-fig3-code1-v2.zip
-
Figure 3—source code 2
Neuronlist object composed of dotprops, called by the NBLAST clustering code.
- https://cdn.elifesciences.org/articles/71679/elife-71679-fig3-code2-v2.zip
-
Figure 3—source code 3
Template surface data file for visualizing brain neuropil, called in the NBLAST clustering code.
- https://cdn.elifesciences.org/articles/71679/elife-71679-fig3-code3-v2.zip
-
Figure 3—source data 1
Metadata for the neuronlist in Figure 3—source code 2.
- https://cdn.elifesciences.org/articles/71679/elife-71679-fig3-data1-v2.csv
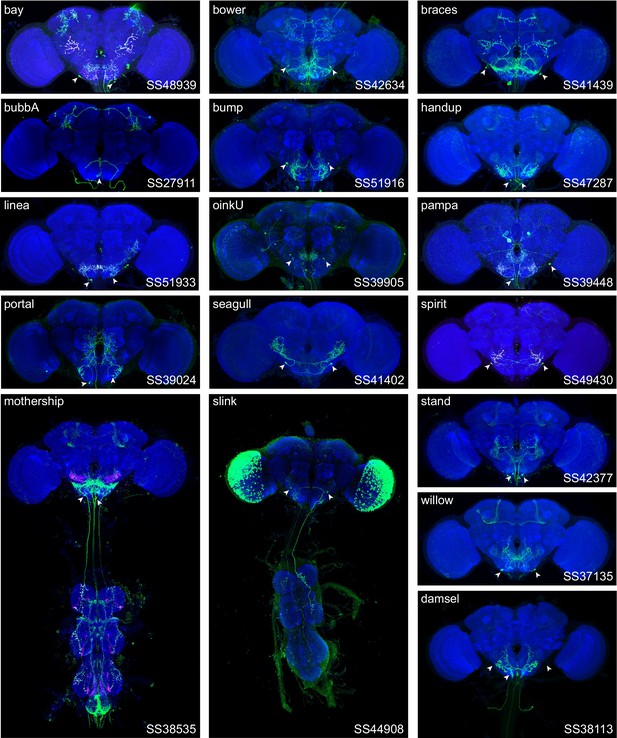
Expression patterns of split-GAL4 lines targeting neuronal cell types not included in NBLAST clustering.
Neuropil morphology is shown with nc82 in blue. Expression pattern of the UAS reporter is shown in green. UAS-Synaptotagmin staining to indicate location of synaptic outputs is shown in fuchsia where available. The cell type covered is indicated in the top right of each panel, while the unique line identifier is indicated in the bottom left of each panel. Filled arrowheads indicate targeted cell type somas. See https://splitgal4.janelia.org/ for image data.
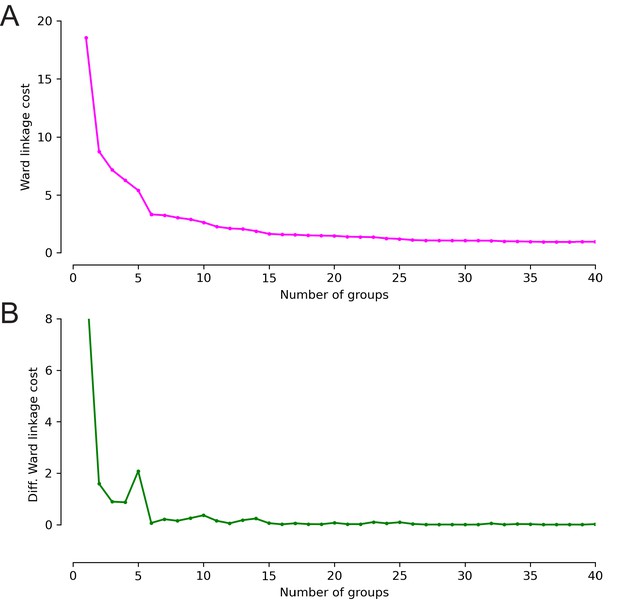
Ward’s joining cost and the differential of Ward’s joining cost for hierarchical clustering of subesophageal zone (SEZ) neuronal cell types with NBLAST.
(A) Ward’s joining cost for clustering into 0–40 groups (fuchsia). Ward’s joining cost declines sharply when clustering with six groups as compared to clustering with fewer than six groups. (B) Differential of Ward’s joining cost for clustering into 0–40 groups (green). The differential is high when clustering into five groups or fewer but does not decline notably after six groups is reached.
-
Figure 3—figure supplement 2—source data 1
Source data for panel A.
- https://cdn.elifesciences.org/articles/71679/elife-71679-fig3-figsupp2-data1-v2.csv
-
Figure 3—figure supplement 2—source data 2
Source data for panel B.
- https://cdn.elifesciences.org/articles/71679/elife-71679-fig3-figsupp2-data2-v2.csv
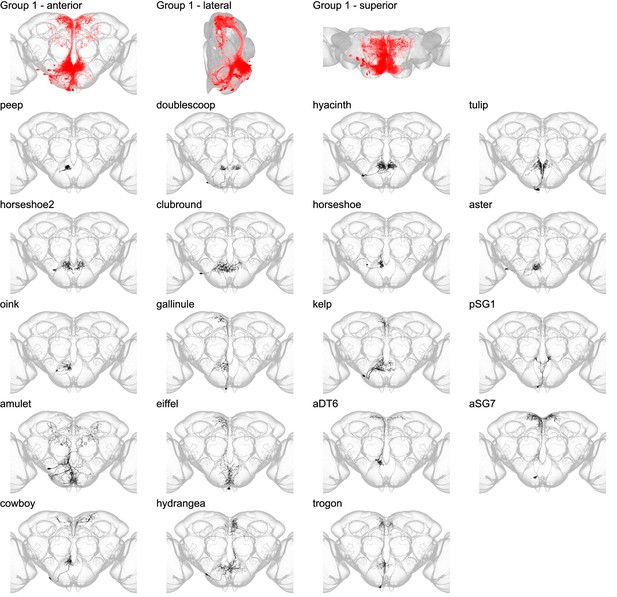
Morphology of neuron types in group 1.
Segmented example images for each neuron type in group 1. The top row shows the morphology of all neuron types in group 1 (red) overlaid in the JRC 2018 unisex coordinate space (gray) in anterior, lateral, and superior views. Below, the morphology of individual group members is shown separately. Individual neuron morphology is shown in black while the outline of the JRC 2018 unisex template is shown in gray. In Figures 3—8, the segmented neurons were imaged with a 63× objective and registered to the full-size JRC 2018 unisex template. The optic lobes have been partially cropped out of each panel.
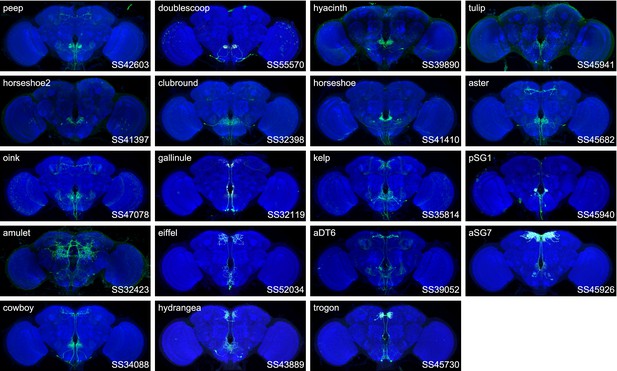
Expression patterns of the best split-GAL4 for each neuron type in roup 1.
Neuropil morphology is shown with nc82 in blue throughout. Expression pattern of the UAS reporter is shown in green. UAS-Synaptotagmin staining indicates the location of synaptic outputs in fuchsia where available. The cell type covered is indicated in the top right of each panel, while the unique line identifier is indicated in the bottom left of each panel. See https://splitgal4.janelia.org/ for image data.
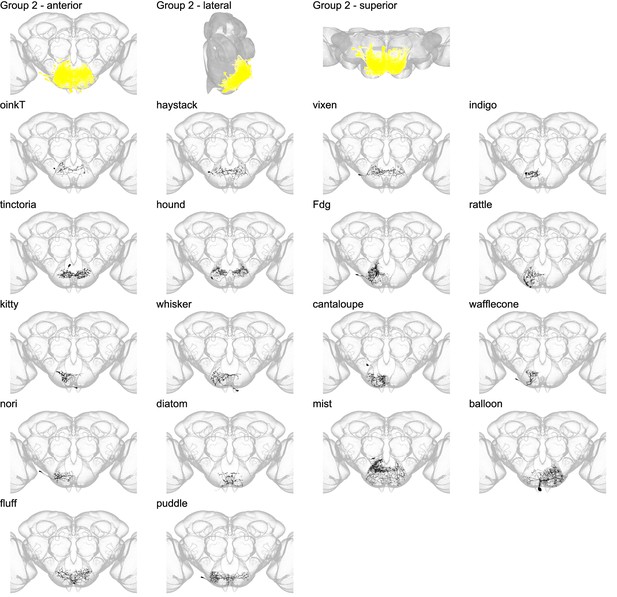
Morphology of individual neuron types in group 2.
The top row shows the morphology of all neuron types in group 2 (yellow), with the morphology of individual group members shown below.
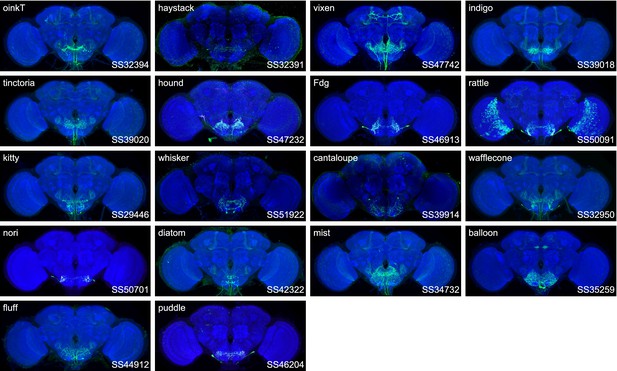
Expression patterns of the best split-GAL4 for each neuron type in group 2.
Neuropil morphology is shown with nc82 in blue throughout. Expression pattern of the UAS reporter is shown in green. UAS-Synaptotagmin staining indicates the location of synaptic outputs in fuchsia where available. The cell type covered is indicated in the top right of each panel, while the unique line identifier is indicated in the bottom left of each panel. See https://splitgal4.janelia.org/ for image data.
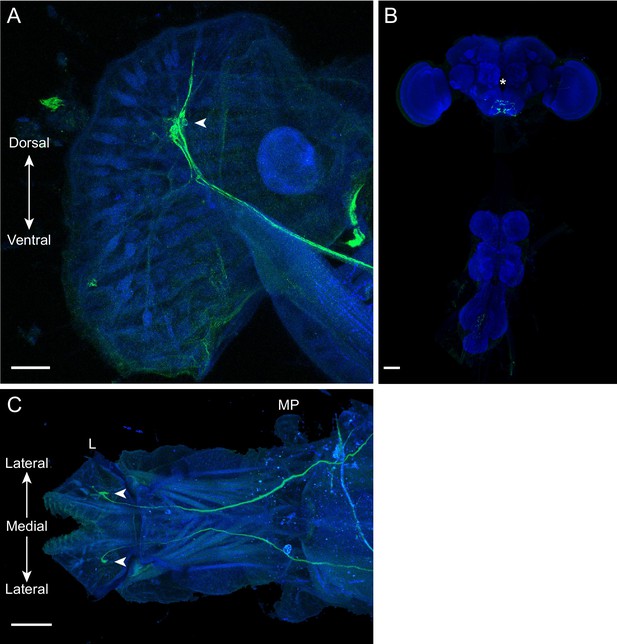
Diatom morphology in the CNS and proboscis.
(A) View from the lateral side of the proboscis labellum showing the expression pattern of SS40945. The dendrites of diatom project toward the dorsal surface of the labellum from the cell bodies (arrowhead). Scale bar is 20 μm. (B) Expression pattern of SS40945 in the CNS. Scale bar is 50 μm. The axons of diatom arborize in the subesophageal zone (SEZ). The location of the esophageal foramen is indicated with an asterisk. (C) View from the dorsal side of a whole-mount proboscis showing the expression pattern of SS40945. The labellum (left) contains the cell bodies of diatom (arrowheads). The axons project through the labellar nerve into the SEZ. The locations of the labellum (L) and the maxillary palps (MP) are annotated. Scale bar is 50 μm. All images show nc82 in blue, while the expression of the UAS-CsChrimson reporter is shown in green. Nc82 staining in (A) and (C) reveals presynaptic sites impinging on proboscis musculature.
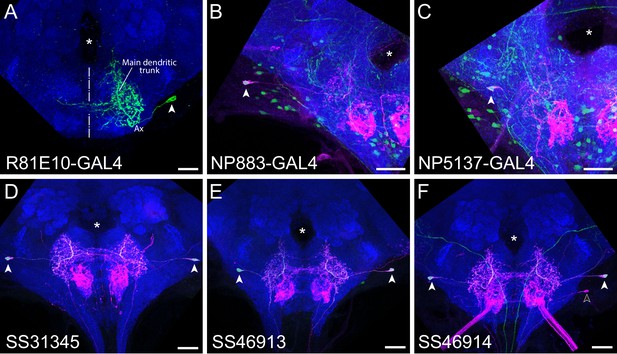
Novel Fdg split-GAL4 lines label the previously identified Fdg cell type.
(A) Single Fdg neuron morphology (green) as seen with MultiColor FlpOut (MCFO) of the 81E10-GAL4 line, which was previously identified as labeling Fdg (Pool et al., 2014). Characteristic features of Fdg, including the main dendritic trunk (labeled), midline-crossing largely dendritic region, the lateral and inferior more axonal region (labeled ‘Ax’), and the lateral cell body (filled arrowhead) can be readily appreciated (Flood et al., 2013). Midline is denoted with a dashed line. (B–F) Like 81E10-GAL4, 81E10-LexA (red) is expressed in a limited number of types in the subesophageal zone (SEZ), including Fdg (filled arrowheads). Fdg can be recognized in the overall pattern by the lateral and superior location of its cell body, as well as its distinctive main dendritic trunk. A second SEZ cell type is occasionally present (F, outlined arrowhead) but can be distinguished by its more inferior cell body. 81E10-LexA expression in sensory axons in the inferior and medial SEZ can also be appreciated, but only slightly obscures Fdg morphology in maximum projection images, as displayed here. (B) Coexpression of 81E10-LexA (red) and NP883-GAL4 (green) in the cell body of Fdg (filled arrowhead). Fdg was originally identified in the NP883-GAL4 line (Flood et al., 2013). (C) Coexpression of 81E10-LexA (red) and NP5137-GAL4 (green) in the cell body of Fdg (filled arrowhead). NP5137-GAL4 was also used to access Fdg by Flood et al., 2013. (D) Coexpression of 81E10-LexA (red) and the SS31345 split-GAL4 line (green) in the cell bodies of Fdg (filled arrowheads). (E) Coexpression of 81E10-LexA (red) and the SS46913 split-GAL4 line (green) in the cell bodies of Fdg (filled arrowheads). (F) Coexpression of 81E10-LexA (red) and the SS46914 split-GAL4 line (green) in the cell bodies of Fdg (filled arrowheads). Neuropil morphology is shown with nc82 in blue throughout. Location of the esophageal foramen is denoted with an asterisk. Scale bars 20 μm.
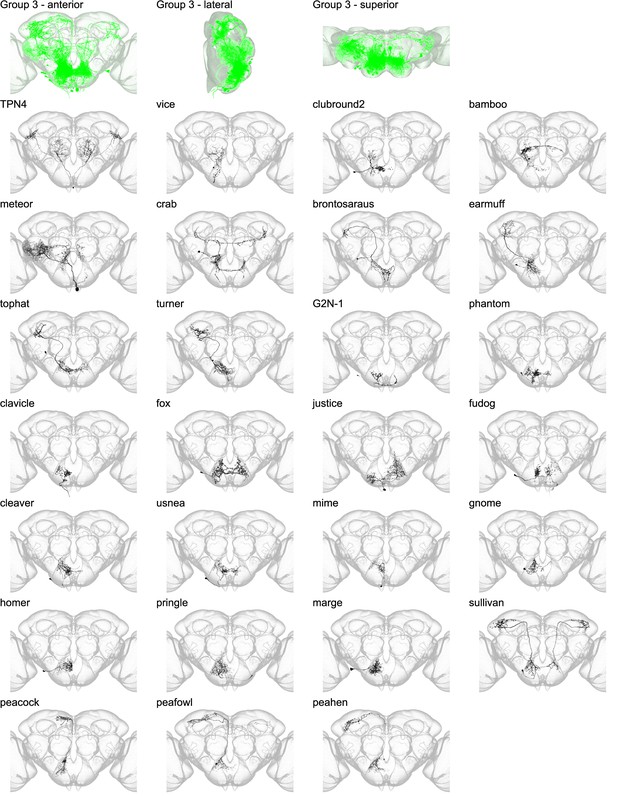
Morphology of individual neuron types in group 3.
The top row shows the morphology of all neuron types in group 3 (green), with the morphology of individual group members shown below.
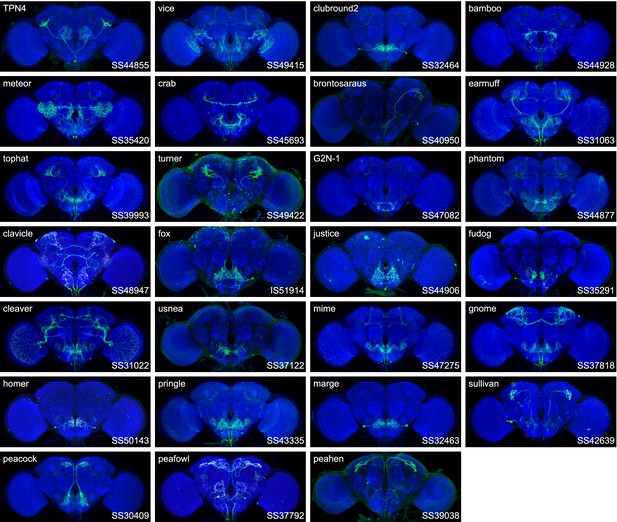
Expression patterns of the best split-GAL4 for each neuron type in group 3.
Neuropil morphology is shown with nc82 in blue throughout. Expression pattern of the UAS reporter is shown in green. UAS-Synaptotagmin staining indicates the location of synaptic outputs in fuchsia where available. The cell type covered is indicated in the top right of each panel, while the unique line identifier is indicated in the bottom left of each panel. See https://splitgal4.janelia.org/ for image data.
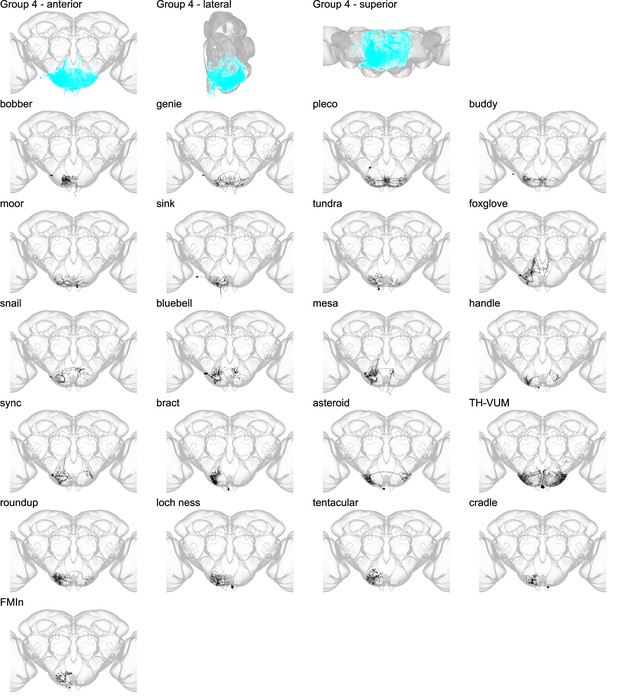
Morphology of individual neuron types in group 4.
The first three panels show the morphology of all neuron types in group 4 (cyan), with the morphology of individual group members shown below.
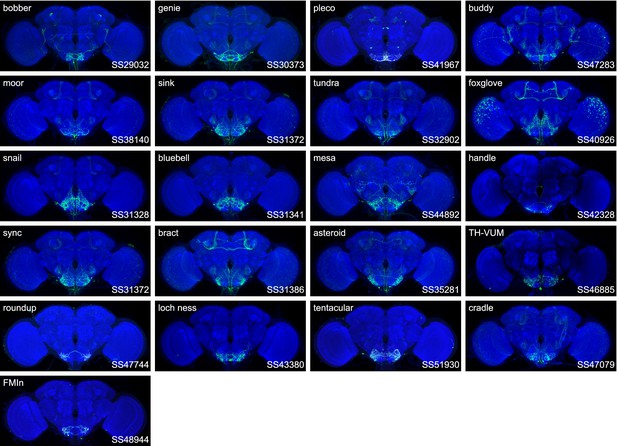
Expression patterns of the best split-GAL4 for each neuron type in group 4.
Neuropil morphology is shown with nc82 in blue throughout. Expression pattern of the UAS reporter is shown in green. UAS-Synaptotagmin staining indicates the location of synaptic outputs in fuchsia where available. The cell type covered is indicated in the top right of each panel, while the unique line identifier is indicated in the bottom left of each panel. See https://splitgal4.janelia.org/ for image data.
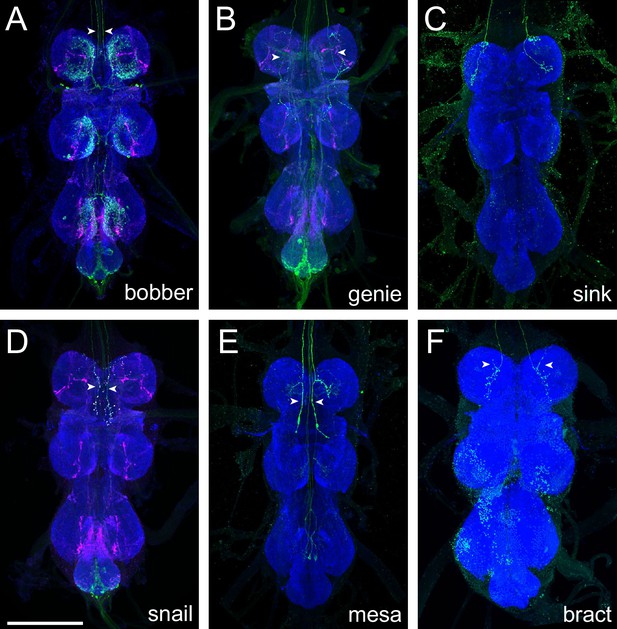
Axonal morphology of descending interneurons clustered into group 4.
(A) Bobber axonal projections in the pattern of SS29032. (B) Genie axonal projections in the pattern of SS30373. (C) Sink axonal projections in the pattern of SS31372. (D) Snail cell type in the pattern of SS31328. (E) Mesa axonal projections in the pattern of SS31369. (F) Bract axonal projections in the pattern of SS31386. Some non-specific background can be seen in the inferior ventral nerve cord (VNC). Neuropil morphology is shown with nc82 in blue throughout. Expression pattern of the UAS reporter is shown in green. UAS-Synaptotagmin staining indicates the location of synaptic outputs in fuchsia where available (A, C, but note non-specific background throughout the VNC). White arrowheads denote the axons of the cell type of interest where other cell types are present. All images are unregistered VNCs. Scale bar is 100 μm.
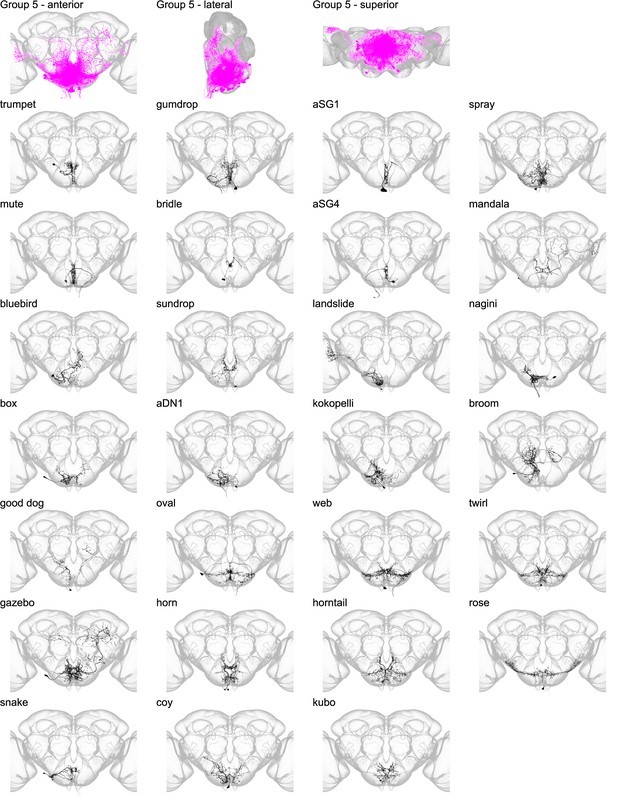
Morphology of individual neuron types in group 5.
The top row shows the morphology of all neuron types in group 5 (fuchsia), with the morphology of individual group members shown below.
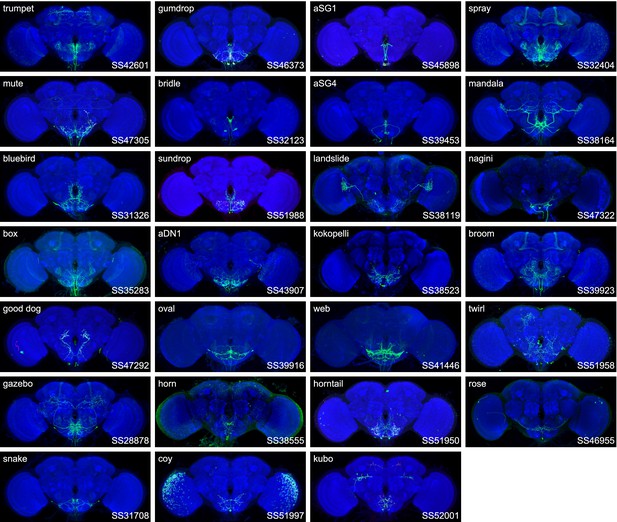
Expression patterns of the best split-GAL4 for each neuron type in group 5.
Neuropil morphology is shown with nc82 in blue throughout. Expression pattern of the UAS reporter is shown in green. UAS-Synaptotagmin staining indicates the location of synaptic outputs in fuchsia where available. The cell type covered is indicated in the top right of each panel, while the unique line identifier is indicated in the bottom left of each panel. See https://splitgal4.janelia.org/ for image data.
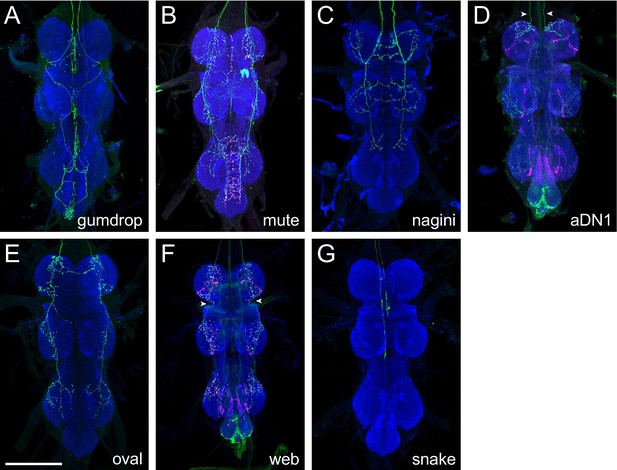
Axonal morphology of descending interneurons clustered into group 5.
(A) Gumdrop axonal projections in the pattern of SS46373. (B) Mute axonal projections in the pattern of SS47305. (C) Nagini axonal projections in the pattern of SS47322. (D) aDN1 axonal projections in the pattern of SS43907. (E) Oval axonal projections in the pattern of SS39916. (F) Web axonal projections in the pattern of SS41446. (G) Snake axonal projections in the pattern of SS31714. Neuropil morphology is shown with nc82 in blue throughout. Expression pattern of the UAS reporter is shown in green. UAS-Synaptotagmin staining indicates the location of synaptic outputs in fuchsia where available (B, D, F, but note non-specific background throughout the ventral nerve cord [VNC]). White arrowheads denote the axons of the cell type of interest where other cell types are present. All images are unregistered VNCs. Scale bar is 100 μm.
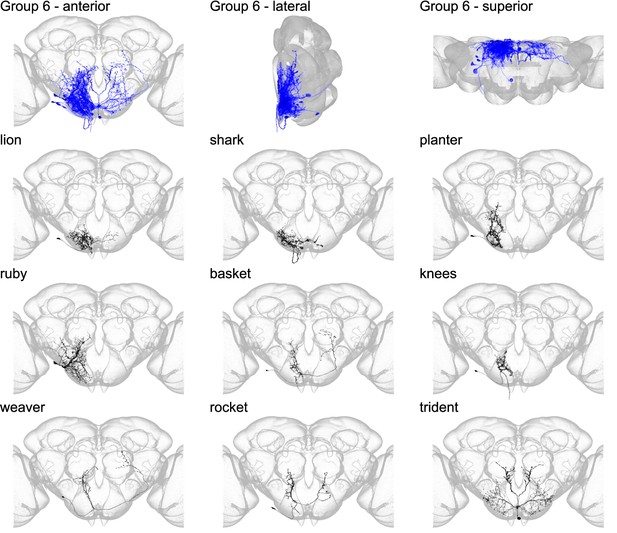
Morphology of individual neuron types in group 6.
The top row shows the morphology of all neuron types in group 6 (royal blue), with the morphology of individual group members shown below.
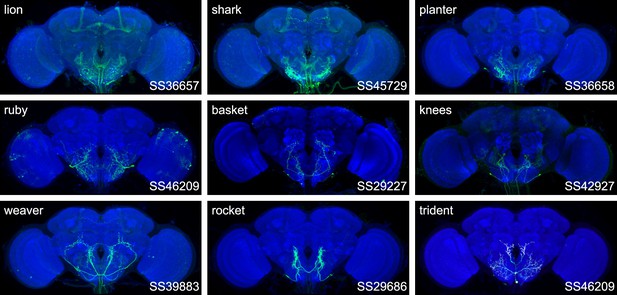
Expression patterns of the best split-GAL4 for each neuron type in group 6.
Neuropil morphology is shown with nc82 in blue throughout. Expression pattern of the UAS reporter is shown in green. UAS-Synaptotagmin staining indicates the location of synaptic outputs in fuchsia where available. The cell type covered is indicated in the top right of each panel, while the unique line identifier is indicated in the bottom left of each panel. See https://splitgal4.janelia.org/ for image data.
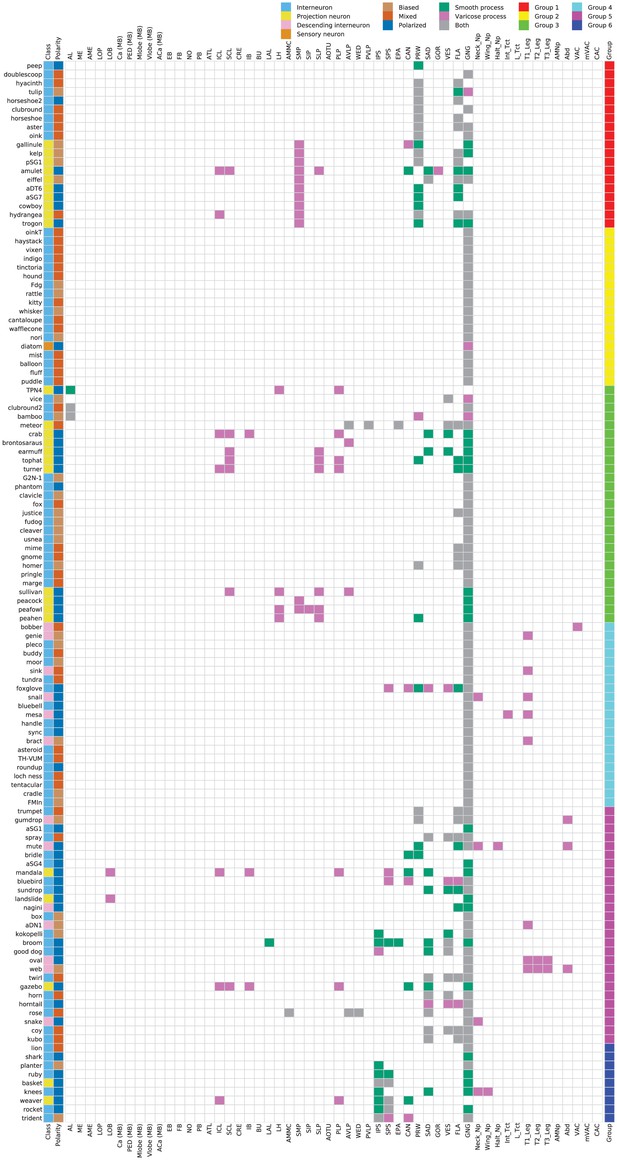
Innervation profile of subesophageal zone (SEZ) neuron types.
(Leftmost column) Cell types are members of one of four cell type classes: interneuron (light blue), projection neuron (yellow), descending interneuron (light pink), or sensory neuron (orange). Interneurons are confined within the SEZ, while projection neurons project from the SEZ to higher neuropils in the central brain. Descending interneurons project their axons from the SEZ through the neck connective to the ventral nerve cord (VNC). Sensory neurons project their axons from elsewhere in the body into the SEZ. Class is indicated for each neuron type by the filled pixel to the right of each neuron type name. (Second-to-leftmost column) Neuron types are polarized in a biased (light brown), mixed (red-orange), or polarized (dark blue) manner. The polarity class for each neuron type is indicated. (Center) The innervation profile for each neuron type is indicated by the filled pixels in its corresponding row. Brain region abbreviations follow the definitions and naming conventions of Ito et al., 2014 for the central brain and Court et al., 2020 for the VNC. The locations of smooth processes (dendrites, green), varicose processes (axons, dark pink), or both smooth and varicose processes (axons and dendrites, gray) are indicated by defined neuropil region. VNC neuropil regions are grouped on the right of the figure. Innervation of the VNC was varicose in all cases. (Far right) Cell group as defined by NBLAST clustering is indicated for each cell type. Group 1: red; group 2: yellow; group 3: green; group 4: cyan; group 5: fuchsia; group 6: royal blue.
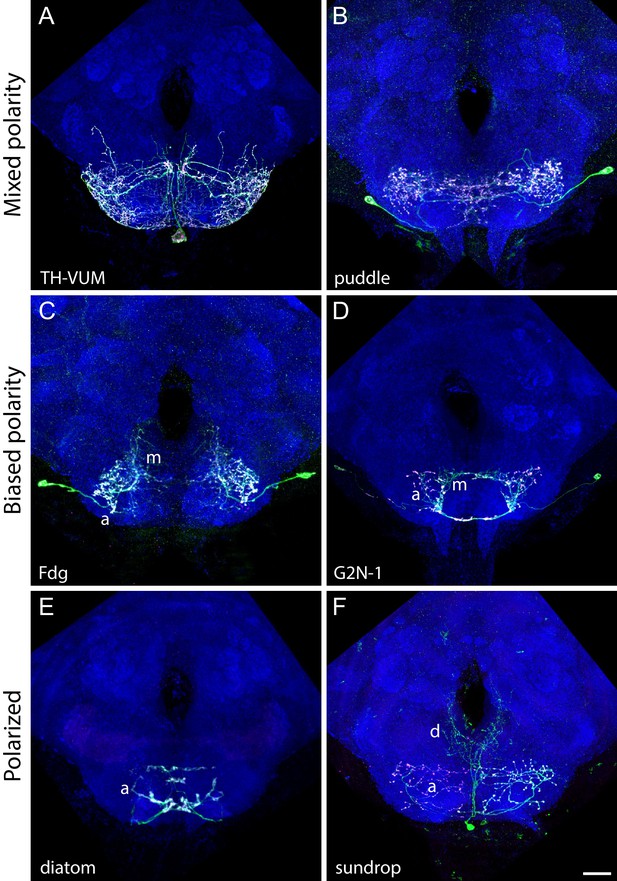
Example images of cell types in the mixed, biased, and polarized polarity classes.
(A–F) Neuropil morphology is shown with nc82 in blue throughout, and the pattern of the UAS reporter is shown in green. UAS-Synaptotagmin staining indicates the location of synaptic outputs (fuchsia). Scale bar is 20 μm. (A, B) Examples of neuronal cell types in the mixed class. TH-VUM (A, previously described) and puddle (B, described here) have synaptic outputs distributed uniformly throughout their arborizations. Dedicated dendritic or axonal regions are not distinguishable. (C, D) Examples of neuronal cell types in the biased class. Previously described Fdg (C) and G2N-1 (D) cell types have synaptically mixed (‘m’) regions of their arbors but still retain a distinct arbor region where synaptic outputs are concentrated (labeled with ‘a’). (E, F) Examples of neuronal cell types in the polarized class. Novel cell types diatom (E) and sundrop (F) have clearly separated dendritic (‘d’) and axonal (‘a’) regions of their arbors as indicated by the clear separation of varicose processes studded with synaptotagmin puncta (fuchsia) and smooth processes lacking synaptotagmin staining. Note that the dendrites of diatom are located in the proboscis labellum as shown in Figure 5—figure supplement 2. (A), (D), and (E) were segmented to remove background staining and clearly separated off-target cell types.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Genetic reagent (Drosophila melanogaster) | Polarity reporter, w; +; 3xUAS-Syt:: smGFP-HA(su(Hw)attP1),5xUAS-IVS- myr::smGFP-FLAG(VK5) | Aso et al., 2014b | ||
| Genetic reagent (D. melanogaster) | csChrimsonReporter/Optogenetic effector,20xUAS- csChrimson::mVenus in attP18 | Klapoetke et al., 2014 | BDSC:55134; FLYB:FBst0055134 | |
| Genetic reagent D. melanogaster | UAS-Syt-HA;; | Robinson et al., 2002 | Recombined with 20XUAS-CsChrimson-mVenus trafficked in attP18 when used for polarity analysis experiments | |
| Genetic reagent (D. melanogaster) | pBPhsFLP2:PEST in attP3; 13xLexAop2-> dSTOP>-myr::smGFP-OLLAS in su(Hw)attP5, 13xLexAop2-> dSTOP>-myr::smGFP-V5 in attP40/CyO; 13xLexAop2-> dSTOP>-myr::smGFP-FLAG in attP2/TM2 | This work | LexA-based MCFO line with heat shock flippase | |
| Genetic reagent (D. melanogaster) | R57C10-Flp2::PEST in su(Hw)attP8;; pJFRC201-10XUAS-FRT>STOP>FRT-myr::smGFP-HA in VK00005,pJFRC240-10XUAS-FRT>STOP >FRT-myr::smGFP-V5-THS-10XUAS-FRT>STOP>FRT-myr::smGFP-FLAG in su(Hw)attP1/TM2 | Nern et al., 2015 | BDSC:64089; FLYB:FBst0064089 | Short name: MCFO-3 |
| Genetic reagent (D. melanogaster) | pBPhsFlp2::PEST in attP3;; pJFRC210-10XUAS-FRT>STOP >FRT-myr::smGFP-OLLAS in attP2, pJFRC201-10XUAS-FRT>STOP>FRT-myr::smGFP-HA in VK0005, pJFRC240-10XUAS-FRT>STOP >FRT-myr::smGFP-V5-THS-10XUAS-FRT>STOP>FRT-myr::smGFP-FLAG in su(Hw)attP1/ TM2 | Nern et al., 2015 | BDSC:64086; FLYB:FBst0064086 | Short name: MCFO-2 |
| Genetic reagent (D. melanogaster) | ;;Dfd-LexA | Simpson, 2016 | ||
| Genetic reagent (D. melanogaster) | ;;Scr-LexA | Simpson, 2016 | ||
| Genetic reagent (D. melanogaster) | Labial-GAL4 | Hirth et al., 2001 | BDSC:43652;FLYB:FBst0043652 | |
| Genetic reagent (D. melanogaster) | ;LexAop-nls-GCaMP6s in VIE-260b; | This work | ||
| Genetic reagent (D. melanogaster) | ;;His2Av-mRFP | Pandey et al., 2005 | BDSC:23650;FLYB:FBst0023650 | |
| Genetic reagent (D. melanogaster) | ; UAS-Syn21-nlsGCaMP6s-p10 in VIE-260b; | This work | ||
| Genetic reagent (D. melanogaster) | ;;UAS-His::mRFP | Emery et al., 2005 | FLYB:FBtp0022240 | |
| Genetic reagent (D. melanogaster) | ;81E10-LexAp65 in JK22C; | This work | Approach and promoter have been previously described (Jenett et al., 2012; Pfeiffer and Homberg, 2014) | |
| Genetic reagent (D. melanogaster) | NP883-GAL4 | Yoshihara and Ito, 2000 | Kyoto:103803;FLYB:FBst0302671 | Line in which Fdg was originally identified (Flood et al., 2013) |
| Genetic reagent (D. melanogaster) | NP5137-GAL4 | Yoshihara and Ito, 2000 | Kyoto:113602;FLYB:FBst0316329 | Line which also labels Fdg (Flood et al., 2013) |
| Genetic reagent (D. melanogaster) | 13XLexAop2-CsChrimson-tdT (attP18), 20XUAS-IVS-Syn21-opGCaMP6f p10 (Su(Hw)attp8);; | Morimoto et al., 2020 | ||
| Antibody | Anti-Brp (mouse monoclonal) | DSHB, University of Iowa, USA | DSHB Cat# nc82, RRID:AB_2314866 | (1:40) |
| Antibody | Anti-GFP (chicken polyclonal) | Thermo Fisher Scientific | Thermo Fisher Scientific Cat# A10262, RRID:AB_2534023 | (1:1000) |
| Antibody | Anti-dsRed (rabbit polyclonal) | Takara | Takara Bio Cat# 632496, RRID:AB_10013483 | (1:1000) |
| Antibody | Anti-chicken Alexa Fluor 488 (goat polyclonal) | Thermo Fisher Scientific | Thermo Fisher Scientific Cat# A-11039, RRID:AB_2534096 | (1:1000) |
| Antibody | Anti-rabbit Alexa Fluor 568 (goat polyclonal) | Thermo Fisher Scientific | Thermo Fisher Scientific Cat# A-11036, RRID:AB_10563566 | (1:1000) |
| Antibody | Anti-mouse Alexa Fluor 647 (goat polyclonal) | Thermo Fisher Scientific | Thermo Fisher Scientific Cat# A-21236, RRID:AB_2535805 | (1:500) |
| Software, algorithm | VVDviewer | Otsuna et al., 2018 | RRID:SCR_021708 | https://github.com/JaneliaSciComp/VVDViewer |
| Software, algorithm | Fiji | Schindelin et al., 2012 | RRID:SCR_002285 | http://fiji.sc/ |
| Software, algorithm | Computational Morphometry Toolkit | Rohlfing and Maurer, 2003 | RRID:SCR_002234 | https://www.nitrc.org/projects/cmtk/ |
| Software, algorithm | R Project for Statistical Computing | R Development Core Team, 2018 | RRID:SCR_001905 | https://www.r-project.org/ |
| Software, algorithm | NeuroAnatomy Toolbox | Jefferis and Manton, 2014 | 10.5281/zenodo.1136106,RRID:SCR_017248 | http://jefferis.github.io/nat/ |
| Software, algorithm | Ilastik | Berg et al., 2019 | RRID:SCR_015246 | https://www.ilastik.org/ |
| Software, algorithm | MaMuT Plugin | Wolff et al., 2018 | https://imagej.net/MaMuT | |
| Software, algorithm | Janelia WorkStation | Rokicki et al., 2019 | RRID:SCR_014302 | https://doi.org/10.25378/janelia.8182256.v1 |
Additional files
-
Supplementary file 1
Subesophageal zone (SEZ) Split-GAL4 Collection database.
Each row of the database describes an individual split-GAL4 line generated in this study. The targeted cell type is noted in the ‘Cell type’ column. The ‘AD’ and ‘DBD’ columns note the AD and DBD hemidrivers that compose each line. The unique stable split code identifies each line and can be found in the ‘SS number’ column. ‘SEZ Split-GAL4 Collection code’ provides a shorter, unique identifier for each split-GAL4 line that is specific to this study. ‘Quality: (P)oor, (G)ood, (E)xcellent, or (I)deal’ column ranks the quality of each line, while ‘Quality notes’ provides a short, qualitative description of off-target expression in each line. The ‘Imagery available: (P)olarity (M)CFO or (B)oth’ notes which types of image data are available for each line at https://splitgal4.janelia.org/.
- https://cdn.elifesciences.org/articles/71679/elife-71679-supp1-v2.csv
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/71679/elife-71679-transrepform1-v2.pdf





