Temperature evolution following joint loading promotes chondrogenesis by synergistic cues via calcium signaling
Figures
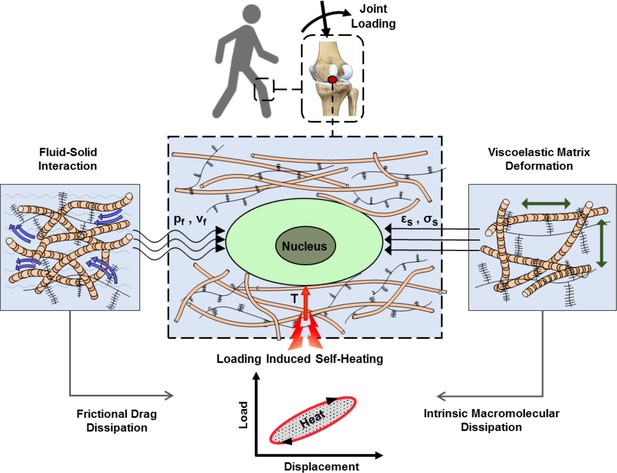
Cartilage loading-induced self-heating during physical activity.
Mechanical hysteresis in cartilage tissue during joint loading generates heat and causes the temperature rise over time. The self-heating of cartilage originates from both, intrinsic matrix viscoelasticity and solid-fluid interaction sources during joint loading. Structural and material characteristics of matrix microenvironment are acting in concert with external stresses to provide direct and indirect biophysical cues for cells such as deformation and temperature variation (pf, vf: fluid pressure and flow velocity; εs, σssolid matrix strain and stress; T: temperature).
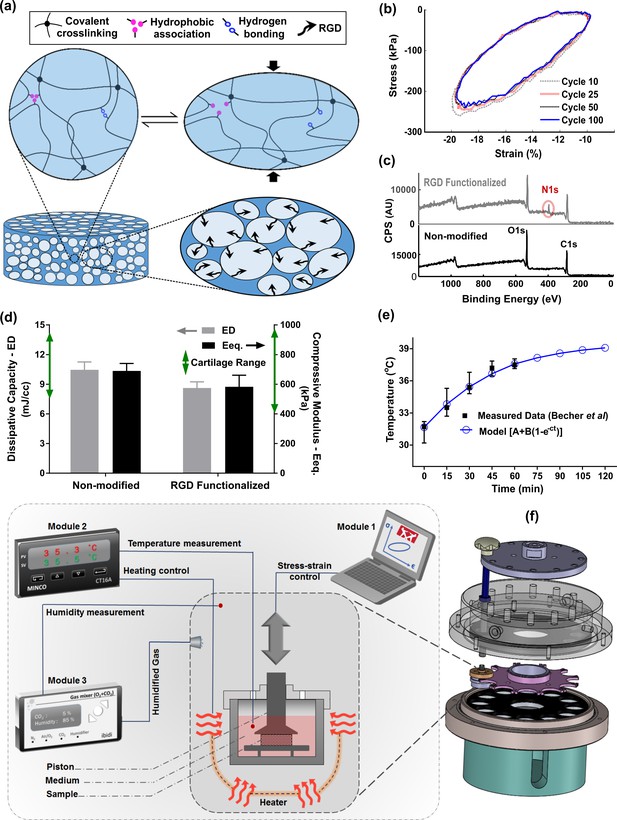
Developed in vitro system to study cartilage thermo-mechanobiology.
(a) Poro-viscoelastic hydrogel with RGD decoration on pores. The polymeric network of the scaffold is hybridly crosslinked with physical hydrophobic associations, hydrogen bonds, and covalent crosslinks between chains. Following an applied deformation, flexible network reorganization and fluid-solid interaction within the porous structure occur resembling cartilage dissipative behavior. (b) The hysteresis loop following loading and unloading steps was preserved over different cycles indicating fatigue resitance capability of the hydrogel. The shrinkage of the loop is mainly at the onset and remains stable after preconditioning cycles, owing to reversible sources of dissipation. (c) X-ray photoelectron spectroscopy (XPS) survey scan of pHEMA porous hydrogels before and after RGD functionalization. The appearance of an N1s peak at 400 eV in the XPS spectra of functionalized samples is the evidence for a successful binding of RGD peptides to the exposed hydroxyl groups of porous pHEMA hydrogel. (d) Mechanical properties of the pure (non-modified) and RGD functionalized hydrogels and reported range of cartilage properties (green arrows) in literature. (e) The measured intra-articular temperature following jogging activity as reported in Becher et al., 2008, and exponential fitted curve to predict the temperature evolution. (f) Modular and custom-designed bioreactor. The apparatus consists of adjustable loading system with embedded screws, chamber cap with different inlets/outlets, culture wells and pistons, wells carrier, chamber base and support. The left schematic illustrates conceptual design of the modular bioreactor for thermo-mechanical stimulation of cell-laden hydrogels.
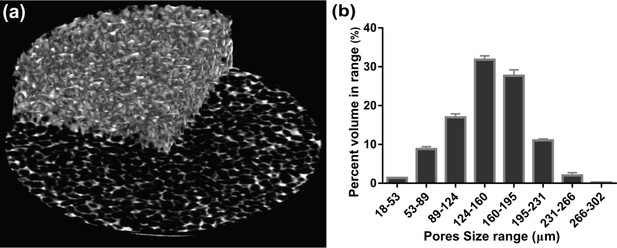
Morphological structure of the dissipative hydrogel.
(a) Reconstructed micro-CT scans. (b) Distribution of pores size obtained by CTAn software. The pore size distribution is mainly between 100 and 200 µm, the strain free permeability (k0) is in order of 10–14 m2, and the porosity (φ) is approximately 68%.
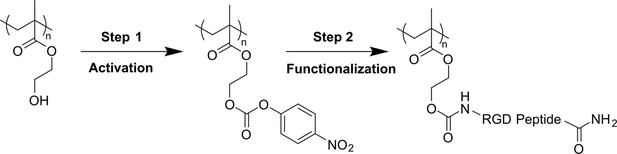
Applied two-step bio-conjugation process (hydroxyl group activation and RGD peptide grafting) for functionalization of pHEMA-based hydrogels.
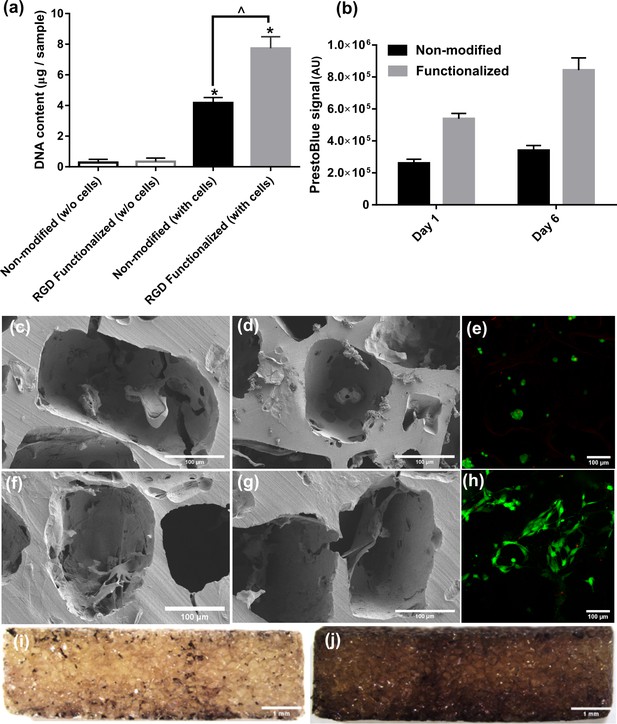
Cell attachment, proliferation, morphology, and distribution inside pure (non-modified) and RGD functionalized hydrogels.
(a) DNA content assay for cell-seeded hydrogels demonstrated significantly higher cell attachment in RGD functionalized samples compared to pure hydrogels (significant differences with control group (*) or between specific groups (^) p < 0.05, Student’s t-test, n = 3). (b) Cell proliferation was significantly higher in RGD functionalized hydrogels over the static culture period compared to non-modified pure samples. (c–e) SEM and live/dead images of the cell-seeded pure hydrogels showing morphology and distribution of cells within. (f–h) SEM and live/dead images of the cell-seeded functionalized hydrogels showing morphology and distribution of cells within. (i, j) Cell distribution on central cross section of the functionalized hydrogel discs after 1 (left) and 10 days (right), respectively.The cells could penetrate inside porous hydrogels and proliferate overtime due to available binding sites by RGD peptides.
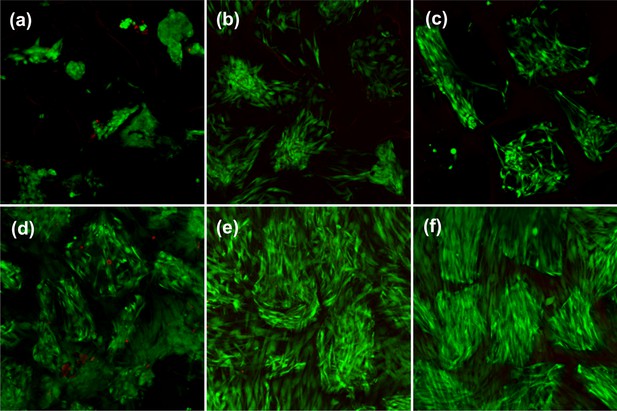
Cell viability and attachment before and after thermo-mechanical stimulation inside the bioreactor for non-modified (top) and RGD functionalized (bottom) hydrogels.
(a, d) Cell viability for free-swelling samples at 32°C on day 2. (b, e) Cell viability for free-swelling samples at 32°C on day 10. (c, f) Cell viability after thermo-mechanical stimulation during 1.5 hr of 10% cyclic compression at 1 Hz over 10% pre-strain along with temperature increase from 32°C to 39°C.
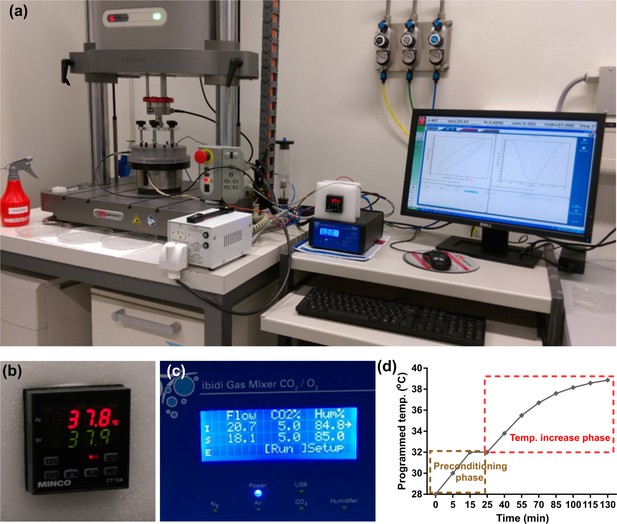
Function evaluation of the developed bioreactor for simulation of cartilage loading-induced self-heating.
(a) Validation of performance of the developed device for thermo-mechanical stimulation. Modular design of the bioreactor permitted independent control over applied mechanical load, culture temperature, CO2/O2 gas concentration, and humidity level. The Instron device was programmed to apply controlled cyclic strain on samples according to reported values in the literature for deformation of knee cartilage during stance phase of walking at 1 Hz frequency. Smooth curve of hysteresis loop shows that samples are uniformly loaded and unloaded. (b) The culture temperature closly tracked the programmed temperature based on the temperature evolution model. (c) The gas mixer was set on a normaxia condition to regulate standard 5% CO2 and 85% humidity levels inside the chamber. (d) Simulation of the temperature increase during cyclic compression with the developed bioreactor after a preconditioning phase. During the preconditioning, the temperature of the medium inside the culture wells reached 32°C after 10–15 min to simulate the cartilage temperature at rest. Meanwhile, the humidified gas injection provided a stable 5% CO2 and 85% humidity inside the chamber. After the equilibration of the system, the mechanical stimulation began and the temperature could either be changed to evolve according to the prediction of the curve fitted data or be kept constant during the cyclic compression period. The error between desired and realized temperatures was less than 0.5°C when the PID coefficients were optimized. It was also verified that actual recorded temperatures inside the wells in different positions were able to closely follow the programmed regime with a maximum error of 0.5°C.
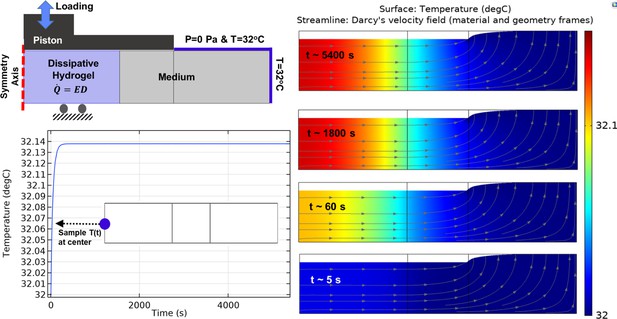
Heat transfer simulation in one culture well of the bioreactor by constant temperature boundary condition (32°C) at the outer interface.
The temperature of the sample and medium was minimally varied over the stimulation period from initial value (32°C) to maximum 32.14°C in core of the sample. The color map illustrates the temperature and the arrowed curves are velocity streamlines. The obtained results confirm that the external heat supply controls the culture wells temperature independently from the applied mechanical loading. The isolated temperature and loading control strategy in our bioreactor design allowed us to decompose direct and indirect effects of mechanical loading on cell responses during self-heating of cartilage.
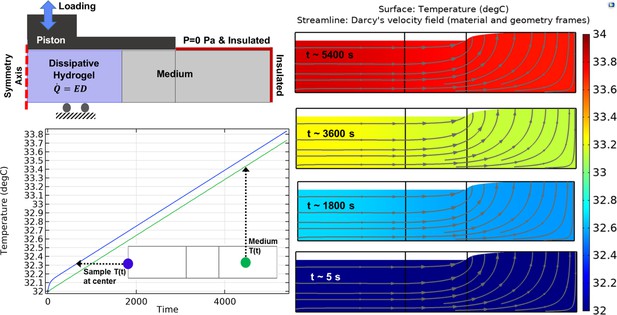
Heat transfer simulation in one culture well of the bioreactor by application of adiabatic boundary condition.
The temperature of the sample and medium was changed around 2°C over the stimulation period showing that hydrogel dissipative capacity can cause self-heating in specific boundary condition. The color map illustrates the temperature and the arrowed curves are velocity streamlines. Since the size of the sample is small compared to the medium, the generated heat by the sample can only increase the temperature from 32°C to approximately 34°C.
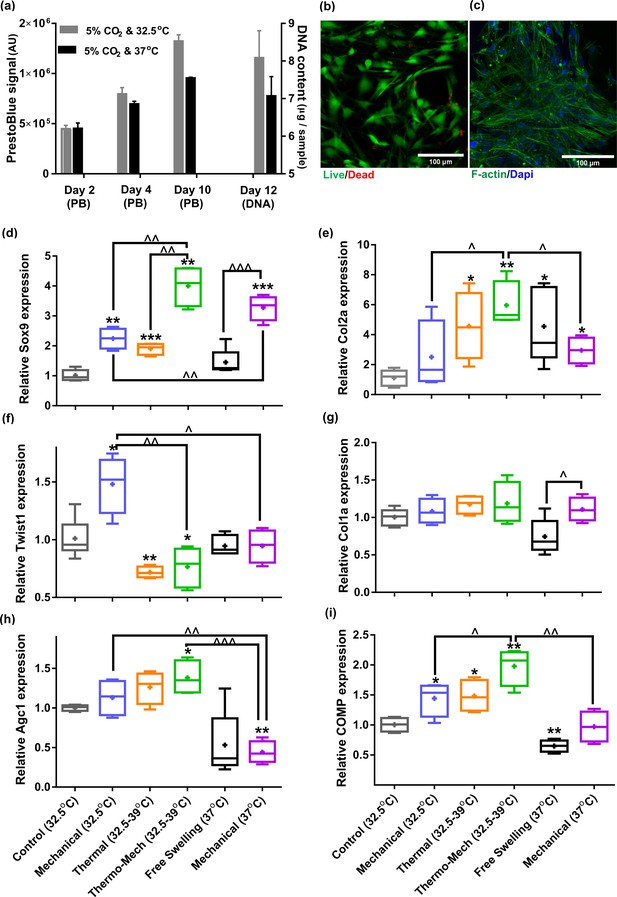
Human chondro-progenitor cells behavior inside functionalized dissipative porous hydrogels.
(a) Metabolic activity (obtained by PrestoBlue [PB] assay) and DNA content of free swelling cell-laden hydrogels in different culture temperatures corresponding to knee intra-articular temperature at rest and core body temperature. (b) Live/dead labeling of cells inside porous hydrogels 4 days post seeding cultured at 32.5°C (green stain demonstrates live cells and red stain shows dead cells). (c) Actin filament and nucleus immunostaining of cells, 6 days after seeding. (d:i) Comparison of the relative gene expression of cells in response to different biophysical cues applied in 3 alternate days for 90 min normalized to control free-swelling samples cultured at 32.5°C (significant differences with control group (*) or between specific groups (^) p < 0.05; n = 4–6). Overall the results indicate that loading-induced self-heating, designated as Thermo-Mech (32.5–39°C), optimally promoted chondrogenesis.
-
Figure 3—source data 1
Fold change of samples in different studied groups.
- https://cdn.elifesciences.org/articles/72068/elife-72068-fig3-data1-v1.xlsx
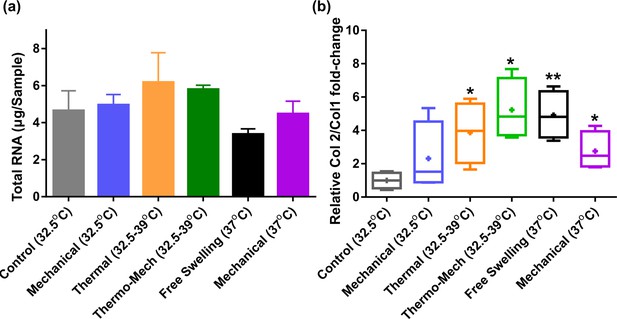
Extracted RNA and Col2/Col1 fold-change ratio.
(a) Total RNA as an indicator for cell metabolism was minimally varied by intermittent thermo-mechanical stimulation when culture baseline temperature was set at 32°C (knee temperature at rest).
A decrease of total RNA for continuous incubation of cells at 37°C (core body temperature) was also observed. (b) Relative Col2/Col1 fold-change in the studied groups shows that applied thermo-mechanical stimulus is chondro-inductive (significant differences with control group (*) p < 0.05). The superior effect of combined thermo-mechanical stimulation compared to thermal and mechanical loading is also noticeable.
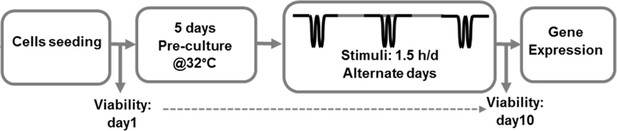
Schematic workflow of the performed in vitro thermo-mechanobiological experiment.
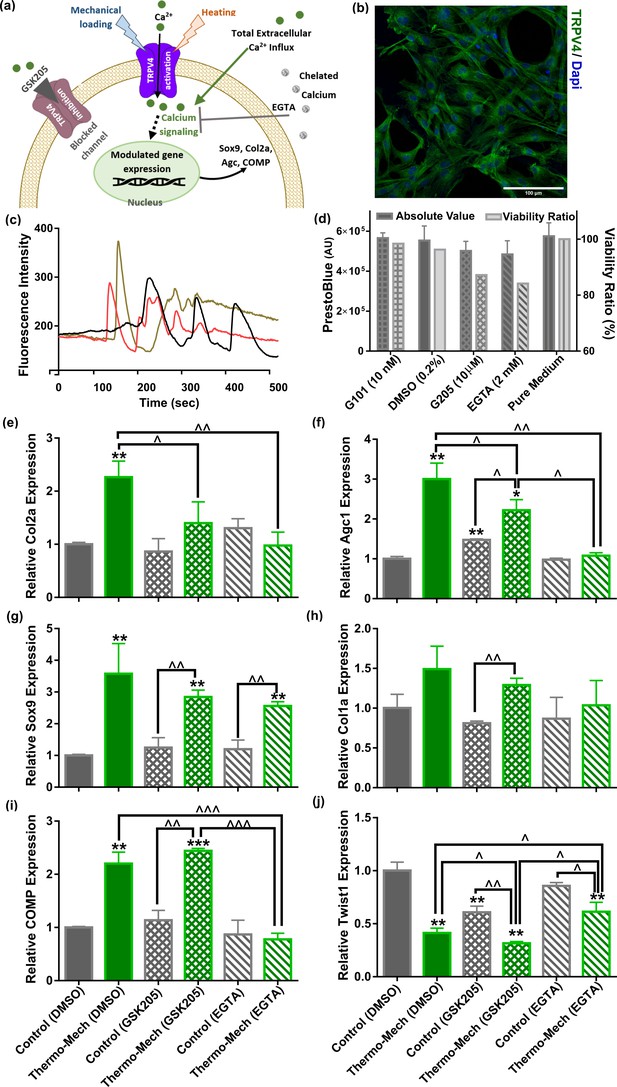
Calcium signaling mechanism, functional characterization of transient receptor potential vanilloid 4 (TRPV4) ion channels expression in human chondro-progenitor cells, and gene expression results with manipulated pathways regulating cytosolic Ca2+ variation.
(a) Schematic of TRPV4-mediated calcium signaling for transduction of thermo-mechanical cues which could be inhibited by GSK205 (TRPV4 antagonist). Generally, the intracellular calcium can be varied through different pathways and all of them are affected when extracellular Ca2+ ions are chelated with EGTA. (b) Successful binding of the specific-TRPV4 antibody to human chondro-progenitor cells confirmed expression of TRPV4 channels on cells seeded in porous hydrogels. (c) Response of cells to TRPV4 agonist (10 nm GSK101) confirmed functionality of the gates for modulation of intracellular calcium content. (d) Viability of cells in presence of pertinent agonsit/antagonist of calcium pathways. (e:j) Relative gene expression of cells in different groups normalized to free-swelling control group with DMSO as drug carrier when employing specific antagonist for inhibiting TRPV4 channels (GSK205) or chelating extracellular calcium sources (EGTA). The control groups are free-swelling samples cultured at 32.5°C and stimulated samples receiving combined thermo-mechanical cues at the same culture medium containing DMSO, GSK205, or EGTA (* significant differences with control group with p < 0.05; ^ significant differences between specific groups with p < 0.05; n = 3–4). Extracted results indicated that thermo-mechanotransduction signaling cascade was almost incomplete without extracellular calcium sources and TRPV4 channels played a key role in translation of perceived physical cues to calcium transients.
-
Figure 4—source data 1
Fold change of samples in different groups of thermo-mechanotransduction pathways study.
- https://cdn.elifesciences.org/articles/72068/elife-72068-fig4-data1-v1.xlsx
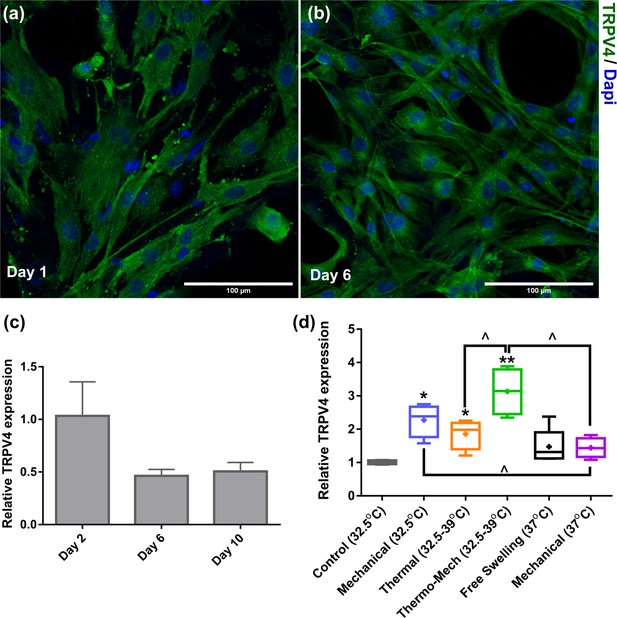
Transient receptor potential vanilloid 4 (TRPV4) expression of chondro-progenitor cells is time and stimuli dependent.
(a,b) Immunostaining of TRPV channels 1 and 6 days after seeding cells in scaffolds. (c,d) When cells were cultured in scaffolds without any stimulation at 32°C, we observed a downregulation of the TRPV4 genes at days 6 and 10 compared to day 2. However, a significant upregulation in expression of TRPV4 gene was detected by application of biomimetic temperature evolution, mechanical loading, and combination thereof (significant differences with control group (*) or between specific groups (^) p < 0.05; n = 3-4).
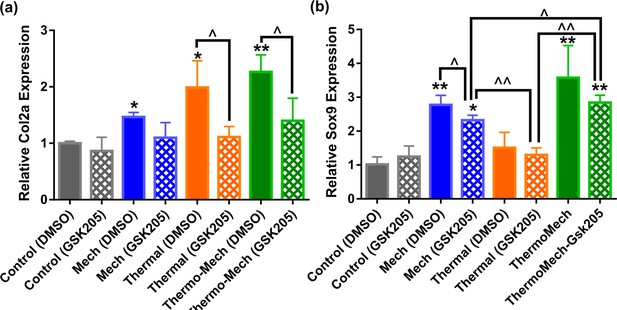
Gene expression results indicated that transient receptor potential vanilloid 4 (TRPV4) acts as a signal integrator for thermal and mechanical stimuli.
Inhibition of TRPV4 channels by GSK205 significantly reduced the expression of Col2a in the thermal group, and slightly in the mechanical group.In parallel, TRPV4 channel disactivation significantly reduced Sox9 expression in mechanically loaded samples and slightly in the thermal group (significant differences with control group (*) or between specific groups (^) p < 0.05; n = 3–4). This data confirms that TRPV4 channels are influential in transduction of thermal and mechanical cues and their contribution could be different depending on the target indicator. In addition, significant upregulation of Sox9 in mechanically loaded samples with and without GSK205 could imply the contribution of other signal mediators such as integrins through cell-scaffold interaction with respect to incorporated RGD motifs.
Videos
Transient receptor potential vanilloid 4 (TRPV4) channel activation in human chondro-progenitor cells cultured in 2D by using 10 nM GSK01 agonist.
Most of the cells are responsive to delivery of TRPV4 activator.
Transient receptor potential vanilloid 4 (TRPV4) channel inhibition in human chondro-progenitor cells in 2D by using 10 µM GSK205 antagonist.
Only a few cells are responsive to delivery of TRPV4 activator.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Cell line (Homo sapiens) | ECPs (human chondro-progenitor cells) | https://doi.org/10.3727/215517912X639324https://doi.org/.10.3390/biom11020250 | Fetal chondro-progenitor cell source (FE002-Cart.) established after standardized processing of a fetal cartilage sample in Prof Lee Ann Applegate lab in 2009. | |
| Antibody | Anti-TRPV4 (Rabbit polyclonal) | Abcam | Cat#: ab191580 | IF (1:200) |
| Commercial assay or kit | Flou 4-AM (Calcium indicators) | Thermo Fisher Scientific | Cat#: F14201 | 5 µM |
| Chemical compound, drug | TRPV4 agonist; TRPV4 activator; GSK101 | Sigma-Aldrich | Cat#: G0798 | 10 nM |
| Chemical compound, drug | TRPV4 antagonist; TRPV4 inhibitor; GSK205 | AOBIOUS | Cat#: AOB1612-5 | 10 µM |
| Commercial assay or kit | NucleoSpin RNA XS | MACHEREY-NAGEL | Cat#: 740902.50 | |
| Commercial assay or kit | Live/dead (The Viability/Cytotoxicity Assay Kit) | Biotium | Cat#: 30002 | |
| Commercial assay or kit | PrestoBlue; PB (Cell Viability Reagent) | Thermo Fisher Scientific | Cat#: A13261 | |
| Software, algorithm | COMSOL Multiphysics | COMSOL Inc | COMSOL 5.4; RRID:SCR_014767 | |
| Software, algorithm | ImageJ | https://imagej.net/ | RRID:SCR_003070 | |
| Software, algorithm | MATLAB | MathWorks | RRID:SCR_001622 | |
| Other | EGTA | Sigma-Aldrich | Cat#: 03777 | |
| Other | RGD peptide (motif for cell attachment via integrin binding) | GL Biochem | GGGRGDS-NH2 This peptide was synthesized by GL Biochem | |
| Other | 4-Nitrophenyl chloroformate, NPC | Sigma-Aldrich | Cat#: 160210 | |
| Other | DAPI stain | Invitrogen | Cat#: D1306 | 2 µg/ml |
| Other | TRIzol Reagent | Thermo Fisher Scientific | Cat#:15596026 | |
| Other | Hoechst 33258 | Thermo Fisher Scientific | Cat#: H3569 | 0.2 µg/ml |
| Other | Loading machine | Instron | Cat#: ElectroPuls E3000 | |
| Other | PID microprocessor | Minco | Cat#: CT16A | |
| Other | Miniature RTD sensor | Minco | Cat#: S308 | |
| Other | Thermo-foil heater | Minco | Cat#: HM6975 | |
| Other | Gas mixer | ibidi | Cat#: 11922 |
Material properties used in the heat transfer model of the hydrogel in the culture well.
| Material properties | Value | Unit |
|---|---|---|
| Equilibrium modulus (Eeq) | 500 | kPa |
| Poisson ratio(ν) | 0.23 | – |
| Porosity (φ) | 68 | % |
| Permeability (k) | 2·1e–14 | m2 |
| Dissipative power of hydrogel | 9000 | w/m3 |
| Heat capacity of pHEMA (Csolid) | 1308 | J/(kg·kelvin) |
| Conductivity of pHEMA (Ksolid) | 0.25 | W/(m·kelvin) |
| Heat capacity of water (Cfluid) | 4200 | J/(kg·kelvin) |
| Conductivity of water (Kfluid) | 0.6 | W/(m·kelvin) |
| Biot–Willis coefficient (α) | 1 | – |
| Relaxation modulus (G1) | 200 | kPa |
| Relaxation modulus (G2) | 58 | kPa |
| Relaxation modulus (G3) | 215 | kPa |
| Relaxation time (τ1) | 0.42 | s |
| Relaxation time (τ2) | 5.82 | s |
| Relaxation time (τ3) | 1600 | s |
Primers data used for quantitative RT-PCR.
| Primer | Sequence | Concent. | Efficiency | Temp. |
|---|---|---|---|---|
| RPL13-F | TAAACAGGTACTGCTGGGCCG | 150 ng | 96% | 60 oC |
| RPL13-R | CTCGGGAAGGGTTGGTGTTC | |||
| Agc-F | GGTACCAGTGCACAGAGGGGTT | 175 ng | 99% | 62 oC |
| Agc-R | TGCAGGTGATCTGAGGCTCCTC | |||
| Twist-F | AGCAGGGCCGGAGACCTAGATGTCA | 250 ng | 95% | 60 oC |
| Twist-R | ACGGGCCTGTCTCGCTTTCTCT | |||
| Comp-F | TGCTTCGGGAACTGCAGGAAAC | 250 ng | 101% | 60 oC |
| Comp-R | GCACGCGTCACACTCCATCACC | |||
| SOX9-F | TGGAAACTTCAGTGGCGCGGA | 225 ng | 108% | 64 oC |
| SOX9-R | AGAGCAAAAGTGGGGGCGCTT | |||
| COL1A1-F | CCTGCGTACAGAACGGCCTCA | 150 ng | 88% | 60 oC |
| COL1A1 -R | CGTCATCGCACAACACCTTGCC | |||
| Col2a- F | GGAATTCGGTGTGGACATAGG | 175 ng | 96% | 60 oC |
| Col2a- R | ACTTGGGTCCTTTGGGTTTG | |||
| TRPV4-F | TCCACCCTATATGAGTCCTCG | 250 ng | 99 | 60 |
| TRPV4-R | TAGGTGCCGTAGTCAAACAGT |





