SOX4 facilitates PGR protein stability and FOXO1 expression conducive for human endometrial decidualization
Figures
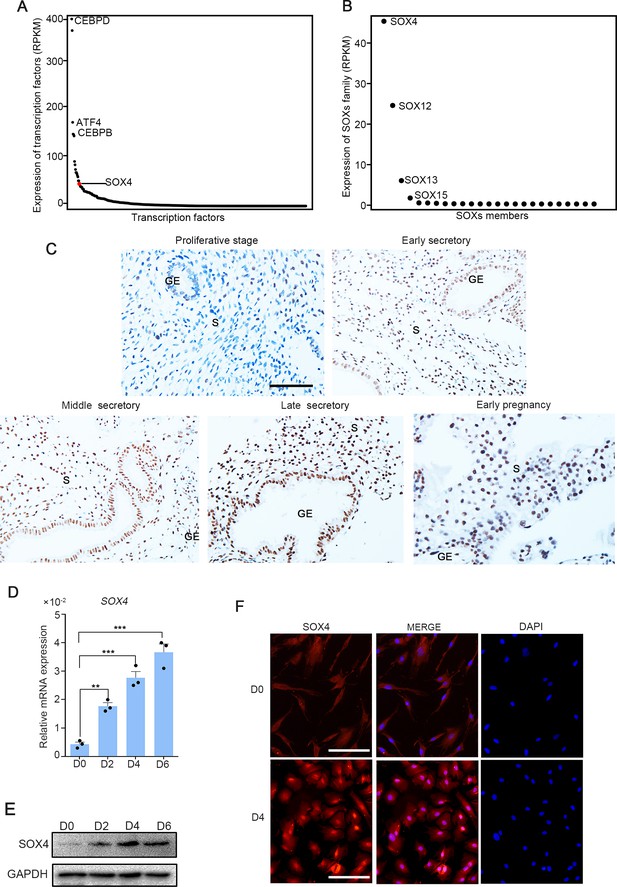
SOX4 is dynamically expressed in human ESCs.
Expression of all transcription factors (A) and SOX family genes (B) in human nondecidualized ESCs by RNA-Seq. The value in Y-axis indicated the RPKM (reads per kilobase per million mapped reads) in RNA-Seq data. (C) Immunohistochemical analysis of endometrial SOX4 protein expression in proliferative, secretory phases (early, middle, and late) of the menstrual cycle and early pregnancy (about 8 weeks). GE: gland epithelium; S: stroma. Scale bar: 100 μm. (D) Expression of SOX4 mRNA levels in decidualized stromal cells at different time points after the E2, MPA, and cAMP treatment. Results are presented as means ± standard error of the mean (SEM); n = 3; **p < 0.005; ***p < 0.0001. (E) Expression of SOX4 protein levels in decidualized stromal cells at different time points after the E2, MPA, and cAMP treatment. (F) Immunofluorescent detection of SOX4 protein localization in the undecidualized (D0) and decidualized (D4) human endometrial stromal cells (HESCs). Scale bar: 100 μm.
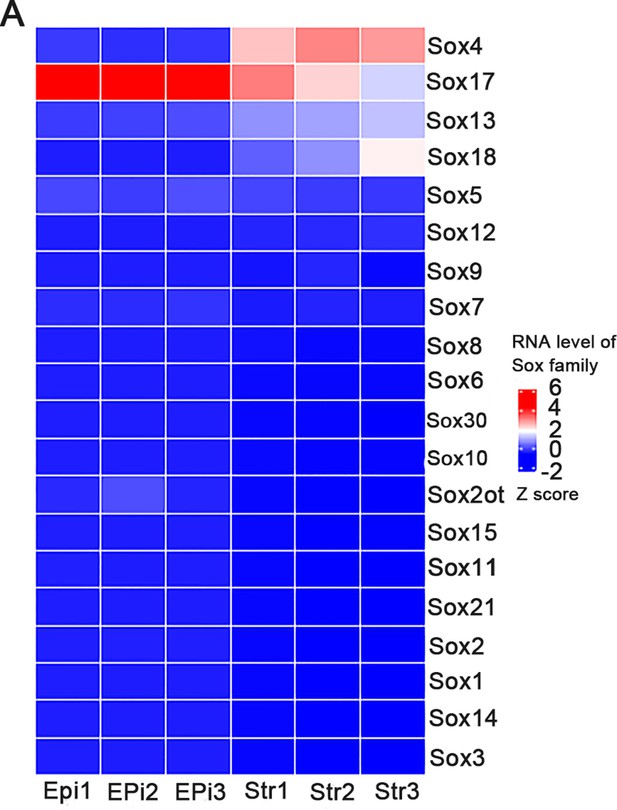
Sox family expression in mouse uterine stromal and epithelial cells.
(A) Heatmap of Sox family members in mouse uterine epithelial and stromal cells by RNA sequencing (RNA-Seq), n = 3.
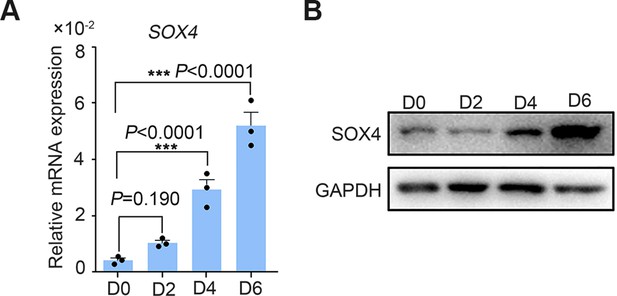
SOX4 expression in the primary stromal cell during the decidualization.
(A, B) Expression of SOX4 mRNA and protein in decidualized primary human endometrial stromal cells (HESCs) from days 0 to 6. Results were presented as means ± standard error of the mean (SEM); n = 3; ***p < 0.001.
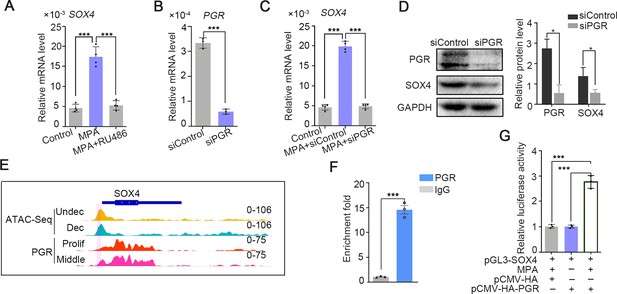
SOX4 is regulated by P4-progesterone receptor (PGR) signaling in human ESCs.
(A) Expression of SOX4 mRNA in the presence of MPA or MPA + RU486 for 2 days in immortalized human endometrial stromal cells (HESCs). Results are presented as means ± standard error of the mean (SEM); n = 3; ***p < 0.0001. (B) RNA level of PGR after siRNA-mediated knockdown. Results are presented as means ± SEM; n = 3; ***p < 0.0001. (C) Expression of SOX4 mRNA in the presence of MPA for 2 days with PGR knockdown. Results were presented as means ± SEM; n = 3; ***p < 0.0001. (D) Protein level of SOX4 and PGR after PGR knockdown in the presence of MPA for 2 days. Band quantification of PGR and SOX4 protein, relative to loading control GAPDH. *p < 0.05 (n = 3). (E) Visualization of PGR binding and chromatin accessibility on SOX4 locus. The chromatin accessibility is depicted in undecidualized and decidualized stromal cells and genome-wide PGR binding is generated from proliferated and middle secretory endometrium as revealed from previous reports. Undec: undecidualized HESCs; Dec: decidualized HESCs; Prolif: proliferative endometrium; Middle: middle phase of secretory endometrium. (F) ChIP assay of potential PGR binding on SOX4 as indicated from (E) in decidualized immortalized HESCs for 2 days. Data are plotted as mean ± SEM; n = 3; ***p < 0.0001. (G) Luciferase activity assay of SOX4 promotor in the presence of MPA and PGR in 293T cells. Results are presented as means ± SEM. n = 3; *p < 0.05; **p < 0.005; ***p < 0.0001.
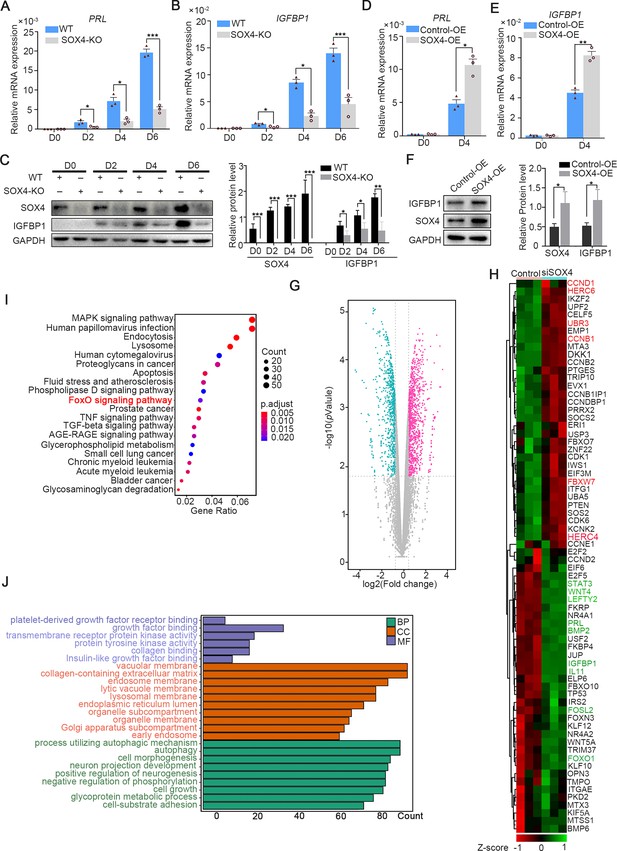
SOX4 regulated genes in decidualized human endometrial stromal cells (HESCs).
mRNA levels of PRL (A) and IGFBP1 (B) in undecidualized and decidualized immortalized HESCs after SOX4 knockout at indicated time points after the E2, MPA, and cAMP treatment. Results are presented as means ± standard error of the mean (SEM); n = 3; *p < 0.05; ***p < 0.0001. (C) Protein levels of IGFBP1 in undecidualized and decidualized HESCs after SOX4 knockout at indicated time points. (D, E) mRNA levels of PRL (C) and IGFBP1 (D) after SOX4 overexpression in undecidualized or decidualized HESCs for 4 days. n = 3; *p < 0.05. (F) Protein levels of SOX4 and IGFBP1 in decidualized HESCs for 4 days with control or SOX4 overexpression. Band quantification of SOX4 and IGFBP1 protein, relative to GAPDH. (G) Differential expressed genes detected by RNA sequencing (RNA-Seq) in immortalized HESCs decidualized for 2 days with control or SOX4 knockdown as visualized by volcano plot. (H) Heatmap of top differential expressed genes from RNA-Seq after SOX4 knockdown in decidualized HESCs. (I, J) KEGG (J) and Gene Ontology (GO) (K) analysis of the differentially expressed genes in RNA-Seq. Results were presented as means ± standard error of the mean (SEM). *p < 0.05; **p < 0.005; ***p < 0.0001. The above experiments were repeated three times.
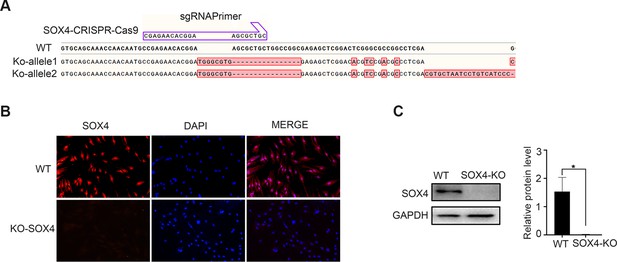
SOX4 knockout in the stromal cell by CRISPR–Cas9.
(A) Schematic diagram of SOX4 knockout by CRISPR/Cas9. (B, C) SOX4 knockout confirmation by immunoblot and immunofluorescence in human endometrial stromal cells (HESCs). n=3; *p < 0.05. Scale bar: 50 μm.
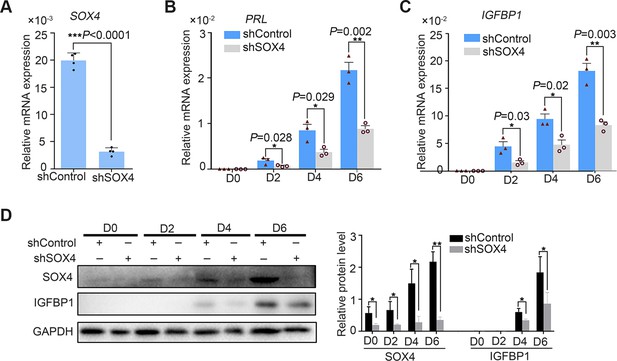
SOX4 knockdown by the shRNA derailed the decidualization in primary stromal cell.
(A) Knockdown efficiency of SOX4 by shRNA. Results were presented as means ± standard error of the mean (SEM); n = 3; ***p < 0.001. (B, C) Expression of PRL and IGFBP1 in the SOX4-knockdown primary decidualized human endometrial stromal cells (HESCs) from days 0 to 6. Results were presented as means ± SEM. n = 3; *p < 0.05; **p < 0.005. (D) Protein levels of SOX4 and IGFBP1 in the SOX4-knockdown primary decidualized HESCs from days 0 to 6.
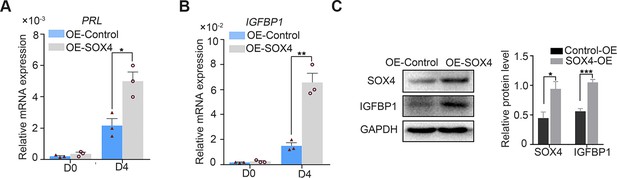
SOX4 overexpression upregulated the expression of decidualization marker genes.
(A, B) Expression of PRL and IGFBP1 in primary decidualized human endometrial stromal cells (HESCs) for 4 days after SOX4 overexpression. Results were presented as means ± standard error of the mean (SEM); n = 3; *p < 0.05; **p < 0.005. (C) SOX4 expression after SOX4 overexpression in the primary decidualized HESCs for 4 days; n=3; *p < 0.05; ***p < 0.00 1.
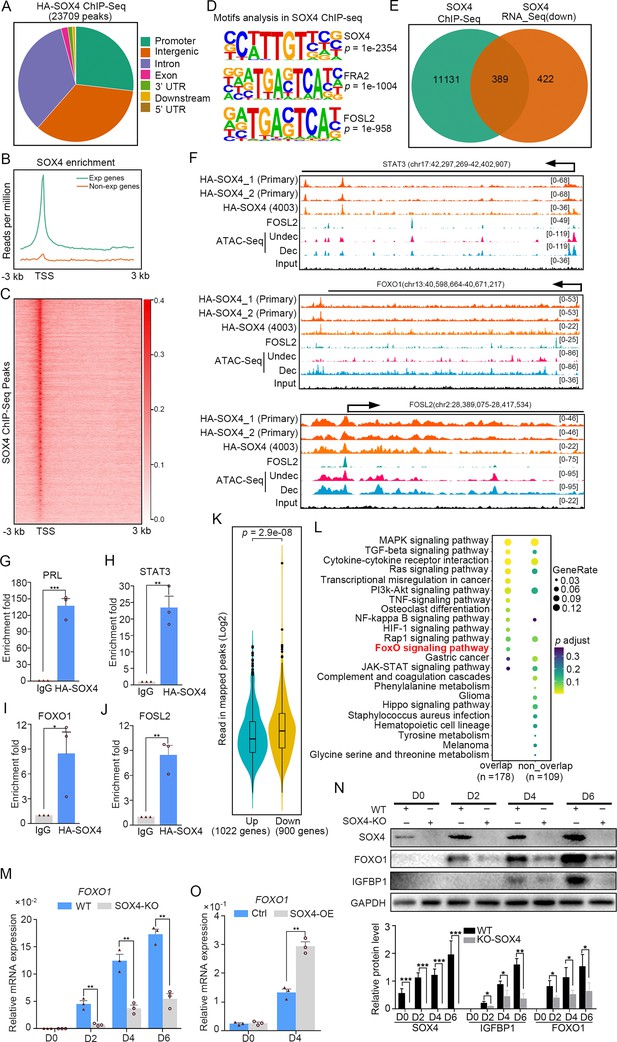
Genome wide binding of SOX4 in decidualized stromal cells.
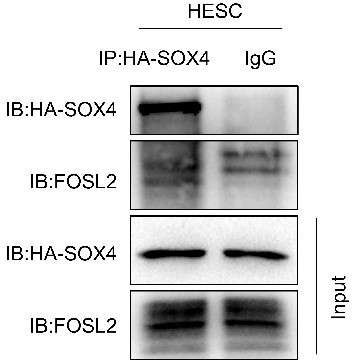
(A) Distribution of SOX4-binding peaks as revealed from SOX4 ChIP-Seq in SOX4 overexpressed (HA-SOX4) immortalized human endometrial stromal cells (HESCs) decidualized for 2 days. (B) Distribution SOX4 binding in genebody. (C) Heatmap of SOX4-binding sites distribution. (D) Motif analysis of SOX4-binding sites. (E) Venn diagram of SOX4 directly binding peaks and SOX4 regulated genes after SOX4 knockdown. (F) SOX4-binding site in the STAT3, FOXO1, and FOSL2 with progesterone receptor (PGR) binding and chromatin accessibility in both primary stromal cell and stromal cell line. The chromatin accessibility is depicted in undecidualized and decidualized stromal cells and genome-wide PGR binding is generated from proliferated and middle secretory endometrium as revealed from previous reports. Undec: undecidualized HESCs; Dec: decidualized HESCs. (G–J) ChIP-qPCR assay of SOX4 binding on PRL, STAT3, FOXO1, and FOSL2 in HA-SOX4 overexpressed decidualized HESCs. Results were presented as means ± standard error of the mean (SEM); n = 3; *p < 0.05; **p < 0.005; ***p < 0.0001. (K) Read number of SOX4 binding in SOX4 regulated genes after SOX4 knockdown. (L) KEGG analysis of overlapped genes of SOX4 directly binding and SOX4 downregulated genes as well as nonoverlapped genes. (M) mRNA levels of FOXO1 in SOX4 knockout undecidualized and decidualized HESCs at indicated time points. Results were presented as means ± SEM; n = 3; **p < 0.005. (N) Protein levels of SOX4, FOXO1, and IGFBP1 in SOX4 knockout undecidualized and decidualized HESCs at indicated time points. Band quantification of indicated proteins, relative to loading control GAPDH. *p < 0.05; ***p < 0.0001. n = 3. (O) mRNA levels of FOXO1 after SOX4 overexpression in undecidualized or decidualized HESCs for 4 days. Results were presented as means ± SEM; n = 3; **p < 0.005. The above experiments were repeated three times.
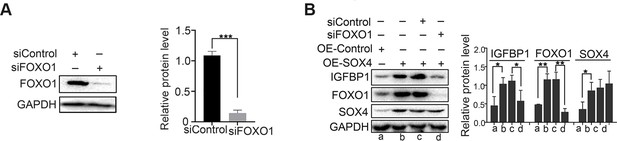
FOXO1 knockdown abolished the effect of SOX4 overexpression on decidualization.
(A) FOXO1 expression after FOXO1 knockdown in the primary decidualized human endometrial stromal cells (HESCs). (B) Protein levels of FOXO1, IGFBP1, and SOX4 after overexpression of SOX4 in the presence of FOXO1 knockdown or not in decidualized HESCs for 4 days; n=3; *p < 0.05; **p < 0.005.
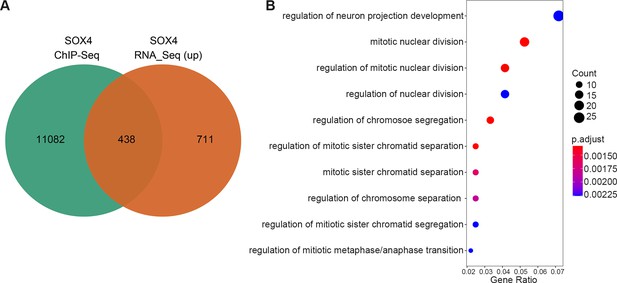
The upregulated genes after SOX4 knockdown with SOX4-binding sites in their regulatory regions.
(A) Venn diagram of SOX4 directly binding peaks and upregulated genes after SOX4 knockdown. (B) Gene Ontology (GO) analysis of the overlapped gene list in A.
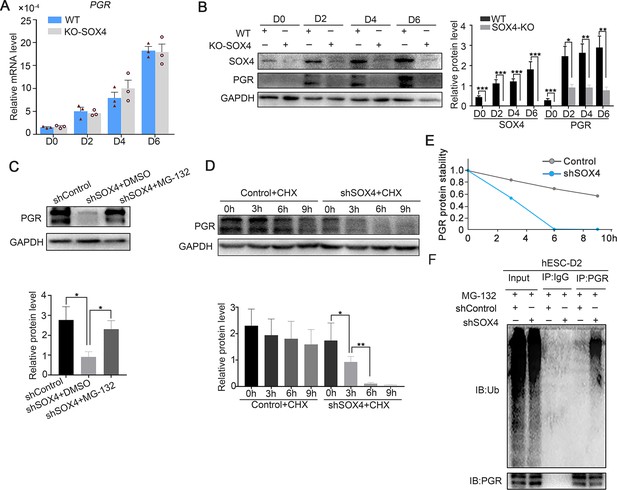
SOX4 stabilizes progesterone receptor (PGR) through repressing the ubiquitin–proteasome pathway.
(A) mRNA levels of PGR after SOX4 knockout in decidualized immortalized human endometrial stromal cells (HESCs) at indicated time points after the E2, MPA, and cAMP treatment. Results were presented as means ± standard error of the mean (SEM); n = 3. (B) Protein levels of PGR and SOX4 after SOX4 knockout in decidualized HESCs at indicated time points after the E2, MPA, and cAMP treatment. (C) Protein levels of PGR in the presence of shSOX4 or MG132. MG-132 is added 6 hr before collecting the immortalized cells. (D, E) Protein levels of PGR in the presence of protein synthesis inhibitor cycloheximide (CHX) in SOX4-knockdown cells. (F) PGR ubiquitination after immunoprecipitation with PGR antibody and blotted with ubiquitin antibody in SOX4-knockdown immortalized HESCs decidualized for 2 days. Cells were treated with MG-132 before cell lysis. The value for relative protein level is band quantification of indicated protein, relative to GAPDH. n=3. *p < 0.05; **p < 0.005; ***p < 0.001.
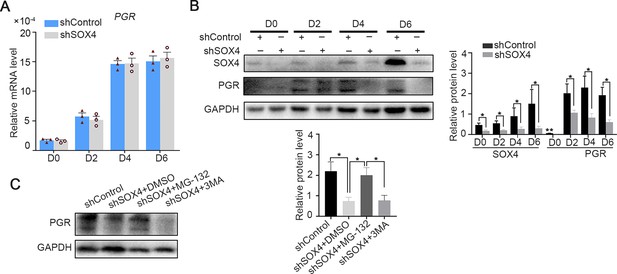
Regulation of SOX4 on progesterone receptor (PGR) in primary decidualized stromal cells.
(A) Expression of PGR mRNA after SOX4 knockdown in primary decidualized human endometrial stromal cells (HESCs) cultured with EPC from 0 to 6 days. Results were presented as means ± standard error of the mean (SEM); n = 3. (B) Protein levels of PGR after SOX4 knockdown in primary decidualized HESCs cultured with EPC from 0 to 6 days. (C) Protein levels of PGR and SOX4 in the presence of proteasome inhibitor MG132 or autophagy inhibitor 3MA. The inhibitors were added 6 hr before collecting the immortalized cells. n=3; *p < 0.05; **p < 0.005.
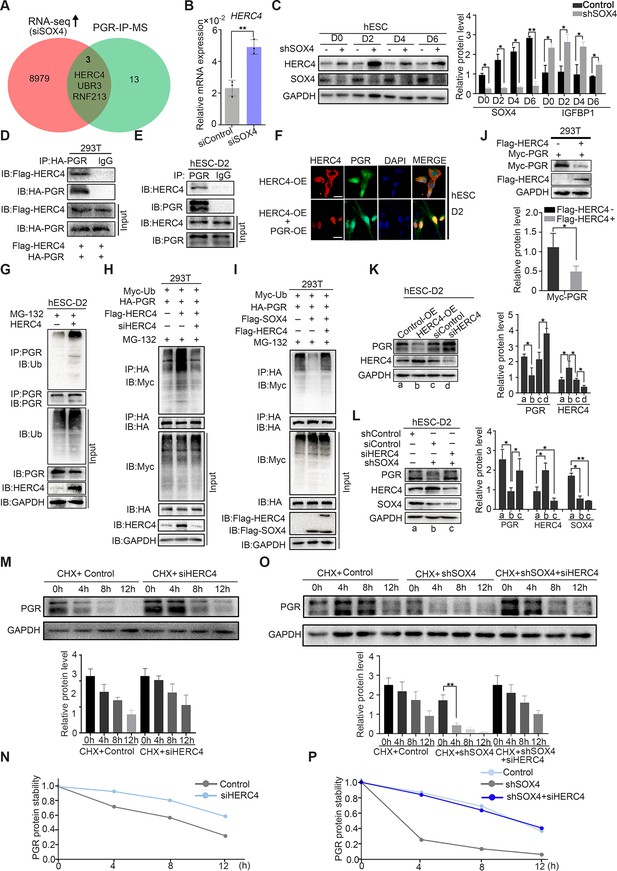
SOX4 inhibits progesterone receptor (PGR) degradation by regulating E3 ligase HERC4.
(A) Venn diagram of overlapping genes between upregulated genes in SOX4 knockdown from RNA sequencing (RNA-Seq) and PGR-associated protein form IP-MS. mRNA and protein levels of HERC4 in the absence of SOX4 by qPCR (B) and western blot (C). Results were presented as means ± standard error of the mean (SEM); n = 3. (D) Protein interaction between PGR and HERC4 after overexpression of HA-PGR and Flag-HERC4 in 293T cells. (E) Protein interaction between PGR and HERC4 after overexpression of HA-PGR and Flag-HERC4 in decidualized immortalized human endometrial stromal cells (HESCs) for 2 days, D2: 2 days. (F) Localization of PGR and HERC4 after overexpression of PGR and HERC4 in decidualized HESCs for 2 days, scale bar: 100 μm. (G) PGR ubiquitination at the present of MG-132 after overexpression of HERC4 in decidualized HESCs for 2 days.(H) PGR ubiquitination at the present of MG-132 and ubiquitin with HERC4 overexpression or knockdown in 293T cells. (I) PGR ubiquitination at the present of MG-132, ubiquitin, and SOX4 with or without HERC4 in 293T cells. (J) Degeneration of PGR after HERC4 overexpression in 293T cells. (K) PGR protein levels at the present of HERC4 overexpression and in HERC4 knockdown decidualized immortalized HESCs for 2 days. (L) Protein levels of PGR, HERC4, and SOX4 after SOX4 and/or HERC4 knockdown in decidualized immortalized HESCs for 2 days. (M, N) The protein half-life of PGR after HERC4 knockdown at the present of protein synthesis inhibitor cycloheximide (CHX) in 2 days decidualized immortalized HESCs. (O, P) The protein half-life of PGR after SOX4 and/or HERC4 knockdown at the present of protein synthesis inhibitor CHX in 2 days decidualized immortalized HESCs. The value for relative protein level is band quantification of indicated protein, relative to GAPDH. The above experiments were repeated three times. n=3; *p < 0.05; **p < 0.005.
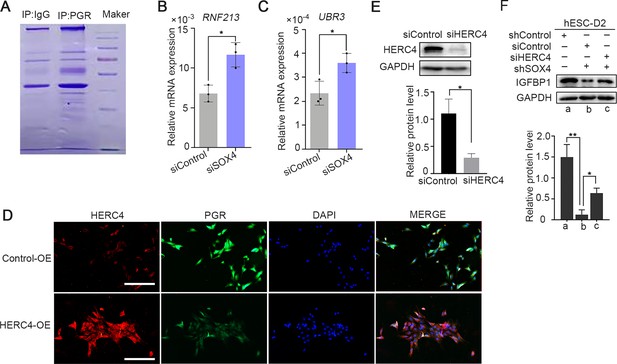
The SOX4 regulated ubiquitin E3 ligase expression during the endometrial stroma decidualization.
(A) Coomassie blue staining of PAGE-Gel with progesterone receptor (PGR) immunoprecipitated complex. (B, C) Expression of RNF213 and UBR3 in SOX4-knockdown human endometrial stromal cells (HESCs). Results were presented as means ± standard error of the mean (SEM); n = 3; *p < 0.05. (D) PGR expression after HERC4 overexpression in HESCs as detected by immunofluorescence, scale bar: 100 μm. (E) Knockdown efficiency of HERC4 by siHERC4. The above experiments were repeated three times. (F) Protein levels of IGFBP1 in the indicated treatment. n=3; **p < 0.005.
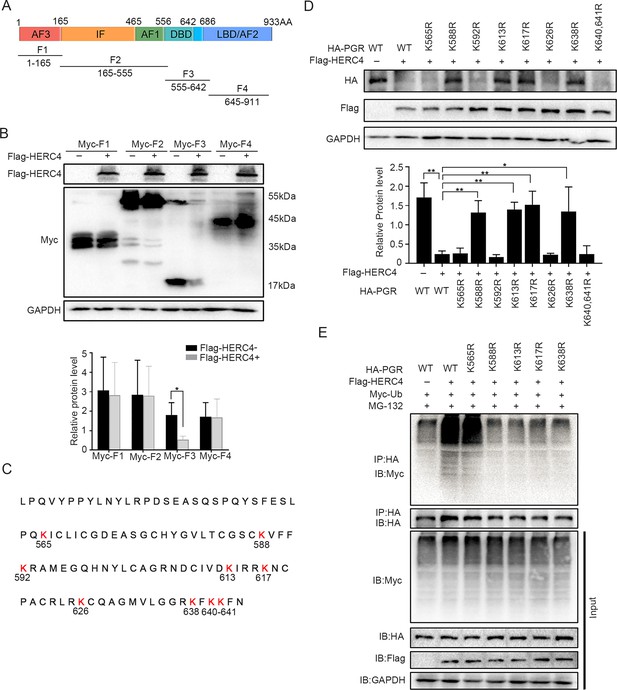
HERC4 mediates progesterone receptor (PGR) ubiquitination at K588, K613, K617, and K638.
(A) Schematic diagram of PGR structure. F1–F4 represents four different functional domains, respectively. (B) Protein levels of different fragments of PGR (Myc-tagged F1–F4) at the present of HERC4 in 293T cells. (C) Lysine (K) sites in PGR DBD domain. (D) Protein levels of PGR after Lysine mutant to Arginine in DBD with HERC4 overexpression in 293T cells.The value for relative protein level is band quantification of indicated protein, relative to GAPDH. (E) PGR ubiquitination after Lysine mutant to Arginine in DBD at the present of HERC4, ubiquitin and MG-132 in 293T cells. n=3; *p < 0.05; **p < 0.005.
The predicted lysine sites for the ubiquitin modification in progesterone receptor (PGR) protein by the E3 ligase HERC4.
(A) UbiBrowser (http://ubibrowser.ncpsb.org) was applied to predict the potential site recognized by E3 ligase HERC4.
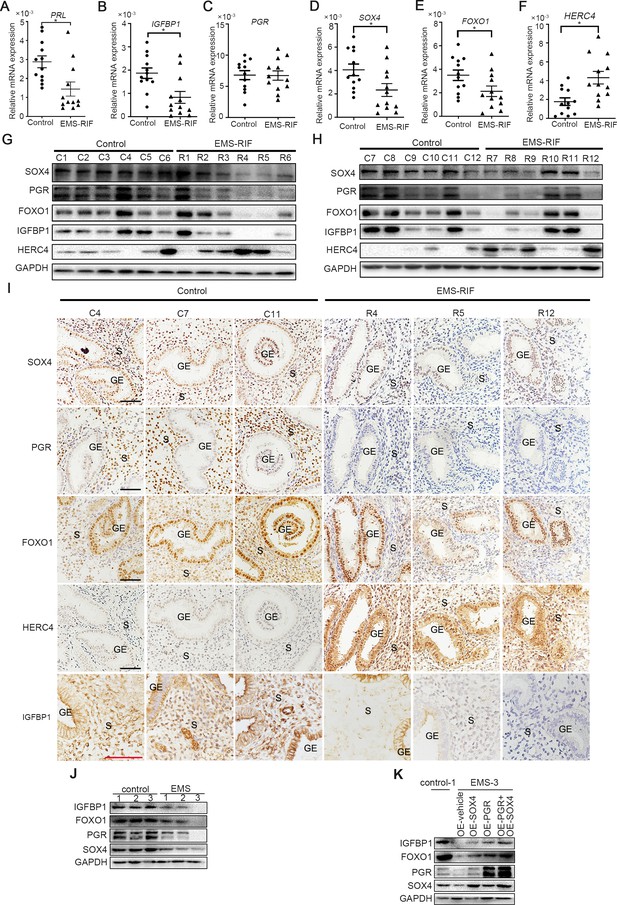
Endometrial SOX4 expression in RIF of women with EMS undergoing IVF treatment.
(A–F) Expression of SOX4, FOXO1, IGFBP1, PRL, PGR, and HERC4 in mid-secretory endometrium from control (n = 12) and EMS-RIF (n = 12). Results were presented as means ± standard error of the mean (SEM). *p < 0.05. (G, H) Protein levels of SOX4, FOXO1, IGFBP1, PGR, and HERC4 in mid-secretory endometrium from control (n = 12) and EMS-RIF (n = 12). C1–C12 and R1–R12 represent tissues from different patient. (I) Localization of SOX4, FOXO1, IGFBP1, PGR, and HERC4 in control (n = 3) and EMS-RIF groups as detected by immunostaining. C4, C7, C11, R4, R5, and R12 represent tissues from different patient. GE: gland epithelium; S: stroma. Scale bar: 100 μm. (J) Protein levels of SOX4, IGFBP1, FOXO1, and PGR in primary human endometrial stromal cells (HESCs) of control and EMS by western blot. (K) Protein levels of IGFBP1, FOXO1, PGR, and SOX4 in decidualized primary endometrial stromal cells from EMS-3 after overexpression of SOX4 and/or PGR.
Histology of pelvic endometriosis tissue.
(A) HE staining of paraffin section from the pelvic endometriosis.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Cell line (H. sapiens) | T HESCs | ATCC | CRL-4003RRID: CVCL_C464 | |
| Antibody | Anti-SOX4(Rabbit polyclonal) | Abcam | Cat # ab80261 RRID:AB_1658989 | WB (1:600)ICC (1:1000)IF (1:300) |
| Antibody | Anti-SOX4(Rabbit polyclonal) | Diagenode | Cat # C15310129 | IP (1:100) |
| Antibody | Anti-PGR(Rabbit monoclonal) | CST | Cat # 8757 RRID:AB_2797144 | WB (1:500)ICC (1:3000)IF (1:200)IP (1:50)ChIP (1:50) |
| Antibody | Anti-HERC4(Rabbit polyclonal) | Protein tech | Cat # 13691-1-AP RRID:AB_10596480 | WB (1:1000)ICC (1:1000)IF (1:300) |
| Antibody | Anti-FOXO1(Rabbit polyclonal) | Abcam | Cat # ab39670 RRID:AB_732421 | WB (1:1000)ICC (1:3000) |
| Antibody | Anti-IGFBP1(Rabbit polyclonal) | Abcam | Cat # ab228741 | WB (1:1000)IHC (1:500) |
| Antibody | Anti-Ub(Mouse monoclonal) | CST | Cat # 3936 RRID:AB_331292 | WB (1:1000) |
| Antibody | Anti-HA(Rabbit monoclonal) | CST | Cat # C29F4 | WB (1:1000)IP (1:100)ChIP (1:100) |
| Antibody | Anti-Myc(Mouse monoclonal) | CST | Cat # 2276 RRID:AB_331783 | WB (1:1000) |
| Antibody | Anti-Flag(Mouse monoclonal) | Sigma | Cat # F9291 RRID:AB_439698 | WB (1:1000) |
| Antibody | Anti-GAPDH(Rabbit polyclonal) | Abmart | Cat # P30008 | WB (1:2000) |
| Chemical compound, drug | E2 | Sigma | E8875 | Final concentration: 10 nM |
| Chemical compound, drug | MPA | Sigma | M1629 | Final concentration: 1 μM |
| Chemical compound, drug | cAMP | MCE | HY-B0764 | Final concentration: 0.5 mM |
| Chemical compound, drug | MG132 | Selleck | S2619 | Final concentration: 20 μM |
| Chemical compound, drug | 3-MA | Selleck | S2767 | Final concentration: 20 mM |
| Chemical compound, drug | CHX | MedChemExpress | HY-12320 | Final concentration: 20 μg/ml |
| Chemical compound, drug | Insulin–transferrin–selenium | Thermo Fisher | 51500-056 | Final concentration: 1% |
| Chemical compound, drug | Puromycin | MedChemExpress | HY-15695 | Final concentration: 500 ng/ml |
| Chemical compound, drug | RNAi MAX | Thermo Scientific | 13778030 | 7.5 μl/6-well |
| Chemical compound, drug | Lipofectamine 2000 | Thermo Scientific | 11668030 | 5 μl/6-well/2500 ng DNA |
| Commercial assay or kit | Dual-Luciferase Reporter Assay System | Promega | E1910 | |
| Commercial assay or kit | ChIP-IT high Sensitivity | Active Motif | 53,040 | |
| Commercial assay or kit | KAPA DNA HyperPrep Kit | Roche | KK8502 |
Additional files
-
Supplementary file 1
Clinical information of all patients and controls.
- https://cdn.elifesciences.org/articles/72073/elife-72073-supp1-v2.docx
-
Supplementary file 2
All primers used in this study.
- https://cdn.elifesciences.org/articles/72073/elife-72073-supp2-v2.docx
-
Supplementary file 3
The plasmids used in this study.
- https://cdn.elifesciences.org/articles/72073/elife-72073-supp3-v2.docx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/72073/elife-72073-transrepform1-v2.docx