Transgenic quails reveal dynamic TCF/β-catenin signaling during avian embryonic development
Figures
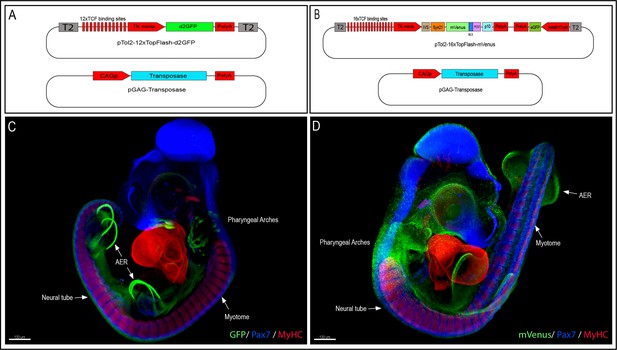
Generation of the 12xTF-d2GFP and the 16xTF-VNP transgenic Japanese quail lines.
(A) Vectors used to generate the 12xTFd2GFP line and (B) the 16xTF-VNP line. (C) An E3.5 12xTFd2GFP embryo and (D) an E3 16xTF-VNP embryo cleared with the 3DISCO method, showing an overview of the TCF/β-catenin reporter activities in these lines. Embryos stained for Pax7 (blue), MyHC (red), and GFP or mVenus (green). Scale bar 100 µm. AER, apical ectodermal ridge.
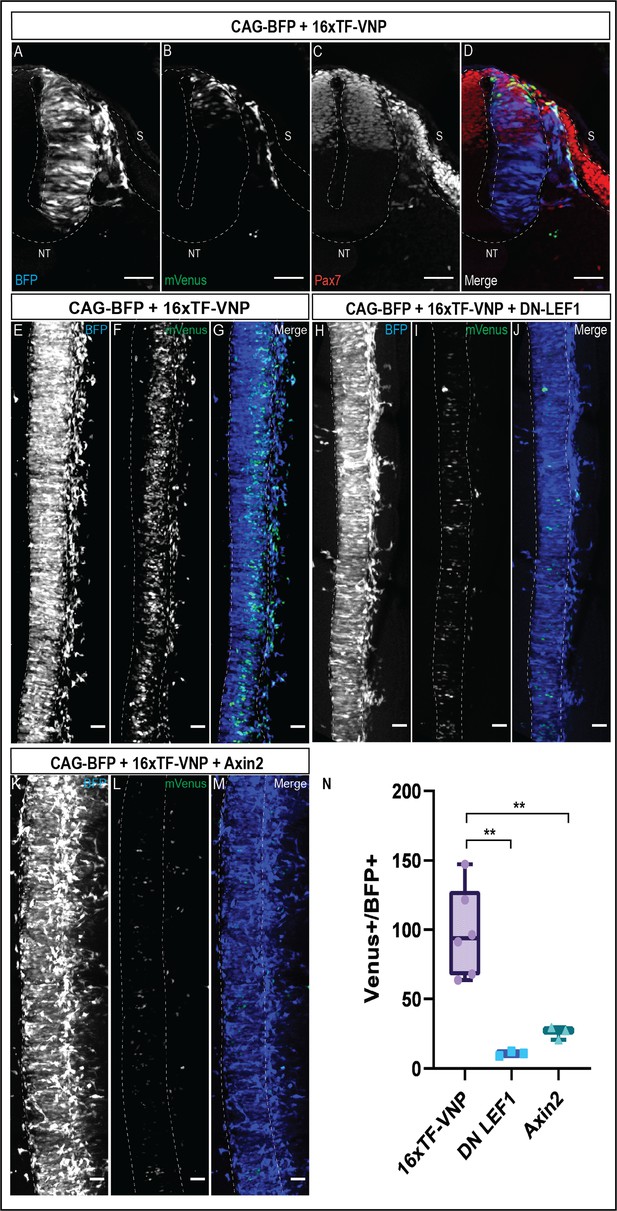
Expression of the 16xTF-VNP in the neural tube and migrating neural crest.
(A–D) Transverse sections of an E2.5 chick embryo electroporated in the NT with a DNA mix containing a CAG-TagBFP construct as a marker for electroporated cells, and the 16xTF-VNP construct. The sections were immunostained for BFP (blue), mVenus (green), and Pax7 (red). It shows that the reporter activity is weaker in the dorsal NT and sharply increases in the migrating NC cells. Scale bar = 30 µm. S, somite; NT, neural tube. (E–G) Confocal stack of a dorsal view of an E2.5 chick embryo electroporated in the NT with a DNA mix containing a CAG-TagBFP construct as a marker for electroporated cells (internal control) and the 16xTFVNP construct. Scale bar 10 µm. (H–J) Confocal stack of a dorsal view of an E2.5 chick embryo electroporated in the NT with a DNA mix containing a CAG-TagBFP construct as a marker for electroporated cells (internal control), the 16xTFVNP construct and the dominant negative (DN) form of LEF1 transcription cofactor. Scale bar 10 µm. (K–M) Confocal stack of a dorsal view of an E2.5 chick embryo electroporated in the NT with a DNA mix containing a CAG-TagBFP construct as a marker for electroporated cells (internal control), the 16xTFVNP construct and the Wnt-inhibiting molecule Axin2. Scale bar 10 µm. (N) Quantification of the mVenus+ cells out of the total BFP+ cells in each electroporated embryo. The quantifications demonstrate a 9-fold decrease in the number of mVenus+/BFP+ cells in embryos co-electroporated with CAG-TagBFP, 16xTF-VNP, and DN-LEF1 and a 3.8-fold decrease in the number of mVenus+/BFP+ cells in embryos co-electroporated with CAG-TagBFP, 16xTF-VNP, and Axin2. *p-value ≤ 0.05. N = 6.
-
Figure 1—figure supplement 1—source data 1
Source data file for the quantification shown in Figure 1—figure supplement 1, panel N.
- https://cdn.elifesciences.org/articles/72098/elife-72098-fig1-figsupp1-data1-v2.xlsx
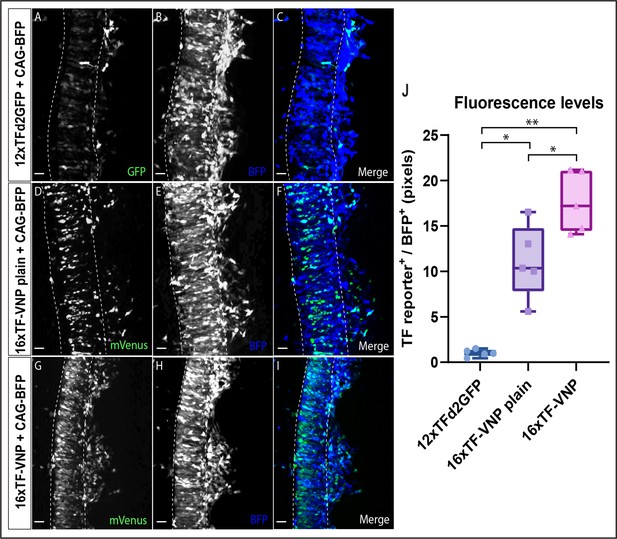
Comparison of reporter fluorescence levels.
(A–C) Confocal stack of a dorsal view of an E2.5 chick embryo electroporated in the NT with a DNA mix containing a CAG-TagBFP construct as a marker for electroporated cells and the 12xTFd2GFP. (D–F) Confocal stack of a dorsal view of an E2.5 chick embryo electroporated in the NT with a DNA mix containing a CAG-TagBFP construct as a marker for electroporated cells and the 16xTFVNP construct without the IVS/Syn21 and p10 translation enhancer sequences, termed here – 16xTF-VNP plain. (G–I) Confocal stack of a dorsal view of an E2.5 chick embryo electroporated in the NT with a DNA mix containing a CAG-TagBFP construct as a marker for electroporated cells and the 16xTF-VNP. (J) A quantification of the fluorescence levels (as measured in pixels) of the GFP+ or mVenus+ cells, normalized to the fluorescence levels of BFP+ cells. Results demonstrate an 11-fold increase in fluorescence levels between the 12xTFd2GFP and the 16xTF-VNP plain constructs, while a 17-fold increase is shown when comparing the 12xTFd2GFP to the 16xTF-VNP construct. An increase of about 1.5-fold is demonstrated between the 16xTH-VNP plain and the 16xTF-VNP constructs. **p-value 0.001–0.01; *p-value ≤ 0.05 N = 5 embryos per treatment. Scale bar 10 µm.
-
Figure 1—figure supplement 2—source data 1
Source data file for the quantification shown in Figure 1—figure supplement 2, panel J.
- https://cdn.elifesciences.org/articles/72098/elife-72098-fig1-figsupp2-data1-v2.xlsx
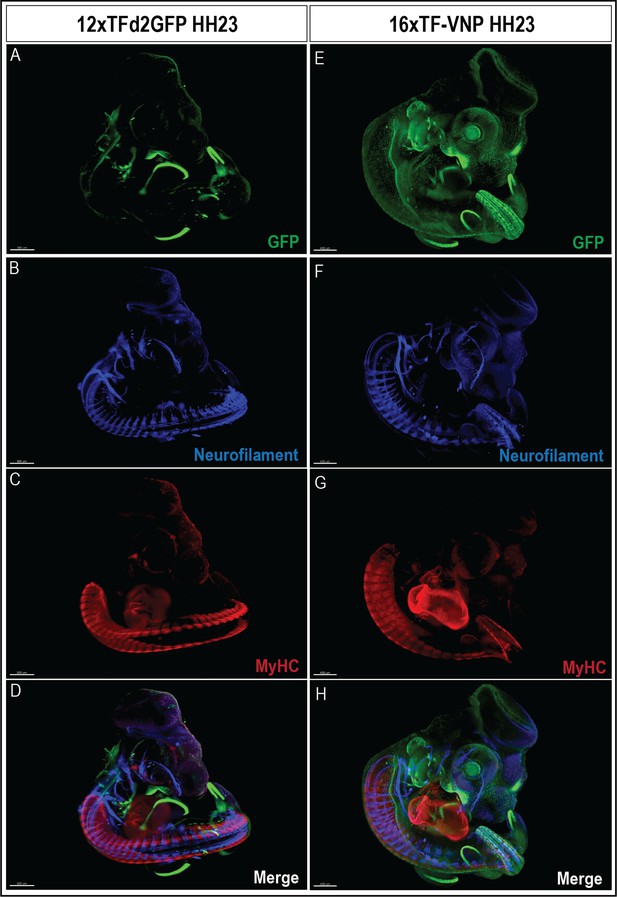
TCF/β-catenin reporter expression in E4 embryos.
(A–D) An HH23 12xTFd2GFP embryo immunostained for GFP (green), neurofilament (blue), and MyHC (red), showing the TCF/β-catenin reporter in the pharyngeal arches, maxilla, AER, and the ventrolateral border of trunk somites (VLL). Scale bar 500 µm. (E–H) An HH23 16xTF-VNP embryo immunostained for mVenus (green), neurofilament (blue), and MyHC (red), showing the TCF/β-catenin reporter in the head, maxilla, pharyngeal arches, AER, neural tube, migrating neural crest cells, and the somite VLL. Note also the GFP expression in the eye, driven by the β-crystallin promoter for easier screening of transgenic embryos. Scale bar 400 µm. Embryonic structure legend as indicated in Figure 1.
The 16xTF-VNP reporter is a sensitive reporter of the TCF/β-catenin signaling activity.
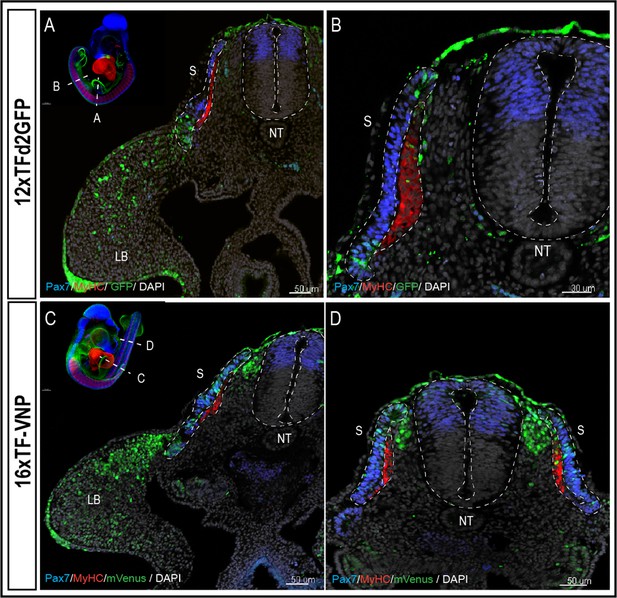
Transverse sections at the levels of the front limb (A, C) and the trunk (B,D) of E3 12xTF-d2GFP (A–B) and 16xTF-VNP (C–D) transgenic embryos stained for GFP or mVenus (green), Pax7 (blue), MyHC (red), and DAPI (gray). Inserts show the levels at which the sections were made. Scale bar 50 µm (A,C,D) or 30 µm (B). S, somite; NT, neural tube; LB, limb bud.
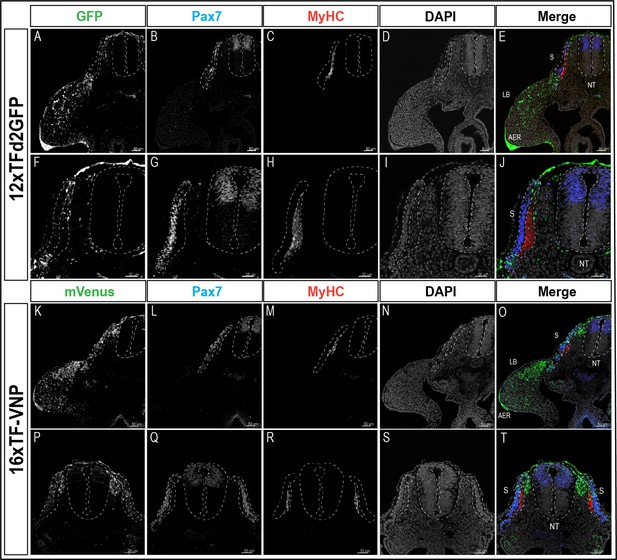
Transverse sections at the levels of the front limb and the trunk of E3 12xTFd2GFP and 16xTF-VNP transgenic embryos stained for GFP or mVenus, respectively (green), Pax7 (blue), MyHC (red), and DAPI (gray).
The image details the different channels for Figure 2. Scale bar 50 µm. S, somite; NT, neural tube; LB, limb bud; AER, apical ectodermal ridge.
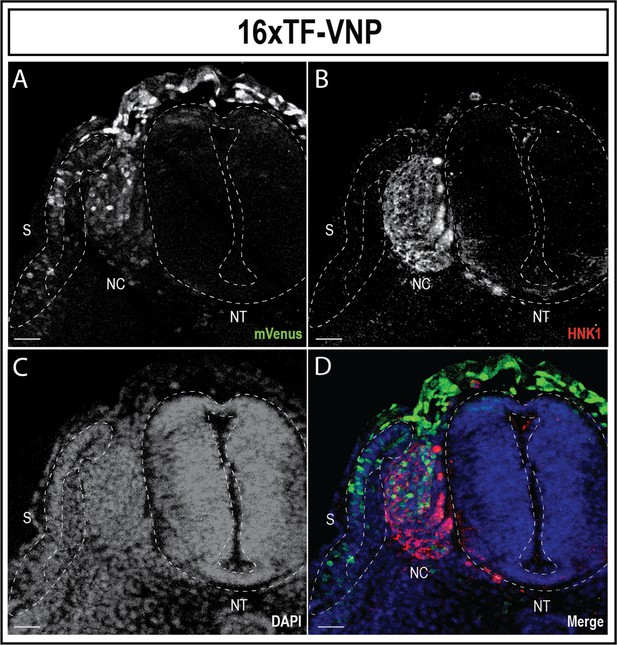
TCF/β-catenin reporter dynamic activity in NC cells.
Transverse sections of E3 (HH19) 16xTF-VNP stained for mVenus (green; A,D), HNK1 (red; B,D), and DAPI (blue; C,D). The TCF/β-catenin reporter is significantly upregulated in emerging NC cells and is rapidly decreased as the NC proceed along their dorso-ventral migration path. Scale bar 50µm. NT, neural tube; NC, neural crest cells; S, somite.
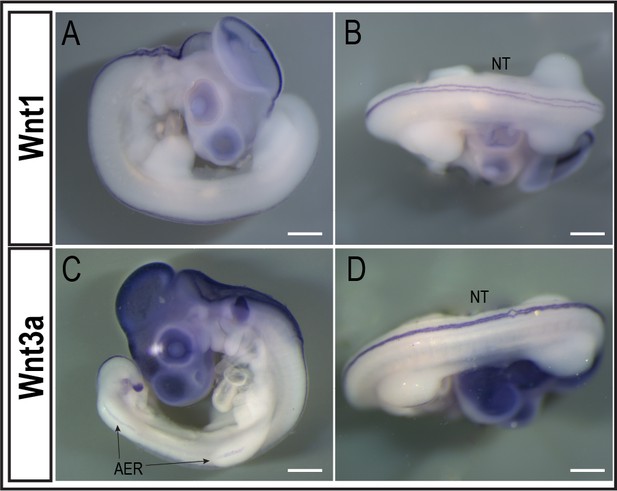
In situ hybridization of Wnt3a and Wnt1 mRNA in quail embryos.
In situ hybridization of Wnt1 (A,B) and Wnt3a (C,D) in E3.5 quail embryos. Both Wnt1 and Wnt3a mRNA transcripts are presented in the NT (A,B,D). Wnt3a mRNA transcript is also present in the AER (C). Scale bar 200µm. NT, neural tube; AER, apical ectodermal ridge.
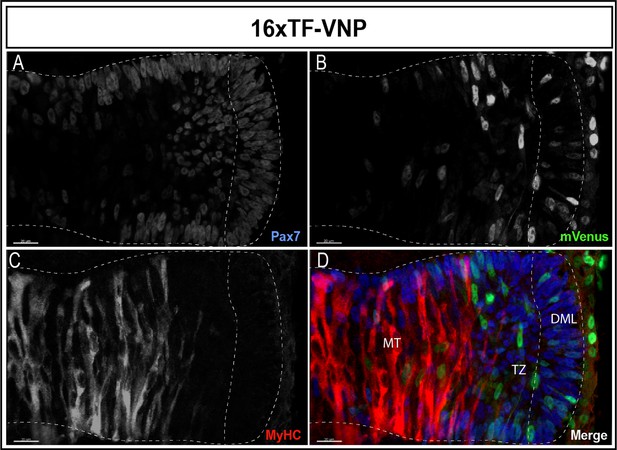
Dynamic TCF/β-catenin signaling activity in somites.
An optical section of an E2.5 embryo somite stained for Pax7 (A,D); blue mVenus (B,D); green, and MyHC (C,D); red reveals high and low reporter expression levels in epithelial cells of the DML, while high expression is seen in elongating myocytes located in the transition zone (TZ). The reporter levels are decreasing in fully elongated myocytes of the myotome. Scale bar 20 µm. DML, dorso-medial lip; MT, myotome.
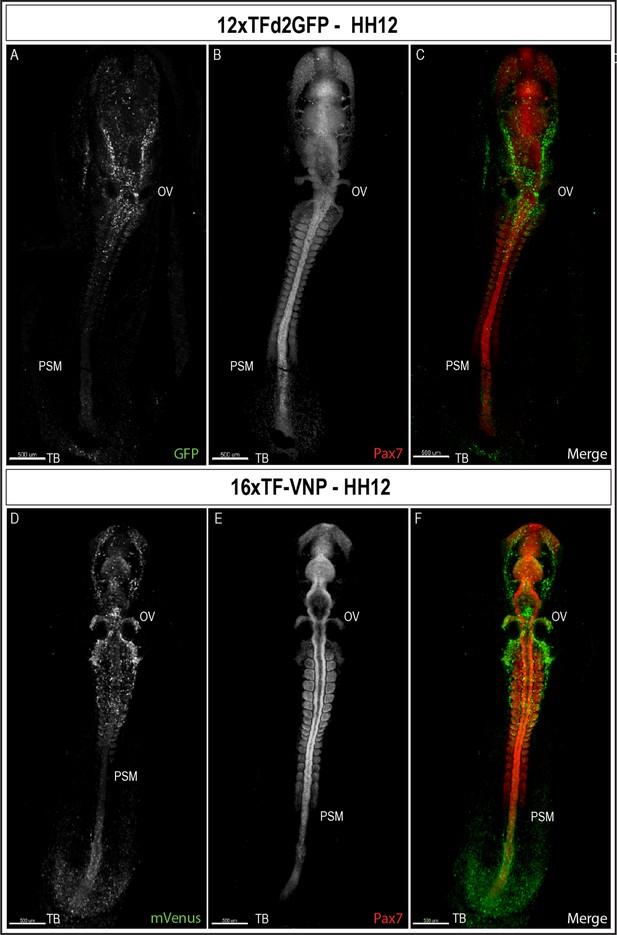
TCF/β-catenin reporter expression in HH12 embryos.
Whole-mount view of HH12 12xTFd2GFP (A–C) and 16xTF-VNP (D–F) embryos immunostained for GFP or mVenus (green) and Pax7 (Red). Both embryos present strong TCF/β-catenin reporter activity in migrating cephalic neural crest in the head area, somites, the posterior neural tube and the tail bud area. Scale bar 500 µm. OV, otic vesicle; PSM, presomitic mesoderm; TB, tail bud.
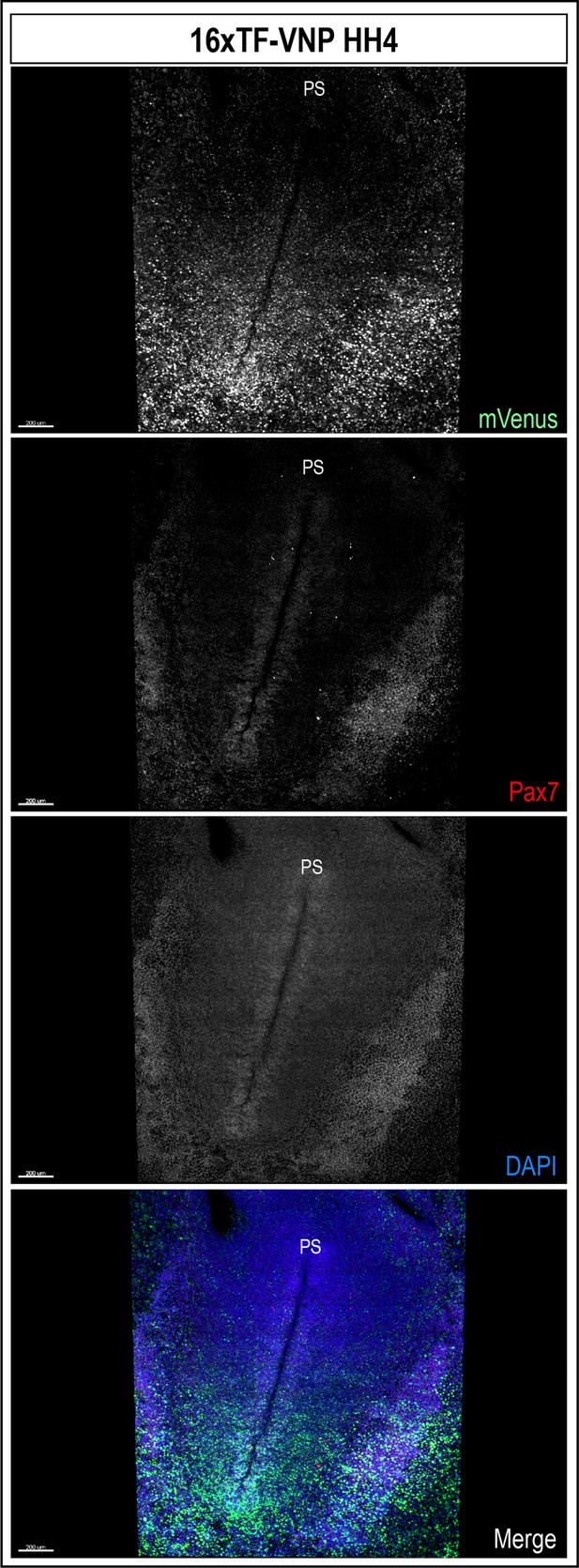
Whole-mount view of HH4 16xTF-VNP embryo immunostained for mVenus (green), Pax7 (red), and DAPI (blue).
The TCF/β-catenin reporter activity is strongly expressed in the posterior half of the gastrulating embryo. Scale bar 200 µm. PS, primitive streak.
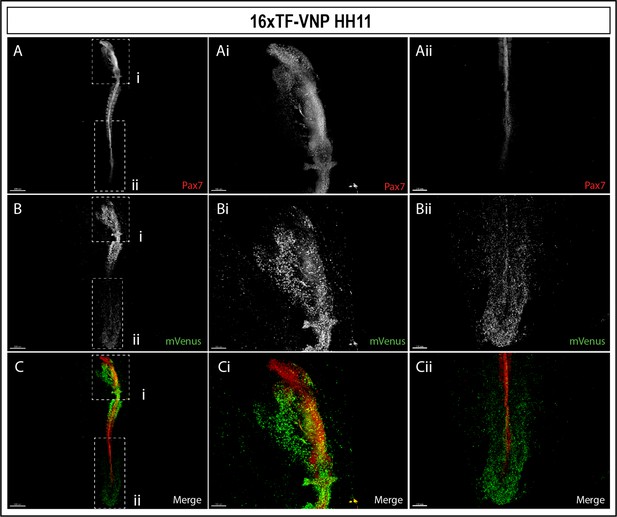
Whole-mount view of HH11 16xTF-VNP embryo immunostained for mVenus (green) and Pax7 (red).
This embryo presents strong TCF/β-catenin reporter activity in migrating cephalic neural crest in the head area, pharyngeal arches, somites, the posterior neural tube, PSM and the tail bud area. Scale bar 500 µm (left panel), 150 µm (middle panel), and 200 µm (right panel). Embryonic structure legend as indicated in Figure 4.
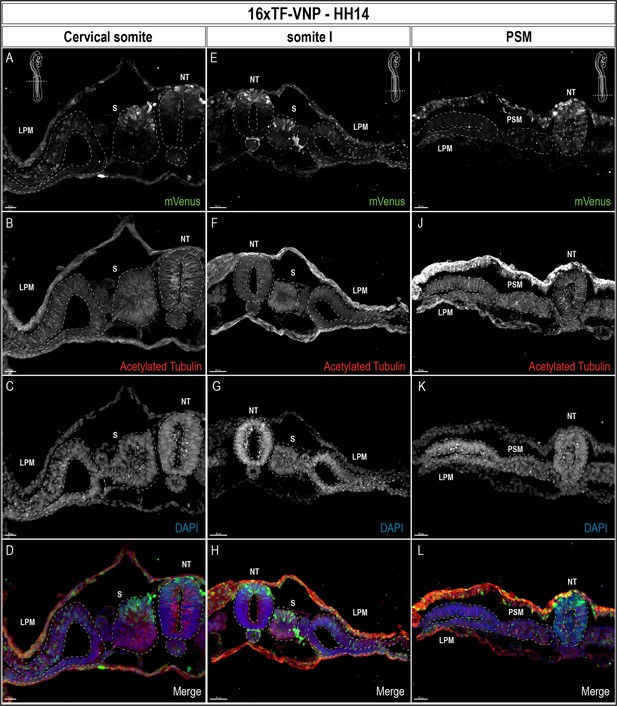
TCF/β-catenin reporter expression pattern in transverse sections of a 14HH 16xTF-VNP embryo.
Transverse sections at the level of the cervical somites (A–D), somite I (E–H), and the anterior PSM (I–L) of a 14HH 16xTF-VNP embryo, immunostained for mVenus (green), Acetylated Tubulin (red), and DAPI (blue). At the PSM level, Venus is expressed in the entire neural tube, in individual ectodermal cells and at low level in the PSM. At somite I level, mVenus is observed in the dorsal neural tube, the dorsal part of the somite, the ectoderm and the lateral plate mesoderm. At the level of cervical somites, mVenus expression is faint in the dorsal neural tube and it is stronger in migrating neural crest cells. The nuclear localization of mVenus is detectable by its colocalization with DAPI. Inserts show the levels at which the sections were made. Scale bar 20 µm (A–D), 50 m µm (E–H), or 30 µm (I–L). NT, neural tube; S, somite; LPM, lateral plate mesoderm.
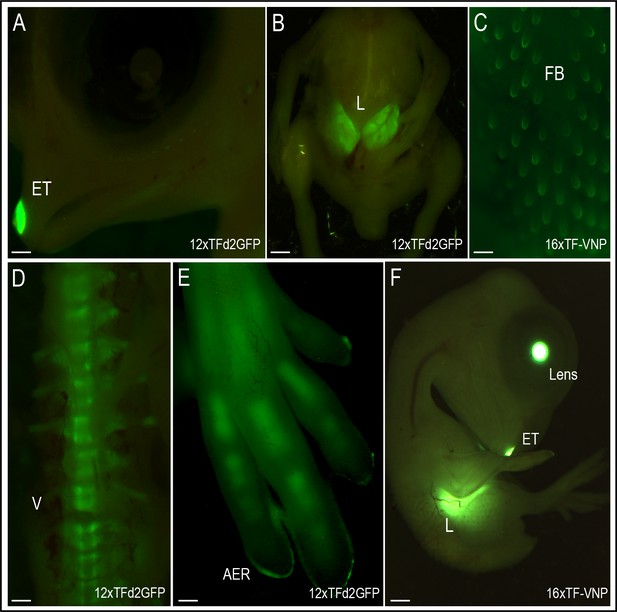
TCF/β-catenin reporter activity in late developmental stages.
Native TCF/β-catenin signaling reporter activity in E9 12xTF-d2GFP (panels A,B,D,E) and E8 16xTF-VNP embryos (C,F). In the 12xTFd2GFP embryo the TCF/β-catenin reporter is strongly expressed in the egg tooth (ET, panel A), liver (L; panel B), AER (panel E) and differentiating bones (vertebrae, V, panel D) and digits, panel (E). In the 16xTF-VNP embryo, the reporter activity is also observed in the feather follicles (FB; panel C), the egg tooth and the liver (panel F). The transgenic embryo Crystallin-EGFP marker is also visible in the lens of the 16xTF-VNP embryo. Scale bar 300µm.
Videos
A 3D reconstruction of E3.5 12xTFd2GFP embryo cleared with the 3DISCO method.
The embryo is stained for GFP (green), Pax7 (blue), and MyHC (red).
A 3D reconstruction of E3 16xTF-VNP embryo cleared with the 3DISCO method.
The embryo is stained for GFP (green), Pax7 (blue), and MyHC (red).
A 9 h time-lapse confocal analysis of an E2.5 12xTFd2GFP embryo somite.
The movie shows a single Z-plane of the somite (10 μm) and focuses on two cells in the dorsal DML which increase the TCF/β-catenin reporter activity (magenta arrowheads). We also show a cell division event in the caudal DML (white arrowheads).
A 9 hr time-lapse movie of an E2 16xTF-VNP embryo showing the growing hind limb bud.
Ectodermal cells, strongly expressing the TCF/β-catenin reporter, are seen as they migrate toward the AER region where they condensate.
The migrating NC and somitic cells are also highly visible.





