DNA-damage induced cell death in yap1;wwtr1 mutant epidermal basal cells
Figures
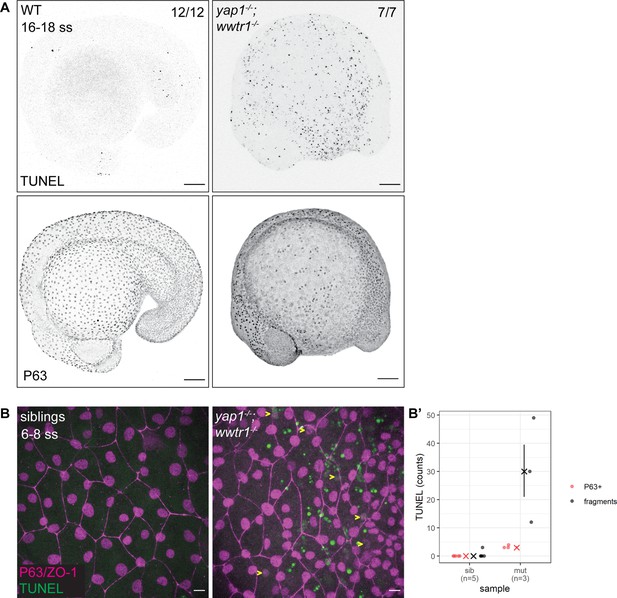
Developing zebrafish yap1;wwtr1 double mutants show aberrant epidermal basal cell death.
(A) Maximum intensity projections of 16–18 ss WT and mutant embryos stained with TUNEL and P63. Scale bars, 100 µm. (B) Maximum intensity projections of the epidermis on the lateral yolk of 6–8 ss mutant and normal siblings stained with TUNEL, P63, and ZO-1. Yellow arrowheads indicate TUNEL and P63 double positive nuclei. Scale bars, 10 µm. (B’) Quantification of TUNEL-positive EBC nuclei (P63+) and apoptotic fragments (fragments).
-
Figure 1—source data 1
TUNEL count data for Figure 1B’.
- https://cdn.elifesciences.org/articles/72302/elife-72302-fig1-data1-v3.xlsx
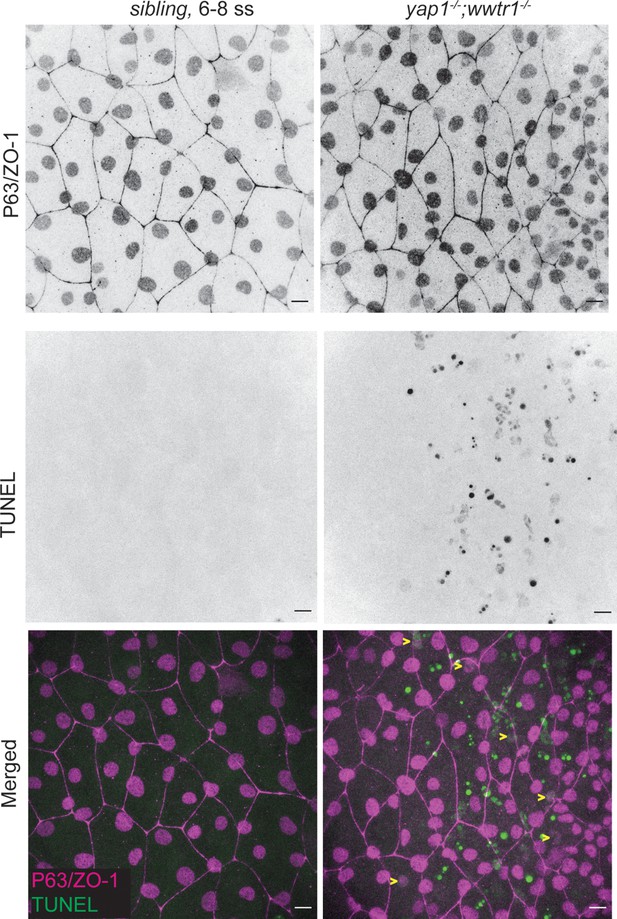
TUNEL analysis in early embryo.
Scale bars 10 µm. Individual channels used to generate Figure 1B.
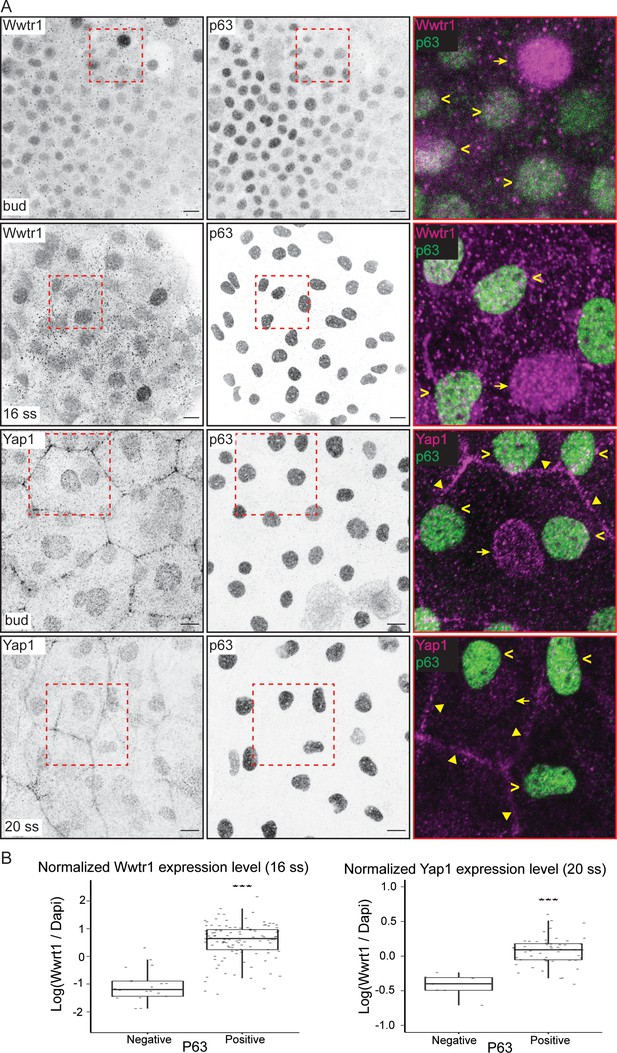
Yap1 and Wwtr1 are localized in epidermal basal cells (EBCs) and EVL cells.
(A) Maximum intensity projections of the epidermis on the lateral yolk stained with P63, and Wwtr1 or Yap1 antibodies at indicated developmental stages. Insets, demarcated in red, show overlay of P63 and Yap1/Wwtr1. Arrowheads – P63-positive basal cells; arrows – peridermal cells; triangles – peridermal cell junctions. Scale bars, 10 µm. (B) Boxplots of normalized intensities of Yap1 and Wwtr1 in the nucleus of P63-positive and P63-negative cells in the basal epidermis. t-Tests were carried out to compare these intensities between the two groups. ***p<0.001.
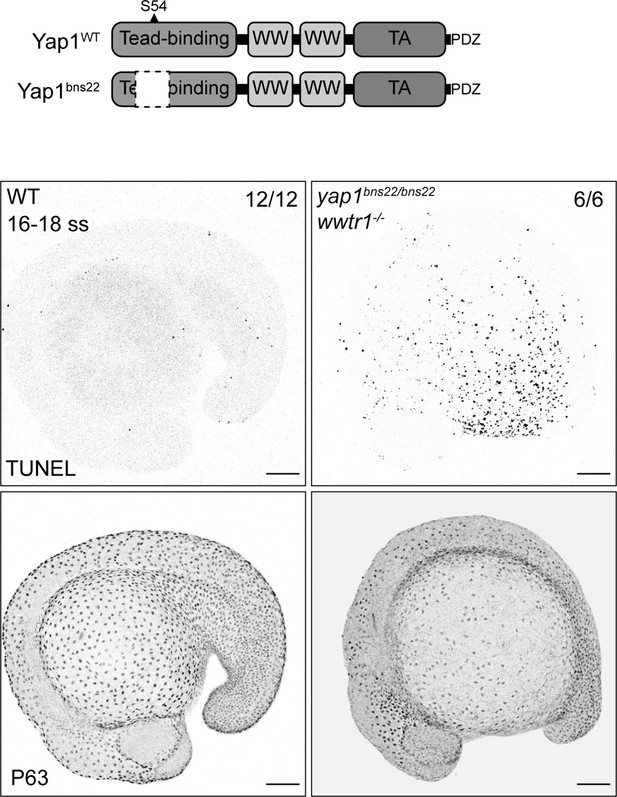
Yap1 and Wwtr1 are localized to the nuclei of epidermal basal cells (EBCs), and Yap1’s Tead-binding domain is essential in EBC survival.
(Top) Schematic of the encoded protein product of the zebrafish yap1 WT and bns22 alleles. S54 is necessary for Yap1 binding to Teads. (Bottom) Maximum intensity projections of 16–18 ss WT and yap1bns22/bns22;wwtr1-/- embryos stained with TUNEL and P63. Scale bars, 100 µm.
-
Figure 2—figure supplement 1—source data 1
Normalized Yap1 and Wwtr1 intensity measurements for Figure 2—figure supplement 1A.
- https://cdn.elifesciences.org/articles/72302/elife-72302-fig2-figsupp1-data1-v3.xlsx
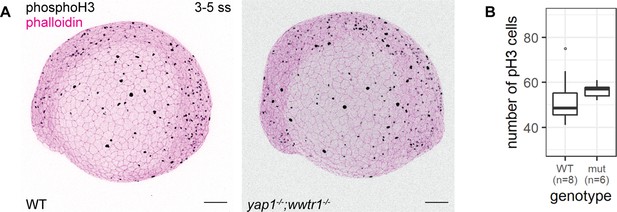
Concurrent loss of yap1 and wwtr1 did not impair epidermal cell proliferation.
(A) Maximum intensity projections of 3–5 ss WT and mutant embryos stained with a proliferation marker, phospho-histone H3 (phosphoH3; pH3), and phalloidin. Scale bars, 100 µm. (B) Boxplot of the number of pH3 cells on the lateral side of the yolk of WT and mutant zebrafish embryos. mut – yap1;wwtr1 double mutants.
-
Figure 3—source data 1
Phospho-histone H3 (pH3) count data for Figure 3B.
- https://cdn.elifesciences.org/articles/72302/elife-72302-fig3-data1-v3.xlsx
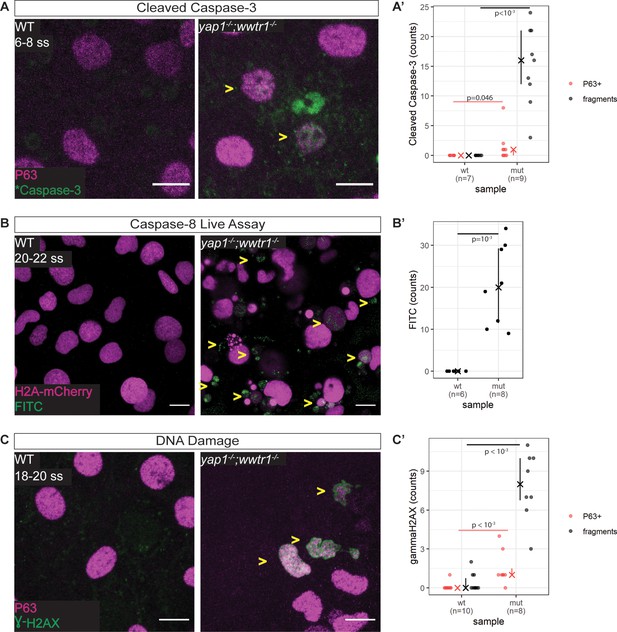
Zebrafish yap1-/-;wwtr1-/- epidermal cells exhibit DNA damage and extrinsic apoptotic cell death.
(A) Maximum intensity projections of 6–8 ss WT and mutant epidermis on the lateral yolk stained with cleaved Caspase-3 (*Caspase-3) and P63. Some *Caspase-3-positive cells are also P63-positive (yellow arrowheads). Scale bars, 10 µm. (A’) Number of epidermal basal cells (EBCs) (P63+) and apoptotic fragments (fragments) expressing cleaved Caspase-3 in WT and mutant embryo epidermis. ‘X’ represents the median, while the whiskers projecting from it represent the interquartile range. (B) Maximum intensity projections of the epidermis on the lateral yolk of 20–22 ss WT and mutant embryos expressing H2A-mCherry. Embryos were incubated in a Caspase-8 chemical probe, FITC-IETD-FMK, prior to imaging. FITC signal indicates Caspase-8 activity in cells (yellow arrowheads). Scale bars, 10 µm. (B’) Number of FITC foci in WT and mutant embryo epidermis. ‘X’ represents the median, while the whiskers projecting from it represent the interquartile range. (C) Maximum intensity projections of 18–22 ss WT and mutant epidermis on the lateral yolk stained with γH2AX and P63. Some nuclei are positive for both markers (yellow arrowheads). Scale bars, 10 µm. (C’) Number of EBCs (P63+) and apoptotic fragments (fragments) expressing γH2AX in WT and mutant embryo epidermis. ‘X’ represents the median, while the whiskers projecting from it represent the interquartile range.
-
Figure 4—source data 1
Casp3, Casp8, and γH2AX count data for Figure 4A’, B’ and C’, respectively.
- https://cdn.elifesciences.org/articles/72302/elife-72302-fig4-data1-v3.xlsx
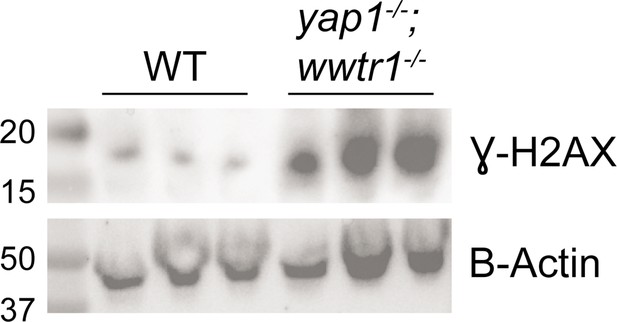
Increased γH2AX levels in mutants.
Western blot of γH2AX and B-actin (loading control). Each lane is a biological replicate of pooled 18–20 ss WT and mutant embryos. Ladder on far left lane with annotated size (in kD).
-
Figure 4—figure supplement 1—source data 1
Raw image files of Western blot assay for Figure 4—figure supplement 1B.
- https://cdn.elifesciences.org/articles/72302/elife-72302-fig4-figsupp1-data1-v3.zip
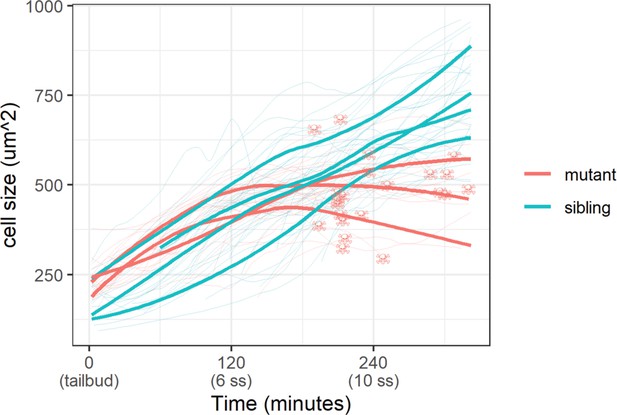
Epidermal cell size of sibling and mutant embryos during development.
Cell size was measured with a membrane marker during live imaging of mutant and sibling embryos from the tailbud stage. Fine lines are the size of individual cells, while bold lines are the average cell size in a single embryo. Skull symbols mark cell size and time before death (DNA condensation).
-
Figure 5—source data 1
Measured cell size from live-imaging experiment for Figure 5.
- https://cdn.elifesciences.org/articles/72302/elife-72302-fig5-data1-v3.xlsx
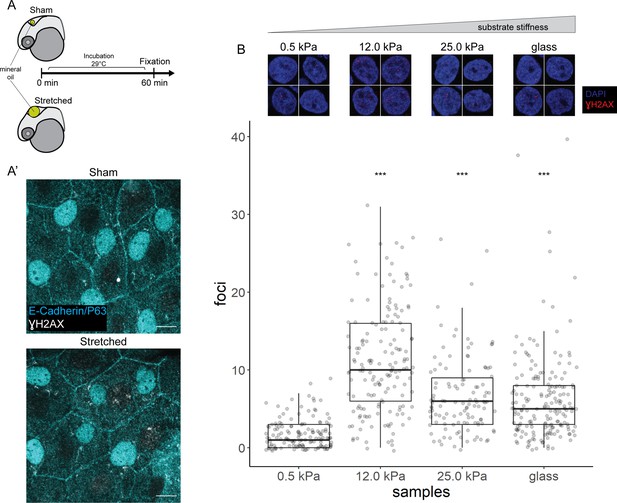
γH2AX in stretched epidermal cells and in keratinocytes cultured on different substrate stiffness.
(A) Schematic of head epidermal cell stretching experiment in zebrafish embryos. (A’) Sham (n=6) and stretched (n=6) head epidermal cells stained with γH2AX and epidermal markers (E-cadherin and P63). P63 is a marker for epidermal basal cells (EBCs). No discernible γH2AX signal was detected in both conditions. Scale bars, 10 µm. (B) Selected nuclei of HaCaT cells cultured on 0.5, 12.0, 25.0 kPa hydrogels and glass, as well as boxplot of the number of γH2AX foci in these nuclei. Number of γH2AX foci in HaCaT cell nuclei were contrasted against HaCaT cells cultured on 0.5 kPa hydrogel using t-tests. Data were collected from three independent experiments. ***p<0.001 adjusted for multiple testing (Tukey method).
-
Figure 6—source data 1
γH2AX foci count data for Figure 6B.
- https://cdn.elifesciences.org/articles/72302/elife-72302-fig6-data1-v3.xlsx
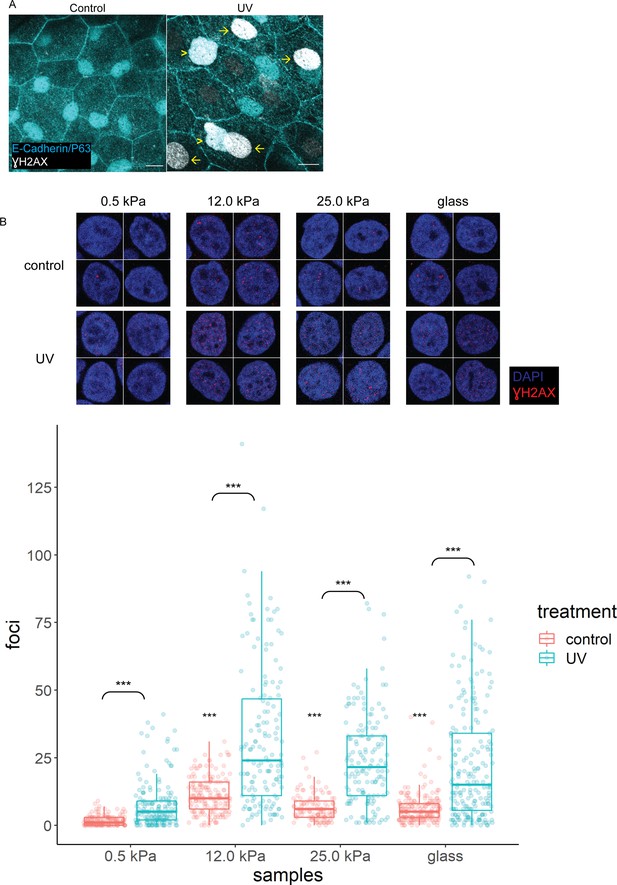
γH2AX in zebrafish head epidermis and HaCaT cells exposed to UV.
(A) Control (n=5) and UV-treated (n=4) head epidermal cells of zebrafish embryos stained with γH2AX and epidermal markers (E-cadherin and P63). P63 is a marker for epidermal basal cells (EBCs). EBCs (yellow arrowheads) and peridermal cells (yellow arrows) exposed to UV exhibit pan-nuclear γH2AX. (B) Selected nuclei of control and UV-treated HaCaT cells cultured on 0.5, 12, 25 kPa hydrogels and glass, and boxplots of the number of γH2AX foci in these nuclei. t-Tests on control groups were compared against control HaCaT cells cultured on 0.5 kPa hydrogel. t-Tests on UV groups were compared against their respective controls (brackets). Data were collected from three independent experiments. ***p<0.001 adjusted for multiple testing.
-
Figure 6—figure supplement 1—source data 1
γH2AX foci count data for Figure 6—figure supplement 1B.
- https://cdn.elifesciences.org/articles/72302/elife-72302-fig6-figsupp1-data1-v3.xlsx
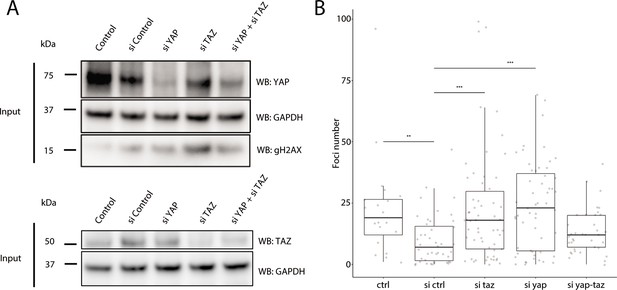
Recruitment of γH2AX in YAP1/WWTR1 knockdown HaCaT cells.
(A) Western blots of control and YAP1/WWTR1 knockdown HaCaT cells. (B) Boxplot of the number of γH2AX foci in control and YAP1/WWTR1 knockdown HaCaT cell nuclei. Number of γH2AX were compared to control knockdowns (sictrl) using t-tests.
Videos
Time lapse imaging of mutant and sibling epidermis.
Maximum intensity projections of the epidermis on the lateral yolk of mutant and sibling embryos. Membranes and nuclei are marked by Lyn-EGFP (green) and H2A-mCherry (red), respectively. Time stamp format, HH:MM; 00:00 is tailbud stage.
Apoptosis of mutant basal cells.
Time lapse of mutant basal cells undergoing apoptosis as captured by live imaging. Membranes and nuclei are marked by Lyn-EGFP (green) and H2A-mCherry (red), respectively. Time stamp format, HH:MM; 00:00 is tailbud stage. Scale bars, 10 µm.





