Autophagosomes fuse to phagosomes and facilitate the degradation of apoptotic cells in Caenorhabditis elegans
Figures
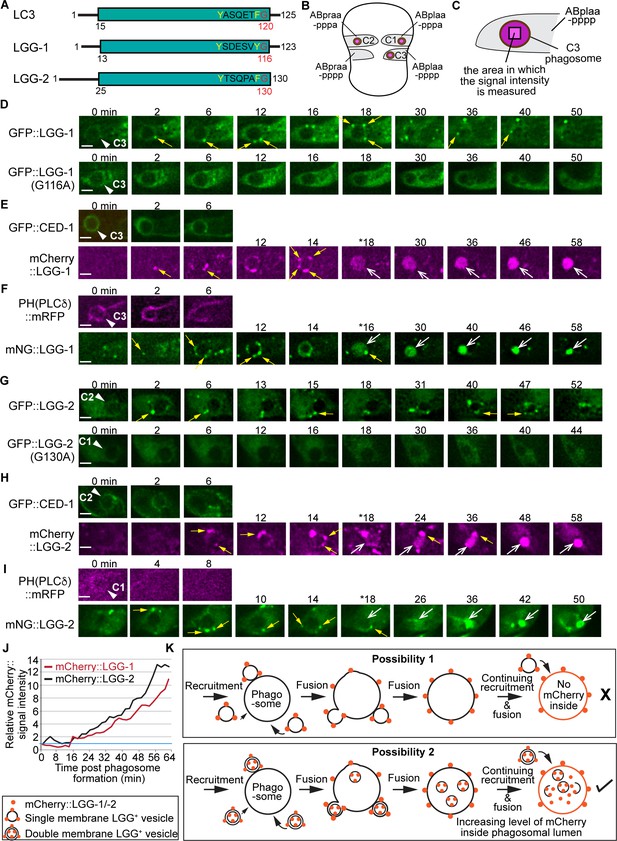
The vesicles labeled with LGG-1 or LGG-2 are recruited to the surface of phagosomes and subsequently fuse to phagosomes.
(A) Domain structures of mammalian LC3 and C. elegans LGG-1 and LGG-2. The green box indicates the conserved ubiquitin-like domain. Residues in white are conserved among the three proteins. The glycine residue in red is the site where the lipid tail is attached to. (B) A diagram illustrating the three phagosomes that contain cell corpses C1, C2, and C3, with which we monitor the dynamic recruitment and fusion of autophagosomes, at ~330 min post-the 1st embryonic division. Both the positions of C1, C2, and C3 (brown dots) and the identities of their engulfing cells are shown. (C) A diagram illustrating that the relative mNG signal in the center of a phagosome is measured over time to create sub-figure (J). At time point T (time after engulfment), the Relative signal intensity T = (Unit Intensity(phagosome center)T – Unit Intensity (background)T) / (Unit Intensity(phagosome center)T0 – Unit Intensity(background)T0). (Figure 1—source data 1). (D–I) Time-lapse images of indicated reporters starting when a nascent phagosome (white arrowheads) just formed (time point ‘0 min’). All reporters were expressed under the control of Pced-1. Scale bars are 2 µm. Solid white arrowheads label nascent phagosomes. Yellow arrows mark a few LGG-labeled puncta on the surface of phagosomes. Open white arrows in (E, F, H, I) label the phagosomal lumen where the LGG signal is observed. ‘*’ is the time point when the LGG signal is first seen inside the phagosomal lumen. CED-1::GFP (E, H) and PH(PLCγ)::mRFP (F, I) are co-expressed markers that label the surfaces of nascent phagosomes. (D) GFP::LGG-1-labeled puncta are observed on the surface of a C3 phagosome, but the GFP signal is not seen inside the phagosomal lumen. No GFP::LGG-1(G116A)-labeled puncta are seen on the surface of phagosomes. (E–F) The mCherry::LGG-1 (E) and mNG::LGG-1 (F) puncta are observed on the surface of a C3 phagosome and subsequently accumulate inside the phagosome lumen. (G) GFP::LGG-2-labeled puncta are observed to attach on the surface of a C2 phagosome, but the GFP signal does not enter the phagosomal lumen, whereas no GFP::LGG-1(G130A)-labeled puncta are seen on the surface of phagosomes. (H–I) The mCherry::LGG-2 (H) and mNG::LGG-2 (I) puncta are observed on the surface of a C2 (H) or C1 (I) phagosome, respectively, and subsequently accumulate inside the phagosome lumen. (J) The relative mCherry::LGG-1 or –2 signal intensity in the center of a phagosome (Y-axis) over time (in the 2 min interval) (X-axis). ‘0 min’ indicates the moment when a nascent phagosome just formed. One blue horizontal line indicates value ‘1’, where no signal enrichment above background level is observed. (K) A diagram illustrating that those double membrane-vesicles labeled with mCherry::LGG on their outer and inner membranes are recruited to phagosomal surfaces and fused to the phagosomal membrane. After the fusion between the outer membrane of these vesicles and the phagosomal membrane, the mCherry::LGG-tagged inner membrane is released into the phagosomal lumen. The continuing incorporation of these double-membrane vesicles to phagosomes increases the mCherry signal level in the phagosomal lumen over time. If the LGG-1 or LGG-2-labeled vesicles are of a single membrane, no fluorescence signal is expected to enter the phagosomal lumen.
-
Figure 1—source data 1
Relative mCherry::LGG-1 and mCherry::LGG-2 signal intesity over time in 1J.
- https://cdn.elifesciences.org/articles/72466/elife-72466-fig1-data1-v2.docx
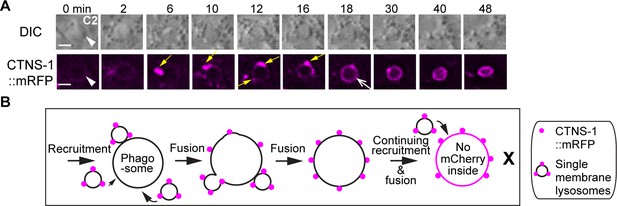
The fusion of lysosomal particles to phagosomes results in the incorporation of lysosomal membrane protein CTNS-1 to the phagosomal membrane but not the lumen.
(A) Time-lapse images of a C2 phagosome following the lysosomal recruitment and fusion. CTNS-1::mRFP is expressed in engulfing cells under Pced-1. ‘0 in’ is the time point when a C2 phagosome (white arrowheads) just forms. Yellow arrows mark CTNS-1::mRFP-labeled lysosomal particles. One open white arrow marks the phagosomal surface that is evenly colored by mRFP. Scale bars are 2 µm. (B) A diagram illustrating that the single membrane-vesicles labeled with CTNS-1::mRFP on their membranes are recruited to phagosomal surfaces and fused to the phagosomal membrane. After the fusion between the membranes of these vesicles and the phagosome, the mRFP signal is evenly distributed to the phagosomal surface. However, no mRFP signal enters the phagosomal lumen.
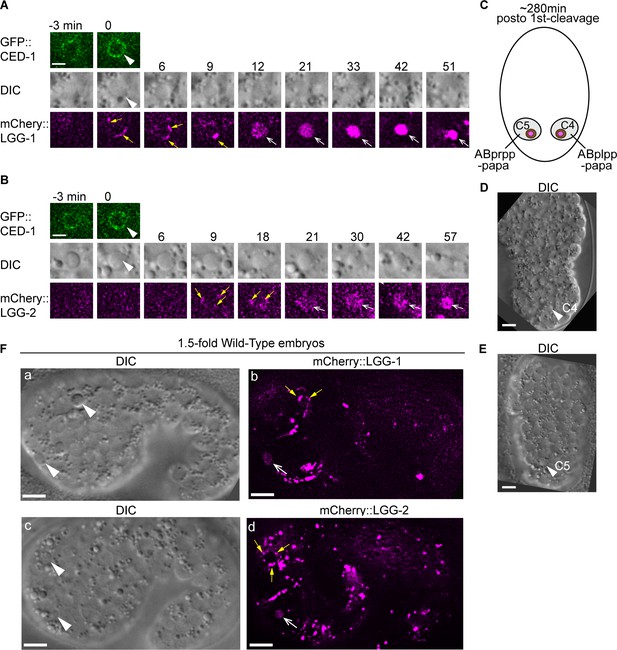
Besides the phagosomes containing C1, C2, and C3, the attachment and fusion of LGG+-vesicles to other phagosomes are also observed during embryonic development.
Wild-type embryos co-expressing Pced-1 ced-1::gfp and Pced-1 mCherry::lgg-1 or Pced-1 mCherry::lgg-2 reporters were analyzed. (A–B) Time-lapse images following the indicated reporters in C4 (A) or C5 (B) phagosomes starting when the nascent phagosome (white arrowheads) just formed (time point ‘0 min’). Scale bars are 2 µm. Yellow arrows mark the LGG+ puncta on phagosome surfaces. Open white arrows mark when mCherry signal is detected in the phagosomal lumen, indicating autophagosome/phagosome fusion events. (C) A diagram illustrating the two phagosomes that contain cell corpses C4 and C5, with which we monitor the dynamic recruitment and fusion of autophagosomes, at ~310 min (A) and ~280 min (B) post-the 1st embryonic division. Both the positions of C4 and C5 (brown dots) and the identities of their engulfing cells are shown. (D–E) Images of wild-type embryos illustrate the positions of the C4 and C5 phagosomes shown in (A) and (B), respectively. Scale bars are 5 µm. (F) Images of two wild-type embryos at a 1.5-fold developmental stage. White arrowheads (a and c) indicate phagosomes in which the mCherry+ puncta are observed to be recruited to their surfaces (yellow arrows in (b and d)), and that mCherry signal is detected in the phagosomal lumen (open white arrows in (b and d)). Scale bars are 5 µm.
mCherry::LGG-1-labeled vesicles are recruited to the surface of a phagosome and subsequently fuse to the phagosome.
Related to Figure 1E. This movie shows time-lapse recording images of a C3 phagosome (white arrowheads) in a wild-type embryo, starting at ~330 min post-1st cleavage. CED-1::GFP labels pseudopods, allowing the visualization of the phagosome formation process. ‘0 min’ is the moment when pseudopods just seal. Yellow arrowheads indicate the mCherry::LGG-1+ puncta, and open white arrows point to the phagosome lumen containing the mCherry signal.
mCherry::LGG-2-labeled vesicles are recruited to the surface of a phagosome and subsequently fuse to the phagosome.
Related to Figure 1H. This movie shows time-lapse recording images of a C3 phagosome (white arrowheads) in a wild-type embryo, starting at ~330 min post-1st cleavage. CED-1::GFP labels pseudopods, allowing the visualization of the phagosome formation process. ‘0 min’ is the moment when pseudopods just seal. Yellow arrowheads indicate the mCherry::LGG-2+ puncta, and open white arrows point to the phagosome lumen containing the mCherry signal.
mNG::LGG-1-labeled vesicles are recruited to the surface of a phagosome and subsequently fuse to the phagosome.
Related to Figure 1F. This movie shows time-lapse recording images of a C3 phagosome in a wild-type embryo, starting at ~330 min post-1st cleavage. PH(hPLCγ)::mRFP labels pseudopods, allowing the visualization of the phagosome formation process. ‘0 min’ is the moment when pseudopods just seal. Yellow arrowheads indicate mNG::LGG-1+ puncta, and open white arrows point to the phagosome with mNG signal in its lumen.
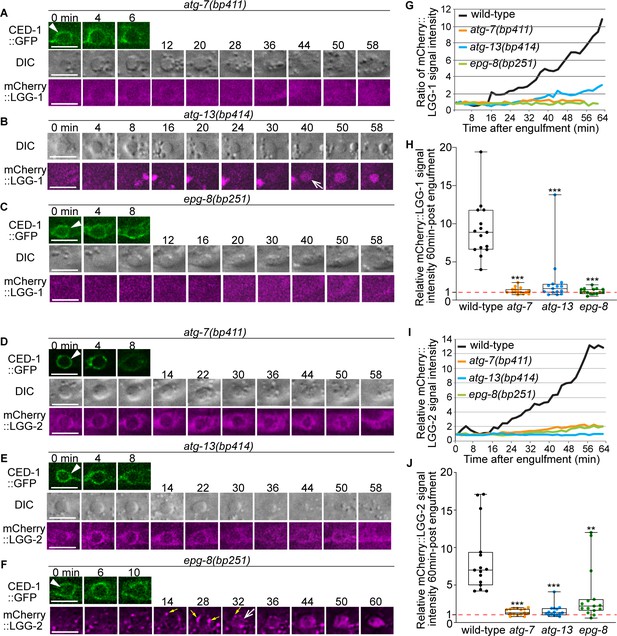
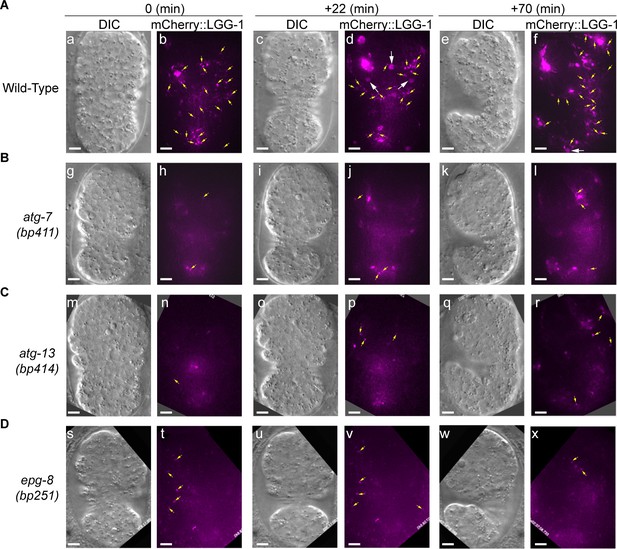
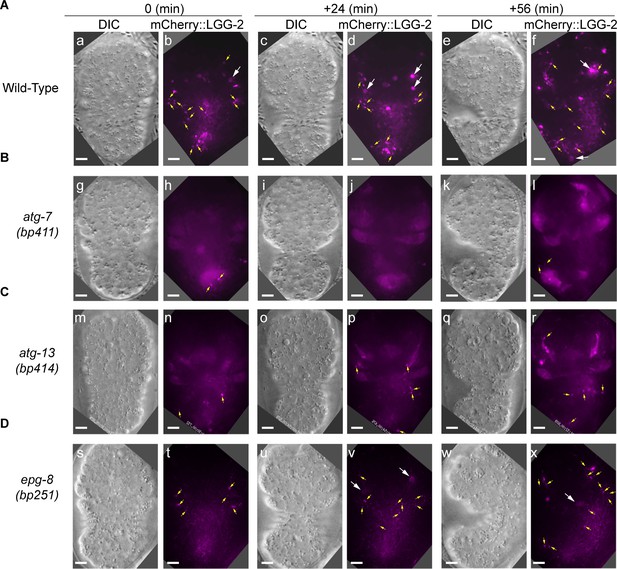
In autophagosome-formation mutants, the enrichment of the LGG+ vesicles on the phagosomal surface and the entry of the LGG signal into the phagosomal lumen are severely defective.
(A–F) Time-lapse image series monitoring the enrichment of the puncta labeled with mCherry-tagged LGG-1 (A–C) or LGG-2 (D–F) on phagosomes (white arrowheads) and the subsequent entry of mCherry signal into the phagosomal lumen in atg-7, atg-13, and epg-8 mutant embryos. ‘0 min’ is when a phagosome is just sealed (determined by CED-1::GFP). Open white arrow denotes the time point that the mCherry signal starts to appear inside the phagosomal lumen. Scale bars are 2 µm. Yellow arrows in (F) mark mCherry::LGG-2 puncta on the surface of a phagosome. (G and I) The relative mCherry::LGG-1 or –2 signal intensity in the center of a phagosome (Y-axis) over time (in the 2 min interval) (X-axis). “0 min” indicates the moment when a phagosome is just sealed. (G) The data for the wild-type, atg-7(bp411), atg-13(bp414), and epg-8(bp251) mutant embryos are from Figure 1E and 2(A-C), respectively. (I) The data for the wild-type, atg-7(bp411), atg-13(bp414), and epg-8(bp251) mutant embryos are from Figure 1I and 2(D-F), respectively. (Figure 2—source data 1). (H and J) Box-and-Whiskers plots of the relative mCherry signal intensity measured in the center of phagosomes 60 min-post the formation of nascent C3 phagosomes from 15 each of wild-type, atg-7(bp411), atg-13(bp414), and epg-8(bp251) mutant embryos. Red dashed lines indicate the position of value 1, which represents no signal enrichment relative to the background signal. (Figure 2—source data 2). ***, p < 0.001, **, < 0.001 p < 0.01, Student t-test of each mutant comparing to the wild-type value.
-
Figure 2—source data 1
Relative mCherry::LGG-1 and mCherry::LGG-2 singnal intesity over time in Figure 2G and I.
- https://cdn.elifesciences.org/articles/72466/elife-72466-fig2-data1-v2.docx
-
Figure 2—source data 2
Relative mCherry::LGG-1 and mCherry::LGG-2 signal intensity at 60min-post engulfment in Figure 2H and J.
- https://cdn.elifesciences.org/articles/72466/elife-72466-fig2-data2-v2.docx
The atg-7, atg-13, and epg-8 mutants are severely defective in the production of LGG-1-labeled autophagosomes.
Images of wild-type (A), atg-7(bp411) (B), atg-13(bp414) (C), and epg-8(bp251) mutant (D) embryos expressing Pced-1 mCherry::lgg-1 are presented. ‘0 min’ labels embryos that are ~330 min post-1st cleavage. The mCherry images are 2-D projections of 14 Z-sections at 0.5 µm intervals. Yellow arrows mark LGG-1 autophagosomes. White arrows in (A(d)) mark three phagosomes, on the surface of which mCherry signal is recruited in (a–b), and 20 min later, enters the phagosome, demonstrating the autophagosomes/phagosome fusion event. Scale bars are 5 µm.
The atg-7, atg-13, and epg-8 mutants are severely defective in the production of LGG-2-labeled autophagosomes.
Images of wild-type (A), atg-7(bp411) (B), atg-13(bp414) (C), and epg-8(bp251) mutant (D) embryos expressing Pced-1 mCherry::lgg-2 are presented. ‘0 min’ labels embryos that are ~330 min post-1st cleavage. The mCherry images are 2-D projections of 14 Z-sections at 0.5 µm intervals. Yellow arrows mark LGG-2 autophagosomes. White arrows in (A(b, d, and f)) mark phagosomes in which mCherry signal was detected, indicating the existence of autophagosomes/phagosome fusion events. Scale bars are 5 µm.
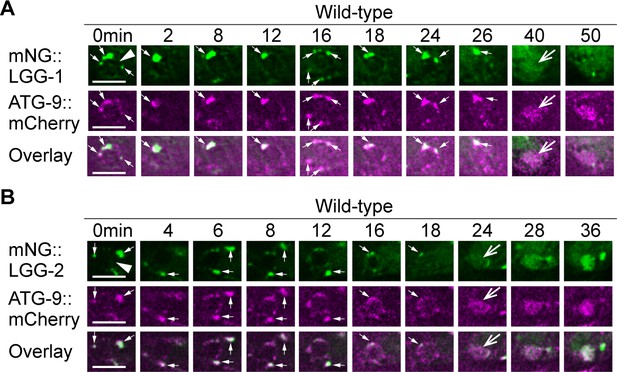
LGG-1+ and LGG-2+ puncta colocalize with ATG-9, a component of autophagosomes.
The mNG- and mCherry-tagged reporters are expressed in wild-type embryos under the control of Pced-1. White arrowheads mark nascent phagosomes. Small white arrows mark the regions where LGG+ and ATG-9+ puncta colocalize. Open white arrows indicate when the fluorescent signal is first detected inside the phagosomal lumen. Scale bars are 5 µm. (A) Time-lapse microscopy showing the localization of mNG::LGG-1 and mCherry::ATG-9. Images are from ABplaapppp, which engulfs C3. (B) Time-lapse microscopy showing the localization of mNG::LGG-2 and mCherry::ATG-9. Images are from ABplaapppa, which engulfs C1.
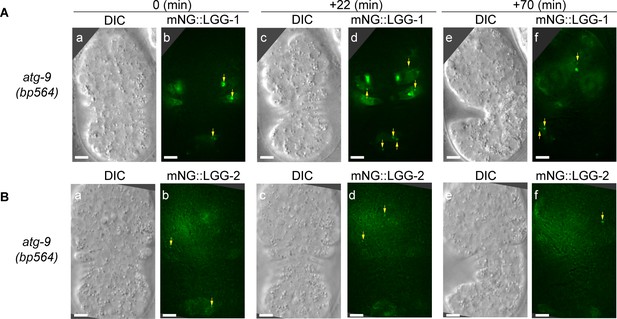
atg-9 is another gene essential for the production of autophagosomes and the incorporation of autophagosomes into phagosomes.
Images of atg-9(bp564) mutant embryos expressing Pced-1 mNG::lgg-1 (A) or::lgg-2 (B) are presented. ‘0 min’ embryos are embryos at ~330 min post-1st cleavage. The mNG images are 2-D projections of 14 Z-sections at 0.5 µm intervals. Green puncta (yellow arrows) represent LGG-1 or LGG-2 autophagosomes. Scale bars are 5 µm.
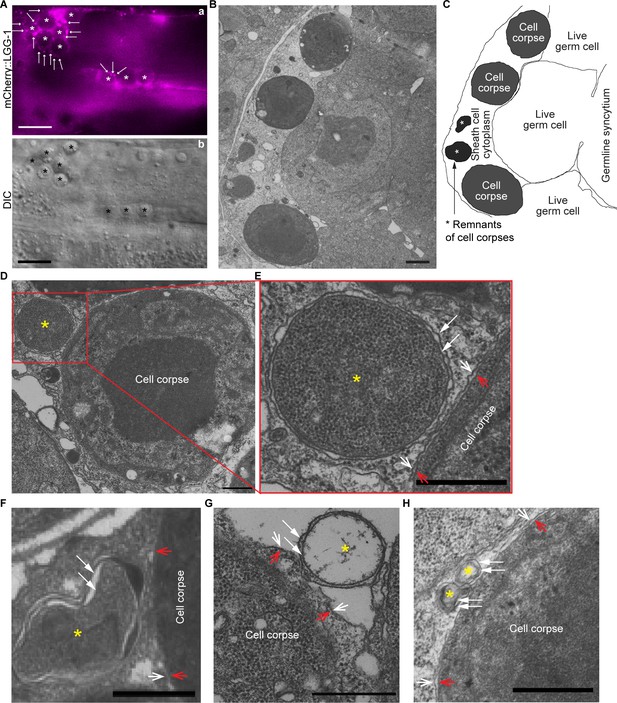
Double-membrane vesicles were observed to attach on the surfaces of phagosomes bearing germ cell corpses All samples are from the distal gonadal arms of rab-7(ok511) adult hermaphrodites.
(A) In the gonadal arm of a rab-7(ok511) hermaphrodite expressing Pced-1 mCherry::lgg-1 in gonadal sheath cells, mCherry+ puncta (arrows) are found on the surfaces of phagosomes carrying germ cell corpses (white (a) and black (b) asterisks). Scale bars are 10 µm. (B) A thin cross-section (50 nm in thickness) TEM image of half of a distal gonad. The scale bar is 1 µm. (C) Traces of membranes corresponding to (B). All three germ cell corpses are inside the gonadal sheath cell. Two asterisks mark the remnants of two engulfed germ cell corpses. (D-H) Scale bars are 500 µm. Examples of five double-membrane vesicles (yellow asterisks) were observed on phagosomal surfaces. White arrows mark each layer of the double-layer membranes of the vesicles of interest. Open arrows mark the phagosomal (white) and germ cell corpse (red) membranes. (E) is an enlarged image of the region framed by the red box in (D). (G) The luminal content of the double-membrane vesicle (*) is missing due to the damage in sample preparation. Due to the same damage, the phagosomal membrane in (G) is unclear.
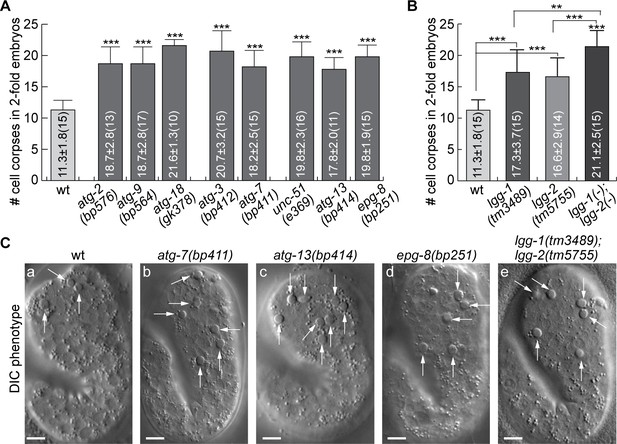
Mutations in autophagy genes impair the clearance of apoptotic cells.
(A–B) Bar graph displaying the average numbers of somatic cell corpses in twofold stage wild-type and various mutant embryos. Bars and error bars represent mean and standard deviation (sd), respectively, the actual values of which are displayed inside the bars. Numbers in parentheses indicate the number of embryos scored. ***, p < 0.001, Student t-test of each mutant comparing to the wild-type value. (Figure 5—source data 1). (C) DIC images of cell corpses in twofold stage embryos of various genotypes. White arrows indicate button-like structures characteristic of cell corpses. Scale bars are 5 µm.
-
Figure 5—source data 1
Cell corpse count and statistical analysis of atg mutants at 2-fold stage.
- https://cdn.elifesciences.org/articles/72466/elife-72466-fig5-data1-v2.docx
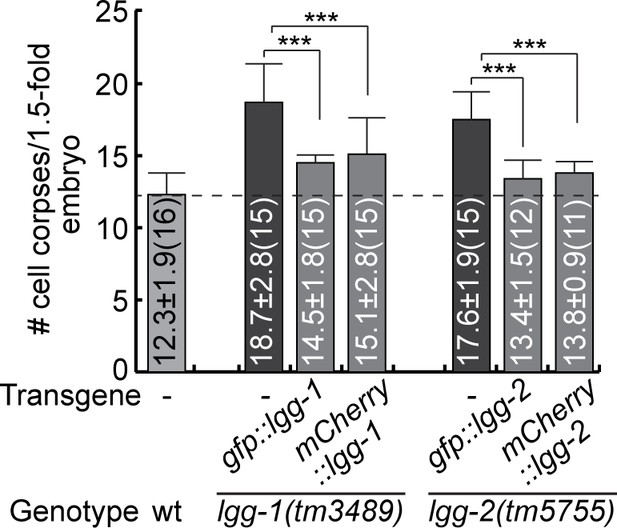
The expression of lgg-1 and lgg-2 cDNA in engulfing cells suppresses the Ced phenotype of lgg-1 and lgg-2 null mutants, respectively.
GFP- and mCherry-tagged reporters for LGG-1 and LGG-2 are expressed in engulfing cells under the control of the Pced-1 promoter in lgg-1(tm3489) and lgg-2(tm5755) mutant embryos, respectively. The bar graph displays the average number of somatic cell corpses in lgg-1(tm3489) and lgg-2(tm5755) mutant embryos at the 1.5-fold stage, both with and without expression rescuing construct. The number of embryos scored for each strain is in parentheses. Bars represent the mean, and the error bars indicate standard deviation. Brackets above the bars indicate the samples compared by the Student t-test. p-values are summarized as such: *, 0.001< p < 0.05; **, 0.0001< p < 0.001; ***, p < 0.0001; ns, no significant difference. (Figure 5—figure supplement 1—source data 1).
-
Figure 5—figure supplement 1—source data 1
Cell corpse count and statistical analysis of atg mutants at 1.5-fold stage.
- https://cdn.elifesciences.org/articles/72466/elife-72466-fig5-figsupp1-data1-v2.docx
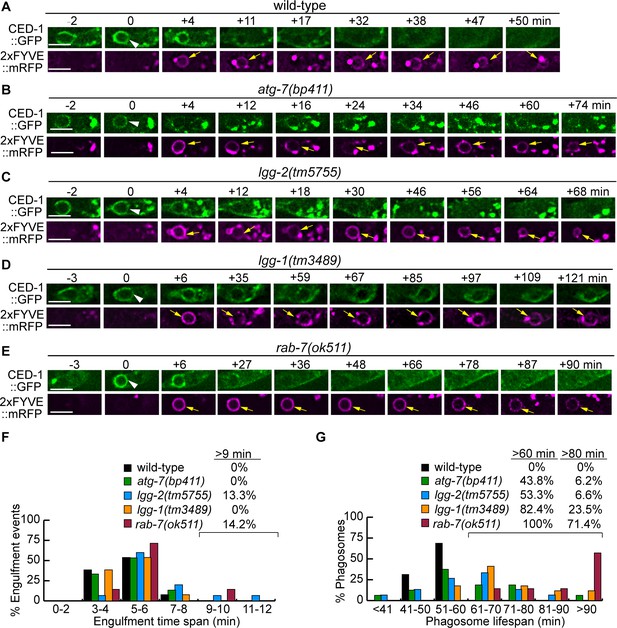
Mutations in rab-7, atg-7, lgg-1, and lgg-2 impair the degradation of cell corpses to different degrees.
(A–E) Time-lapse recording conducted in wild-type and different mutant embryos monitoring the dynamics of the pseudopod marker CED-1::GFP and the phagosome marker 2xFYVE::mRFP (both expressed in engulfing cells) during the engulfment and degradation processes of cell corpse C3 by ABplaapppp. ‘0 min’ is the first time point when a nascent phagosome (white arrowheads) is formed, as indicated by the closure of a green GFP::CED-1 ring. 2xFYVE::mRFP labels the surface of a phagosome (yellow arrows) until it is degraded. Scale bars are 2 µm. (F) Histogram depicting the distribution of the time it takes to engulf 15 C3 cell corpses in wild-type, atg-7, lgg-1, and lgg-2 and 7 cell corpses in rab-7(m-z-) homozygous embryos. The engulfment time is defined as the period between the first time when pseudopods (labeled with CED-1::GFP) are observed and when a full circle is observed forms around C3. (Figure 6—source data 1). (G) Histogram depicting the lifespan distribution of 15 C3 phagosomes in wild-type, atg-7,lgg-1, lgg-2, and seven phagosomes in rab-7(m-z-) homozygous embryos. Phagosome lifespan is measured as the time interval between the ‘0 min’ time point when a nascent phagosome is just sealed and when the phagosome shrinks to one-half of its original diameter. (Figure 6—source data 2).
-
Figure 6—source data 1
Time of engulfment of C3 phagosomes.
- https://cdn.elifesciences.org/articles/72466/elife-72466-fig6-data1-v2.docx
-
Figure 6—source data 2
Time of degradation of C3 cell corpses.
- https://cdn.elifesciences.org/articles/72466/elife-72466-fig6-data2-v2.docx
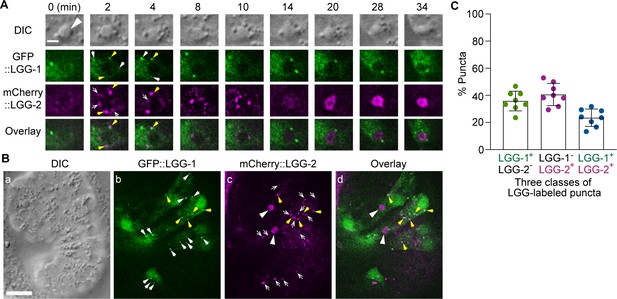
The puncta labeled with LGG-1 and/or LGG-2 define three distinct populations of vesicles The GFP- and mCherry- tagged reporters are expressed in wild-type embryos under the control of Pced-1.
(A) Time-lapse image series of a C2 phagosome (big white arrowhead) monitoring the localization of LGG-1+, LGG-2+, and LGG-1+ - LGG-2+ double-positive puncta on the surface of the phagosome. ‘0 min’ is the moment when a nascent phagosome just seals. The scale bar is 2 µm. White arrows mark GFP+ mCherry- puncta, white open arrows mark GFP- mCherry+ puncta, and yellow arrows mark GFP+ mCherry+ puncta. (B) DIC and fluorescence images of an embryo exhibiting LGG+ puncta outside phagosomes in multiple cells. The scale bar is 10 µm. The white arrows, white open arrows, and yellow arrows mark GFP+ mCherry-, GFP- mCherry+, and GFP+ mCherry+ puncta on the surface of the phagosome, respectively. The big white arrowheads in (c) and (d) indicate the mCherry signal internalization to the phagosomal lumen. (C) Bar graph indicating the distribution of single and double-labeled puncta in the LGG-labeled population in the engulfing cells for C1, C2, and C3, scored immediately prior to the point when the LGG signal was observed inside the phagosomal lumen. Eight engulfing cells were scored. Bars represent the mean, the error bars indicate standard deviation, and each dot represents one sample. (Figure 7—source data 1).
-
Figure 7—source data 1
Percentage distribution of single and double-labeled puncta in the LGG-labeled population in the engulfing cells for C1, C2, and C3.
- https://cdn.elifesciences.org/articles/72466/elife-72466-fig7-data1-v2.docx
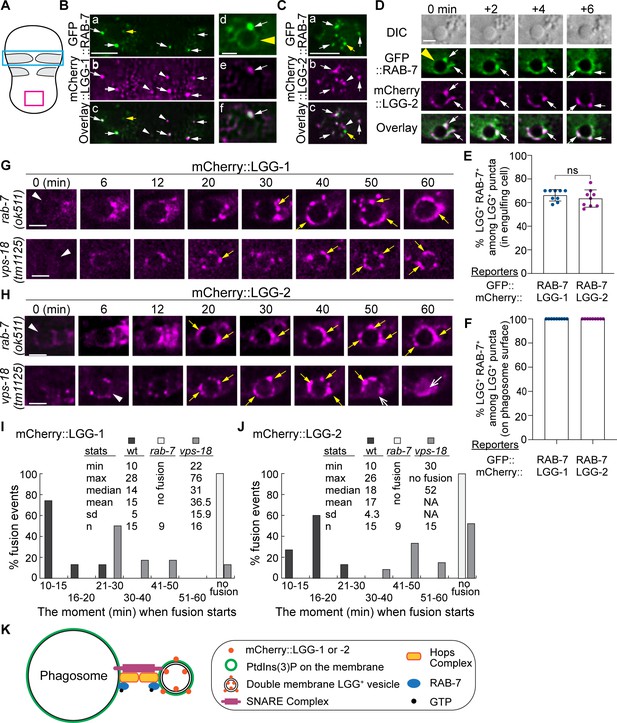
RAB-7 is enriched on a portion of autophagosomes, and RAB-7 and VPS-18 are essential for the fusion between autophagosomes and phagosomes.
(A) Diagram of the ventral surface of an embryo at ~330 min post 1st embryonic division. (B–D) Images of part of the ventral surface of an embryo co-expressing Pced-1gfp::rab-7 and Pced-1mCherry::lgg-1 (B) or Pced-1mCherry::lgg-2 (C–D). B(a-c) depicts the region framed by the blue box in (A). B(d-f) depicts a C1 phagosome (a yellow arrowhead). C(a-c) depicts the region framed by the red box in (A). (D) A time-lapse image series of a C1 phagosome (a yellow arrowhead) indicates the dynamic recruitment and fusion of GFP and mCherry double-positive puncta to the phagosomal membrane. ‘0 min’ is when the first puncta are observed on the phagosomal surface. White arrows mark several puncta that are both GFP+ and mCherry+. Yellow arrows mark puncta that are GFP+ but mCherry-. White arrowheads mark puncta that are GFP- but mCherry+. Scale bars for B(a-c) and (C) are 5 µm, and for B(d-f) and (D) are 2 µm. (E–F) Bar graphs depicting the percentage of LGG-1+ or LGG-2+ puncta that are also RAB-7+ in the cytoplasm of the engulfing cells for C1, C2, and C3 (E) or on the surfaces of the phagosomes 2 min before the autophagosome-phagosome fusion occurs (F). Nine engulfing cells and the phagosomes inside were scored for each of the LGG-1+ and LGG-2+ categories. Bars and error bars represent the mean and standard deviation values. Each dot represents one sample. ns, not significant. (Figure 8—source data 1). (G–H) Time-lapse images monitoring the recruitment and fusion of puncta labeled with mCherry::LGG-1 (E) or::LGG-2 (F) to the C1, C2, and C3 phagosomes in rab-7(ok511) and vps-18(tm1125) mutant embryos. ‘0 min’ represents the moment when a phagosome just seals (white arrowheads). Yellow arrows mark the mCherry+ puncta on the phagosomal surfaces. Open white arrow marks the mCherry signal inside the phagosomal lumen. Scale bars are 2 µm. (I–J) Histograms depicting the distribution of the time it takes for LGG-1+ or LGG-2+ puncta to fuse to phagosomes, measured from the ‘0 min’ point to the time when mCherry was detected in the center of a phagosome. C1, C2, and C3 phagosomes were recorded. n, the number of phagosomes scored. ‘No fusion’: no mCherry signal entry was observed even after 72–114 min post-nascent phagosome formation. NA: not applicable. (Figure 8—source data 2). (K) A Diagram depicts the mechanism driving autophagosome-phagosome fusion. RAB-7 is enriched on the surfaces of both phagosomes and autophagosomes. RAB-7 and VPS-18, a subunit of the HOPs complex, are proven essential for autophagosome-phagosome fusion. Other factors are proposed to play roles in this event based on the general knowledge of intracellular membrane fusion.
-
Figure 8—source data 1
Percentage of distribution of LGG-1+ or LGG-2+ puncta that are also RAB-7+.
- https://cdn.elifesciences.org/articles/72466/elife-72466-fig8-data1-v2.docx
-
Figure 8—source data 2
The time it takes for LGG-1 or LGG-2 autophagosomes fuse to with phagosomes.
- https://cdn.elifesciences.org/articles/72466/elife-72466-fig8-data2-v2.docx
In rab-7 (ok511), mCherry::LGG-1-labeled vesicles fail to fuse with phagosomes.
Related to Figure 8G. This movie shows time-lapse recording images of a C3 phagosome in a rab-7(ok511) m-z- homozygous embryo, starting at ~330 min post-1st cleavage. “0 min” is the moment when a nascent phagosome is just formed. White arrowheads mark the phagosome. Yellow arrows mark mCherry::LGG-1+ puncta.
In rab-7(ok511) mutants, mCherry::LGG-2-labeled vesicles fail to fuse with phagosomes.
Related to Figure 8H. This movie shows time-lapse recording images of a C3 phagosome in a rab-7(ok511) m-z- homozygous embryos, starting at ~330 min post-1st cleavage. ‘0 min’ is the moment when a nascent phagosome is just formed. White arrowheads mark the phagosome. Yellow arrows mark mCherry::LGG-2+ puncta.
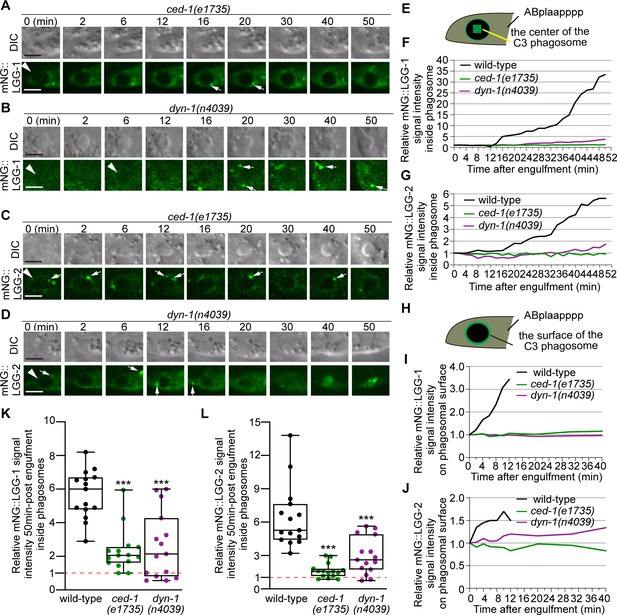
CED-1 and DYN-1 are essential for the incorporation of autophagosomes into phagosomes.
(A–D) Time-lapse image series monitoring the presence or absence of puncta (white arrows) labeled with mNG::LGG-1 (A–B) or –2 (C–D) on C3 phagosomes (white arrowheads) and the subsequent entry of the mNG signal into the lumen in ced-1 and dyn-1 mutant embryos. DIC images mark the position of the cell corpse. ‘0 min’ is the moment when phagosomes are just sealed. Scale bars are 2 µm. (E) A diagram illustrating that the relative mNG signal in the center of a phagosome is measured over time to create sub-figures (F) and (G). At time point t (time after ‘0 min’), the Relative Signal Intensity T = (Unit Intensity(phagosome center)T –Unit Intensity(background)T) / (Unit Intensity(phagosome center)T0- Unit Intensity (background)T0). (F–G) The relative mNG::LGG-1 (F) or –2 (G) signal intensity in the center of a phagosome (Y-axis) over time in the 2 min interval (X-axis). ‘0 min’ is the moment when pseudopods are sealed and a nascent phagosome forms. (F) The data for the wild-type, ced-1(e1735), and dyn-1(n4039) mutant embryos are from Figure 1F and 8(A-B), respectively. (G) The data for the wild-type, ced-1(e1735), and dyn-1(n4039) mutant embryos are from Figure 1I and 8(C-D), respectively. (Figure 9—source data 1) (H) A diagram illustrating that the relative mNG signal on the surface of a phagosome is measured over time to create sub-figures (I) and (J). At time point T (time after ‘0’ min), the Relative signal intensity T = (Unit Intensity(phagosome surface (the green ring))T –Unit Intensity(background)T) / (Unit Intensity(phagosome surface)T0 - Unit Intensity (background)T0). (I–J) The relative mNG::LGG-1 or –2 signal intensity on the surface of a phagosome (Y-axis) over time in the 2 min interval (X-axis). ‘0 min’ indicates the moment when pseudopods are sealed and nascent phagosome forms. (I) The data for the wild-type, ced-1(e1735), and dyn-1(n4039) mutant embryos are from Figure 1F and 8(A-B), respectively. (J) The data for the wild-type, ced-1(e1735), and dyn-1(n4039) mutant embryos are from Figure 1I and 8(C-D), respectively. (Figure 9—source data 2). (K–L) Box-and-Whiskers plots of the relative mNG signal intensity measured in the center of phagosomes 50 min-post the formation of nascent C3 phagosomes from 15 each of wild-type, ced-1(e1735), and dyn-1(n4039) mutant embryos. Red dashed lines indicate the position of value 1, which represents no signal enrichment relative to the background signal. ***, p < 0.001, Student t-test of each mutant compared to the wild-type value. (Figure 9—source data 3).
-
Figure 9—source data 1
Singal intesity over time of mNG::LGG-1 and mNG::LGG-1 in Figure 9F, G.
- https://cdn.elifesciences.org/articles/72466/elife-72466-fig9-data1-v2.docx
-
Figure 9—source data 2
Recruitment of mNG::LGG-1 and mNG::LGG-2 to the surface of phagosomes in Figure 9I, J.
- https://cdn.elifesciences.org/articles/72466/elife-72466-fig9-data2-v2.docx
-
Figure 9—source data 3
Relative mNG::LGG-1 and mNG::LGG-2 signal intensity at 50min-post engulfment.
- https://cdn.elifesciences.org/articles/72466/elife-72466-fig9-data3-v2.docx
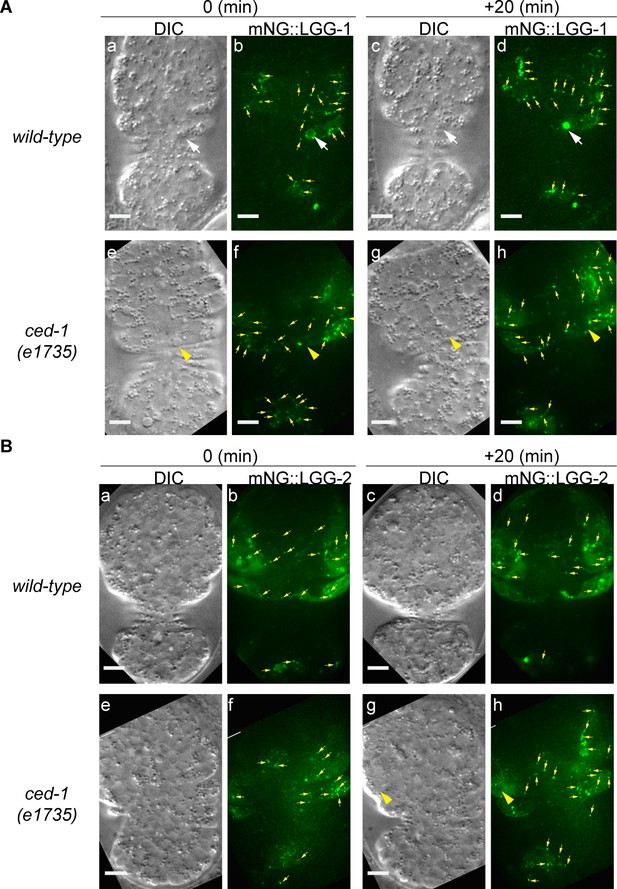
The generation of autophagosomes is normal in ced-1 mutants.
Images of wild-type and ced-1(e1735) mutant embryos expressing Pced-1 mNG::lgg-1 (A) or Pced-1lgg-2::mNG (B) are presented. (a–b and e–f) Embryos are at ~370 min post-1st cleavage; (c–d and g–h) Embryos 20 min later. (a, c, e, g) DIC images. (b, d, f, h) 2-D projection of 14 consecutive Z-sections at 0.5 µm interval each. Green puncta represent LGG autophagosomes (yellow arrows). White arrows in (A(a-d)) marks one C3 phagosome, onto which LGG-1::mNG puncta are recruited in (a–b); 20 min later (c–d), LGG-1::mNG enters this phagosome, showing an autophagosomes/phagosome fusion event. Yellow arrowheads in (A(e-h)) marks one C3 phagosome on the surface, of which only one mNG punctum was found (f), and no mNG signal enters the phagosome (h), indicating the lack of recruitment and fusion. Yellow arrowhead in (B(g-h)) marks one C2 phagosome on the surface, of which one mNG+ punctum is found, and no fusion is visible at 20 min. Scale bars are 5 µm.
In ced-1(e1735) mutants, mNG::LGG-1-labeled vesicles fail to be recruited to the phagosomal surface.
Related to Figure 9A. This movie shows time-lapse recording images of a C3 phagosome in a ced-1(e1735) embryo, starting at ~300 min post-1st cleavage. ‘0 min’ is the moment when a nascent phagosome is just formed. White arrowheads mark the phagosome; yellow arrows label mNG::LGG-1+ puncta.
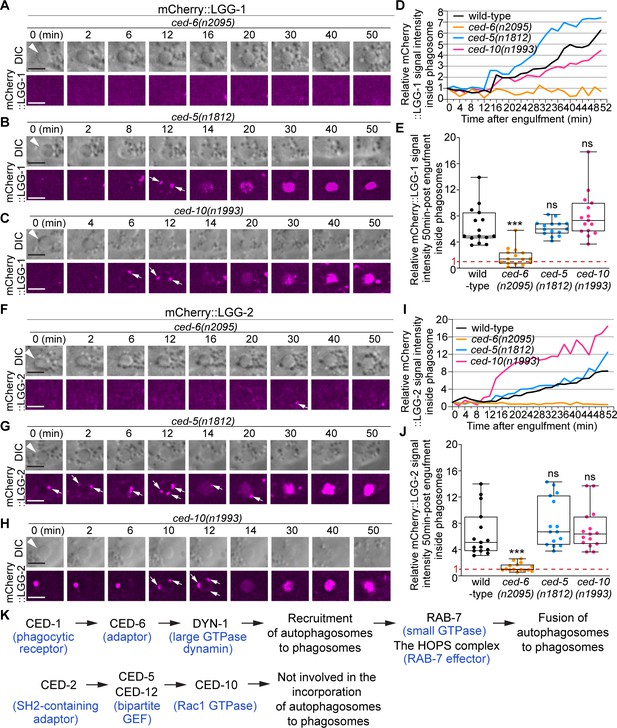
ced-6, but not ced-5 or ced-10, is required for the incorporation of autophagosomes into phagosomes.
(A–C and F–H) Time-lapse image series monitoring the presence or absence of puncta (white arrows) labeled with mCherry::LGG-1 (A–C) or –2 (F–H) on C3 phagosomes (white arrowheads) and the subsequent entry of the mCherry signal into the phagosomal lumen in ced-6, ced-5, and ced-10 mutant embryos. DIC images mark the position of the cell corpse. ‘0 min’ is the moment when a nascent phagosome just seals. Scale bars are 2 µm. (D and I) The relative mCherry::LGG-1 (D) or –2 (I) signal intensity in the center of a phagosome (Y-axis) over time (in the 2 min interval) (X-axis). “0 min” indicates the moment when a nascent phagosome just seals. (D) The data for the wild-type, ced-6(n2095), ced-5(n1812), and ced-10(n1993) mutant embryos are from Figure 1E and 9(A-C), respectively. (I) The data for the wild-type, ced-6(n2095), ced-5(n1812), and ced-10(n1993) mutant embryos are from Figure 1H and 9(F-H), respectively. (Figure 10—source data 1). (E and J) Box-and-Whiskers plots of the relative mCherry signal intensity measured in the center of phagosomes 50 min-post the formation of nascent C3 phagosomes from 15 each of wild-type, ced-6(n2095), ced-5(n1812), and ced-10(n1993) mutant embryos. The red dashed lines indicate where value one is, representing no signal enrichment relative to the background signal. “***”, p < 0.001; ns, not significant, Student t-test against the wild-type samples. (Figure 10—source data 2). (K) A diagram illustrating that between the two parallel pathways that regulate the clearance of apoptotic cells, only the CED-1 pathway, but not the other pathway, plays an essential role in promoting the incorporation of autophagosomes into phagosomes. Blue letters in parentheses are the names of the mammalian homolog of the corresponding C. elegans proteins.
-
Figure 10—source data 1
Relative signal intesity over time mCherry::LGG-1 and mCherry::LGG-2 in Figure 10 D and I.
- https://cdn.elifesciences.org/articles/72466/elife-72466-fig10-data1-v2.docx
-
Figure 10—source data 2
Relative mCherry::LGG-1 and mCherry::LGG-2 signal intensity 50min-post engulfment in Figure 10 E and F.
- https://cdn.elifesciences.org/articles/72466/elife-72466-fig10-data2-v2.docx
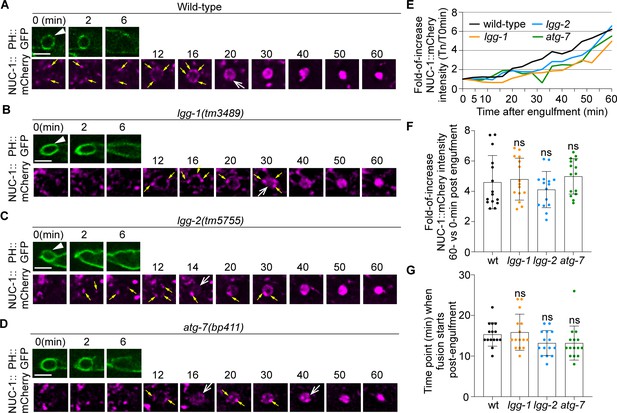
Defects in the formation of autophagosomes do not significantly affect the timing or efficiency of lysosomes incorporation into phagosomes.
The time-lapse recording was conducted on phagosomes containing C1, C2, and C3 in wild-type and named mutant embryos carrying Pced-1nuc-1::mCherry, the lysosomal lumen reporter, and Pced-1PH(PLCγ)::gfp, the marker for extending pseudopods and nascent phagosomes. (A-D) Fluorescence time-lapse images of a C3 phagosome in each strain with the indicated genotype. ‘0 min’ is the moment when a nascent phagosome (white arrowhead) just seals. Yellow arrows mark the lysosomal particles that are located on phagosomal surfaces. White open arrows mark the phagosomes with mCherry signals in the lumen. Scale bars are 2.5 µm. (E) The relative NUC-1::mCherry signal intensity in the center of a phagosome (Y-axis) over time (in the 2 min interval) (X-axis). ‘0 min’ indicates the moment when a nascent phagosome is just sealed. Data are from Figure 10 (A–D). (Figure 11—source data 1) (F) Bar graphs of the average fold-of-increase of the mCherry signal intensity at the center of phagosomal lumen 60 min-post the formation of nascent C3 phagosomes. Bars represent the mean, the error bars indicate standard deviation, and each dot represents a sample. 15 phagosomes of the indicated genotype were scored. Student t-test of each mutant compared to the wild-type value. ns, not significant. (Figure 11—source data 2) (G) Bar graphs of the average time when the NUC-1::mCherry signal is first detected inside the lumen of 15 C3 phagosomes in the indicated genotypes. Bars represent the mean, the error bars indicate standard deviation, and each dot represents a sample. Student t-test of each mutant compared to the wild-type value. ns, not significant. (Figure 11—source data 2).
-
Figure 11—source data 1
NUC-1::mCherry signal intensity over time in Figure 11E.
- https://cdn.elifesciences.org/articles/72466/elife-72466-fig11-data1-v2.docx
-
Figure 11—source data 2
Time of fusion of NUC-1::mCherry and relative signal intesity at 60 min-post in Figure 11 F and G.
- https://cdn.elifesciences.org/articles/72466/elife-72466-fig11-data2-v2.docx
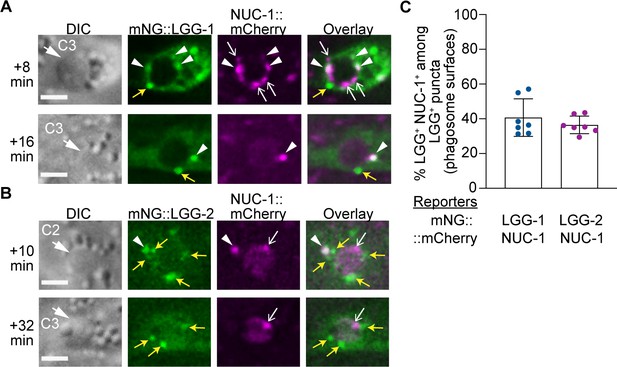
Visualizing the enrichment of lysosomes, autophagosomes, and autolysosomes on phagosomal surfaces.
(A–B) DIC and fluorescence images of C2 and C3 phagosomes in wild-type embryos co-expressing Pced-1 nuc-1::mCherry with Pced-1 mNG::lgg-1 or Pced-1 mNG::lgg-2. Images were captured at the indicated time point after phagosome formation (‘0 min’). DIC images mark the position of the cell corpse (white arrows). Yellow arrows, open white arrows, and white arrowheads label the GFP+ mCherry- puncta (autophagosomes), GFP- mCherry+ puncta (lysosomes), and GFP+ mCherry+ double-positive puncta (autolysosomes), respectively, on phagosomal surfaces. Scale bars are 2 µm. (C) Bar graph depicting the percentage of autolysosomes (GFP+ mCherry+ puncta) among LGG-1+ or –2+ puncta on the surface of phagosomes. Each sample (dot) represents the distribution of the aforementioned puncta collected from the C1, C2, and C3 phagosomes of one embryo. Seven embryos were scored. Bars and error bars represent mean and standard deviation values. (Figure 12—source data 1).
-
Figure 12—source data 1
Percentage distribution percentage of autolysosomes (GFP+ mCherry+ puncta) among LGG-1+ or -2+ puncta on the surface of C1, C2, and C3 phagosomes.
- https://cdn.elifesciences.org/articles/72466/elife-72466-fig12-data1-v2.docx
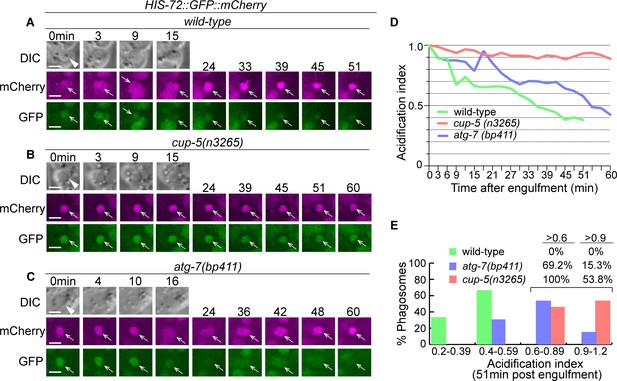
Inactivation of autophagy results in a modest phagosomal acidification defect.
(A–C) Time-lapse imaging series of phagosomes (white arrowheads in DIC images) of wild-type, cup-5, and atg-7 mutant embryos expressing Phis-72 his-72::gfp::mCherry. Open white arrows depict the nuclei of engulfed cell corpses, labeled with both the GFP and mCherry markers. Reduction of the GFP signal intensity over time is indicative of phagosome acidification. ‘0 min’ is when a phagosome is just sealed. Scale bars are 2 µm. (D) The acidification index curves of three phagosomes (Y-axis) over time (in the 3 min interval) (X-axis) in embryos with the labeled genotypes. ‘0 min’ indicates the moment when a phagosome is just sealed. The data of the wild-type, cup-5 (n3265), and atg-7 (bp411) are from A-C, respectively. (Figure 13—source data 1). (E) Histogram depicting the distribution of the acidification index measured at 51-min-post the formation of nascent phagosomes. In wild-type, cup-5 (n3265), and atg-7 (bp411) mutant embryos, 6, 12, and 12 phagosomes were scored. (Figure 13—source data 2).
-
Figure 13—source data 1
The acidification index curves of phagosomes over time in Figure 13D.
- https://cdn.elifesciences.org/articles/72466/elife-72466-fig13-data1-v2.docx
-
Figure 13—source data 2
Distribution of the acidification index measured at 51-min-post the formation of phagosomes in Figure 13E.
- https://cdn.elifesciences.org/articles/72466/elife-72466-fig13-data2-v2.docx
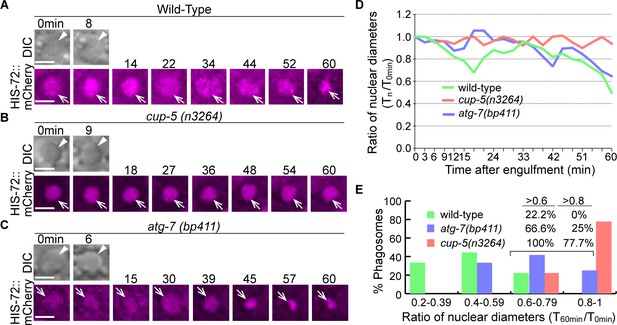
Inactivation of autophagy results in a modest delay of the degradation of apoptotic cell DNA.
(A–C) Time-lapse imaging series monitoring the shrinkage of the apoptotic cell nucleus inside a C1, C2, or C3 phagosome (white arrowhead in DIC images) in three different strains expressing Phis-72 his-72::mCherry. Apoptotic cell nuclei are labeled with HIS-72::mCherry (open white arrows). ‘0 min’ is when a phagosome is just sealed. Scale bars are 2 µm. (D) The ratio of the nuclear diameter of a phagosome (Y-axis) at labeled time points compared to that of the ‘0 min’ diameter (in the 3 min interval) (X-axis). ‘0 min’ indicates the moment when a phagosome is just sealed. The data of the wild-type, cup-5 (n3265), and atg-7 (bp411) are from Figure 1(A–C), respectively. (Figure 14—source data 1). (E) Histogram depicting the distribution of the ratio of nuclear diameters measured at 60min-post phagosome formation. In wild-type, cup-5 (n3265), and atg-7 (bp411) mutant strains, 9, 9, and 12 engulfed apoptotic cells were scored. (Figure 14—source data 2).
-
Figure 14—source data 1
The ratio of the nuclear diameter curves of phagosomes over time in Figure 14D.
- https://cdn.elifesciences.org/articles/72466/elife-72466-fig14-data1-v2.docx
-
Figure 14—source data 2
Distribution of the ratio of nuclear diameters measured at 60min-post phagosome formation in Figure 14E.
- https://cdn.elifesciences.org/articles/72466/elife-72466-fig14-data2-v2.docx
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (E. coli) | OP50 | CGC | OP50 | |
| Strain, strain background (C. elegans) | N2 | CGC | Wild-type Bristol N2 | |
| Strain, strain background (C. elegans) | VC308 | CGC | rab-7(ok511) /mIn1 II | |
| Strain, strain background (C. elegans) | ZH0989 | Yu et al., 2008 | unc-76(e911) enIs36 [punc-76(+), Pced-1 ced-1::gfp, Pced-1 2xFYVE::mRFP] V | Figure 6 Available from the Zhou Lab |
| Strain, strain background (C. elegans) | ZH2059 | This study | unc-76(e911)V; enEx979 [punc-76(+), Phis-72 HIS-72::GFP::mCherry] | Figure 13 Available from the Zhou Lab |
| Strain, strain background (C. elegans) | ZH2105 | This study | cup-5(n3264) III; unc-76(e911) V; enEx979 | Figure 13 Available from the Zhou Lab |
| Strain, strain background (C. elegans) | ZH2573 | This study | lgg-2(tm5755) IV; unc-76(e911) V; enEx1223 [Pced-1mCherry::lgg-2, punc-76(+)] | Figure 5—figure supplement 1 Available from the Zhou Lab |
| Strain, strain background (C. elegans) | ZH2632 | This study | lgg-2(tm5755) IV; unc-76(e911) V; enEx1267 [Punc-76(+), Pced-1::gfp::lgg2] | Figure 5—figure supplement 1 Available from the Zhou Lab |
| Strain, strain background (C. elegans) | ZH2715 | This study | rab-7(ok511) II / mIn1 II; unc-76(e911) V; enEx1320 [punc-76(+), Pced-1mCherry::lgg-2] | Figure 8 Available from the Zhou Lab |
| Strain, strain background (C. elegans) | ZH2782 | This study | rab-7 (ok511) II / mIn1 II; unc-76(e911) V; enEx1376 [punc-76(+), Pced-1 mCherry::lgg-1] | Figure 8 Available from the Zhou Lab |
| Strain, strain background (C. elegans) | ZH2831 | This study | lgg-1(tm3489) II / mIn1 I; lgg-2(tm5755) IV | Figure 5 Available from the Zhou Lab |
| Strain, strain background (C. elegans) | ZH2835 | This study | lgg-1(tm3489) II / mIn1 I; unc-76(e911) enIs36 V | Figure 6 Available from the Zhou Lab |
| Strain, strain background (C. elegans) | ZH2838 | This study | lgg-1(tm3489) II / mIn1 II; unc-76(e911) V; enEx1428 [punc-76(+), Pced-1 gfp::lgg-1] | Figure 5—figure supplement 1 Available from the Zhou Lab |
| Strain, strain background (C. elegans) | ZH2841 | This study | lgg-1(tm3489) II / mIn1 II; unc-76(e911) V; enEx1431 [punc-76(+), Pced-1 mCherry::lgg-1] | Figure 5—figure supplement 1 Available from the Zhou Lab |
| Strain, strain background (C. elegans) | ZH2875 | This study | lgg-2(tm5755) IV; unc-76(e911) enIs36 V | Figure 6 Available from the Zhou Lab |
| Strain, strain background (C. elegans) | ZH2889 | This study | unc-76(e911) V; enEx1459 [punc-76, Pced-1 PH::mrfp,Pced-1 mNG::lgg-2] | Figure 1 Available from the Zhou Lab |
| Strain, strain background (C. elegans) | ZH2903 | This study | atg-9(bp564) him-5(e1490)V; lin-15AB(n765ts) X; enEx1468 [Plin-15(+), Pced-1 PH::mrfp, Pced-1 mNG::lgg-1] | Figure 3—figure supplement 1, Available from the Zhou Lab |
| Strain, strain background (C. elegans) | ZH2907 | This study | vps-18(tm1125) II; enIs80 [punc-76(+), Pced-1 ced-1::gfp, Pced-1mCherry::lgg-1] IV; unc-76(e911) V | Figure 8 Available from the Zhou Lab |
| Strain, strain background (C. elegans) | ZH2916 | This study | ced-1(e1735) I; unc-76(e911) V; enEx1470 [Pced-1 mNG::lgg-1, punc-76(+), Pced-1 PH::mrfp] | Figure 9 Available from the Zhou Lab |
| Strain, strain background (C. elegans) | ZH2919 | This study | enIs82 [unc-76(+), Pced-1 ced-1::gfp, Pced-1mCherry::lgg-1] II; unc-76(e911) V | Figure 1 Available from the Zhou Lab |
| Strain, strain background (C. elegans) | ZH2921 | This study | unc-76(e911) enIs85 [punc-76(+), Pced-1 PH::mrfp, Pced-1mNG::lgg-2] V. | Figure 1 Available from the Zhou Lab |
| Strain, strain background (C. elegans) | ZH2922 | This study | atg-9(bp564) him-5(e1490) V; lin-15AB(n765ts) X; enEx1472 [Plin-15(+), Pced-1 PH::mrfp, Pced-1 mNG::lgg-2] | Figure 3—figure supplement 1, Available from the Zhou Lab |
| Strain, strain background (C. elegans) | ZH2929 | This study | vps-18(tm1125) II; unc-76(e911) V; enIs83 [punc-76(+), Pced-1 ced-1::gfp, Pced-1mCherry::lgg-2] X | Figure 8 Available from the Zhou Lab |
| Strain, strain background (C. elegans) | ZH2934 | This study | ced-1(e1735) I; unc-76(e911) enIs85 V | Figure 9 Available from the Zhou Lab |
| Strain, strain background (C. elegans) | ZH2950 | This study | enIs82 II; atg-7(bp411) IV; unc-76(e911) V | Figure 2 Available from the Zhou Lab |
| Strain, strain background (C. elegans) | ZH2951 | This study | atg-7(bp411) IV; enIs83 X | Figure 2 Available from the Zhou Lab |
| Strain, strain background (C. elegans) | ZH2952 | This study | enIs82 II; atg-13(bp414) III | Figure 2 Available from the Zhou Lab |
| Strain, strain background (C. elegans) | ZH2953 | This study | atg-13(bp414) III; enIs83 X | Figure 2 Available from the Zhou Lab |
| Strain, strain background (C. elegans) | ZH2954 | This study | epg-8(bp251) I; enIs82 II; unc-76(e911) V | Figure 2 Available from the Zhou Lab |
| Strain, strain background (C. elegans) | ZH2955 | This study | epg-8(bp251) I; unc-76(e911) him-5(1490) V; enIs83 X | Figure 2 Available from the Zhou Lab |
| Strain, strain background (C. elegans) | ZH2980 | This study | atg-7(bp411) IV; unc-76(e911) enIs36 V | Figure 6 Available from the Zhou Lab |
| Strain, strain background (C. elegans) | ZH2992 | This study | enIs87 [punc-76, Pced-1 PH::mrfp, Pced-1 mNG::lgg-1] I; unc-76(e911) V | Figure 1 Available from the Zhou Lab |
| Strain, strain background (C. elegans) | ZH2994 | This study | enIs82 II; ced-6(n2095) III; unc-76(e911) V | Figure 10 Available from the Zhou Lab |
| Strain, strain background (C. elegans) | ZH2995 | This study | ced-6(n2095) III; unc-76(e911) V; enIs83 X | Figure 10 Available from the Zhou Lab |
| Strain, strain background (C. elegans) | ZH3009 | This study | ced-5(n1812) IV; unc-76(e911) V; enIs83 X | Figure 10 Available from the Zhou Lab |
| Strain, strain background (C. elegans) | ZH3010 | This study | enIs82 II; ced-5(n1812) IV; unc-76(e911) V | Figure 10 Available from the Zhou Lab |
| Strain, strain background (C. elegans) | ZH3011 | This study | enIs82 II; ced-10(n1993) IV; unc-76(e911) V | Figure 10 Available from the Zhou Lab |
| Strain, strain background (C. elegans) | ZH3012 | This study | ced-10(n1993) IV; unc-76(e911) V; enIs83 X | Figure 10 Available from the Zhou Lab |
| Strain, strain background (C. elegans) | ZH3014 | This study | enIs87 I; unc-76(e911) V; dyn-1(n4039) X; enEx21[Pdyn-1 dyn-1] | Figure 9 Available from the Zhou Lab |
| Strain, strain background (C. elegans) | ZH3015 | This study | unc-76(e911) enIs85 V; dyn-1(n4039) X; enEx21 | Figure 9 Available from the Zhou Lab |
| Strain, strain background (C. elegans) | ZH3485 | This study | unc-76(e911)V; enEx1791 [punc-76(+), Pced-1 mNG::LGG-1, Pced nuc-1::mCherry] | Figure 12 Available from the Zhou Lab |
| Strain, strain background (C. elegans) | ZH3489 | This study | rab-7 (ok511) II / mIn1 II; unc-76(e911) V; enEx1705 [punc-76(+), Pced-1 CED-1::GFP, Pced-1 2xFYVE::mRFP] | Figure 6 Available from the Zhou Lab |
| Strain, strain background (C. elegans) | ZH3492 | This study | atg-7(bp411) IV; unc-76(e911) V, enEx979 | Figure 13 Available from the Zhou Lab |





