A non-bactericidal cathelicidin provides prophylactic efficacy against bacterial infection by driving phagocyte influx
Figures

The nucleotide sequence encoding the precursor of PopuCATH and the deduced amino acid sequence.
(A) The amino acid sequence of mature peptide is underlined, and the putative signal peptide is italic. Asterisk (*) indicates stop codon. Deduced amino acid sequence of PopuCATH precursor was translated in ExPASy Translate Tool (http://web.expasy.org/translate/). Sequence Blast was performed with Blastx (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
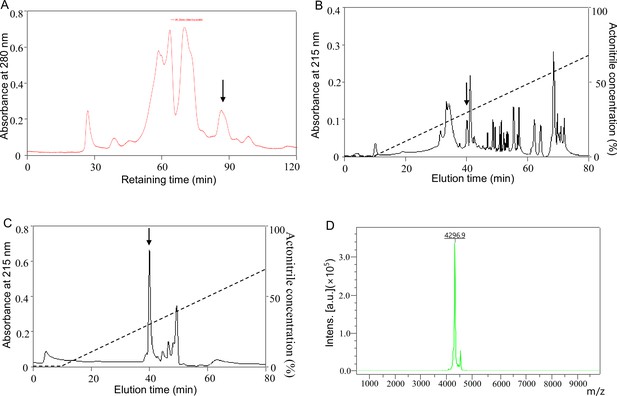
Peptide purification.
(A) The skin secretion was first separated by molecular sieving fast protein liquid chromatography (FPLC). About 1.5 g of lyophilised P. puerensis skin secretion was dissolved in 10 mL phosphate-buffered saline (PBS, 0.1 M, pH 6.0) with one tablet of Complete Mini Protease Inhibitor Cocktail (Roche, USA). After centrifugation (12,000 g, 15 min, 4℃), the supernatant was separated by FPLC on GE ÄKTA pure system. Molecular sieving FPLC was performed on a Superdex 75 10/300 GL column (10 × 300 mm, 24 mL volume, GE, USA) with PBS (0.1 M, pH 6.0) at a flow rate of 0.6 mL/min. (B) Eluted peaks containing objective peptide after FPLC in panel A (indicated by an arrow) were pooled and purified by RP-HPLC. (C) Eluted peaks containing objective peptide after RP-HPLC in panel B (indicated by an arrow) were further purified by RP-HPLC. A C18 column (25 × 0.46 cm, Waters, USA) was used to purify the objective peptide by RP-HPLC in panel A and B. Fractions eluted with a linear gradient from 0% to 60% acetonitrile supplemented with 0.1% trifluoroacetic acid in 60 min at a flow rate of 0.7 mL/min. (D) MALDI-TOF mass spectrometry analysis of the purified peptide. The eluted peak containing objective peptide in panel C (indicated by an arrow) was collected for purity assay, about 1 μL of the eluted peak was spotted onto a matrix-assisted laser desorption ionisation time-of-flight mass spectrometry (MALDI-TOF MS) plate with 1 μL of α-cyano-4-hydroxycinnamic acid matrix (10 mg/mL in 60% acetonitrile) and analyzed by an UltraFlex I mass spectrometer (Bruker Daltonics, Germany).
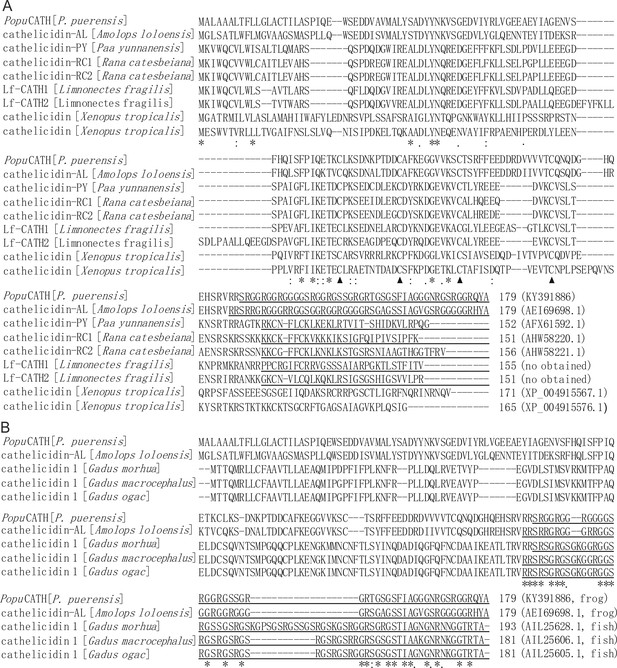
Multiple alignment of the precursor of PopuCATH with other cathelicidins.
(A) Alignment of the precursor of PopuCATH with other amphibian cathelicidins. The four conserved cysteine motifs (▲), identical sites (*), conserved sites (:), and less conserved sites (.) of the precursors were indicated. The GenBank accession number of each cathelicidin was listed at its end. (B) Alignment of the mature PopuCATH with glycine-rich cathelicidins identified from frog and fish. The mature peptide of the glycine-rich cathelicidins were underlined. The identical sites (*), conserved sites (:), and less conserved sites (.) of the mature peptides were indicated. The GenBank accession number of each cathelicidin was listed at its end. Sequence identity was aligned by ClustalW (http://embnet.vital-it.ch/software/ClustalW.html).
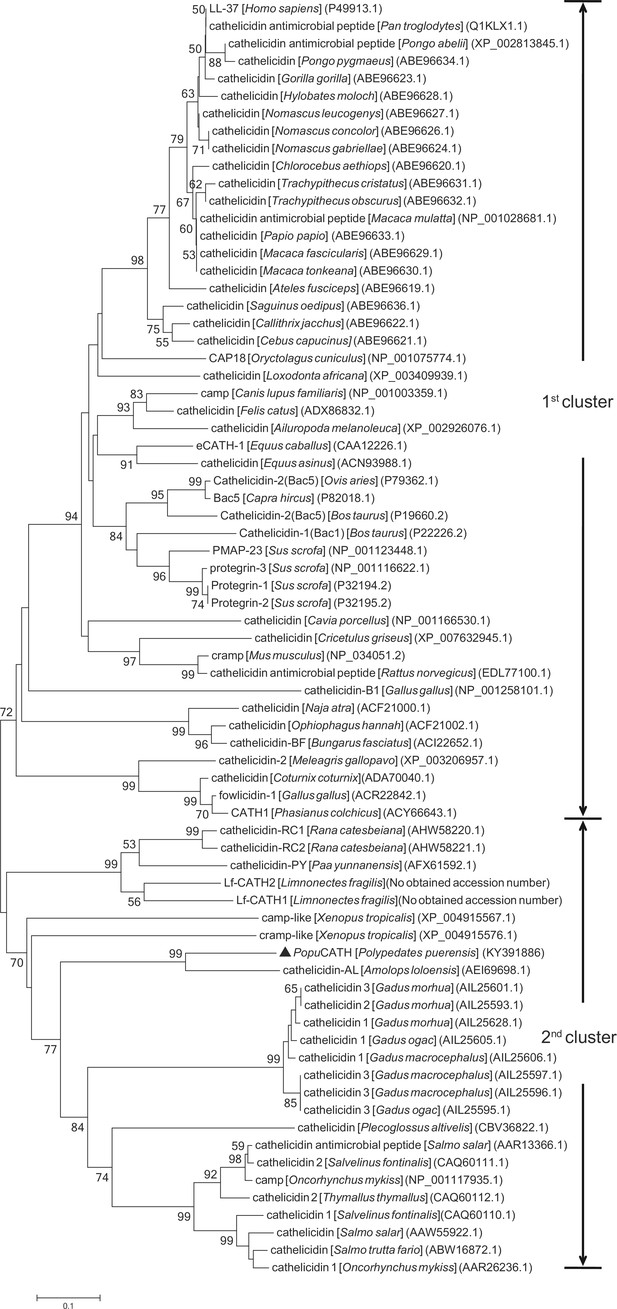
Phylogenetic analysis of the precursor of PopuCATH with other vertebrate cathelicidins.
Phylogenetic dendrogram was constructed by ClustalX program (version 1.81) and the Molecular Evolutionary Genetics Analysis (MEGA) software (version 5.0) using neighbor-joining (NJ) method based on the proportion difference of aligned amino acid sites of the full sequence of precursors. Only branches supported by a bootstrap value of at least 50% (expressed as percentage of 1,000 bootstrap samples supporting the branch) are shown at branching points. Two clusters are partitioned and indicated for two major branches. PopuCATH is marked by a triangle (▲).
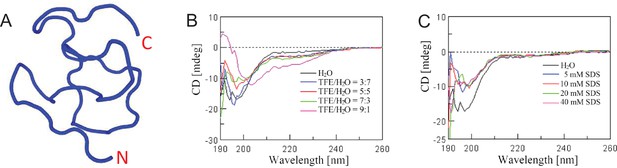
Secondary structural analysis.
(A) The secondary structure of PopuCATH was first predicted by a computational framework PEP-FOLD3 (http://bioserv.rpbs.univ-paris-diderot.fr/services/PEP-FOLD3). (B, C) Circular dichroism (CD) experiments were performed to verify the secondary structural components of PopuCATH in artificial membrane environments, including TFE/H2O (B) and SDS/H2O (C). PopuCATH (0.2 mg/mL) was prepared in H2O, artificial membrane environments of TFE/H2O and SDS/H2O. CD spectra were recorded at 298 K on a Jasco-810 spectropolarimeter (Jasco, Tokyo, Japan) with 1 mm path-length cell and 0.2 nm interval from 190 to 260 nm. Data from three consecutive scans were averaged, smoothed, and expressed as the mean residue ellipticity (θ) in deg·cm2 ·dmol−1.

PopuCATH lacks direct antimicrobial activity but can prevent bacterial infection in tree frogs.
(A) Bacterial killing kinetic assay. E. coli, S. aureus, and C. albicans were diluted in Mueller-Hinton broth, and A. hydrophila were diluted in nutrient broth at density of 105 CFU/mL. PopuCATH (200 μg/mL), PY (cathelicidin-PY, 1× MIC, positive control) or PBS was added and incubated at 37℃ or 25℃. At indicated time points, the CFUs were counted. (B) Microbial metabolic activity assay. E. coli, S. aureus, C. albicans, and A. hydrophila were diluted in DMEM at density of 105 CFU/mL, and PopuCATH (200 μg/mL), PY (cathelicidin-PY, 1× MIC, positive control) or PBS was added. Microbial dilution (100 μL/well) and WST-8 (10 μL/well) was added to 96-well plates, respectively, and incubated at 37℃ or 25℃. At indicated time points, absorbance at 255 nm was monitored. Metabolic activity was expressed as the percentage of the PBS-treated group. (C) SEM assay. E. coli ATCC25922 and S. aureus ATCC25923 were washed and diluted in PBS (105 CFU/mL). PopuCATH (200 μg/mL), PY (1× MIC, positive control) or PBS was added into the bacterial dilution and incubated at 37℃. After incubation for 30 min, bacteria were centrifuged at 1000 g for 10 min, and fixed for SEM assay. The bacterial surface morphology was observed using a Hitachi SU8010 SEM. (D) Anti-bacterial activity in tree frogs. PopuCATH (10 mg/kg) was intraperitoneally injected into P. puerensis (n = 5, 21–30 g) at 8 or 4 hr prior to (–8 or –4 hr), or 4 hr after ( + 4 hr) S. aureus ATCC25923 inoculation (108 CFU/frog, intraperitoneal injection). At 18 hr post bacterial challenge, peritoneal lavage was collected for bacterial load assay. **p < 0.01, ***p < 0.001, ns, not significant.
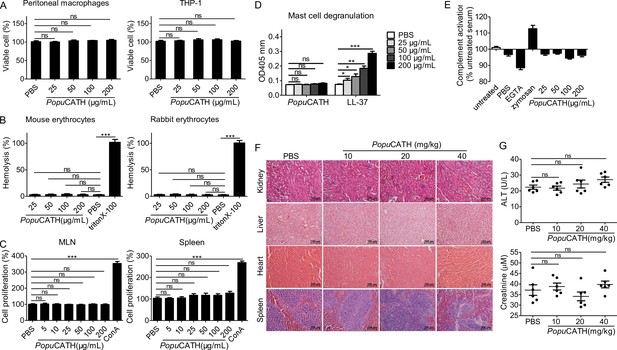
PopuCATH exhibits low toxic side effects to mammalian cells and mice.
(A) Cytotoxicity assay. Peritoneal macrophages or THP-1 cells were seeded in 96-well plates (5 × 105 cells/well, 200 µL). PopuCATH (25, 50, 100, or 200 μg/mL) was added and cultured for 24 hr. CCK-8 reagent (10 µL/well) was added and incubated for 1 hr. The absorbance at 450 nm was recorded. Viable cells was expressed as the percentage of the PBS-treated group. (B) Hemolysis assay. Mouse erythrocytes and rabbit erythrocytes were washed with 0.9% saline and incubated with PopuCATH (25, 50, 100, and 200 μg/mL), PBS or triton X-100 (1%, positive control) at 37℃. After incubation for 30 min, the erythrocytes were centrifuged at 1000 g for 5 min. The absorbance at 540 nm was measured. Hemolytic activity was expressed as the percentage of the triton X-100-treated group. (C) Immunogenicity assay. Lymphocytes isolated from mesenteric lymph node (MLN) and spleen of mice were suspended in RPMI 1640 (2%FBS) and seeded in 96-well plates (5 × 104 cells/well, 200 µL). PopuCATH (25, 50, 100, or 200 μg/mL) or concanavalin A (Con A, 2 μg/mL) was added and incubated at 37℃ for 24 hr. CCK-8 reagent (10 µL/well) was added. After incubation at 37℃ for 1 hr, the absorbance at 450 nm was measured. Cell proliferation was expressed as the percentage of PBS-treated group. (D) Hypersensitivity assay. RBL-2H3 cells were seeded in 96-well plates (2 × 104 cells/well, 200 µL) and cultured overnight. PopuCATH (25, 50, 100, or 200 μg/mL), PBS or LL-37 (25, 50, 100, or 200 μg/mL, positive control) was added and incubated at 37℃ for 0.5 hr. The supernatant was collected for mast cell degranulation assay. (E) Complement activation assay. Mouse serum was treated with PBS, EGTA inhibitor (10 mM), zymosan (0.5 mg/mL), PopuCATH (25, 50, 100, and 200 μg/mL) at 37℃ for 1 hr. C3a des-Arg was measured by ELISA. (F, G) In vivo acute toxicity assay. C57BL/6 mice (18–20 g, n = 6) were intraperitoneally injected with PopuCATH at dose of 10, 20 and 40 mg/kg. At 24 hr post injection, kidneys, livers, hearts and spleens were collected for H&E staining (F), scale bar: 100 μm. Serum was collected for the creatinine and alanine aminotransferase (ALT) measurement (G). *p < 0.05, **p < 0.01, ***p < 0.001, ns, not significant.
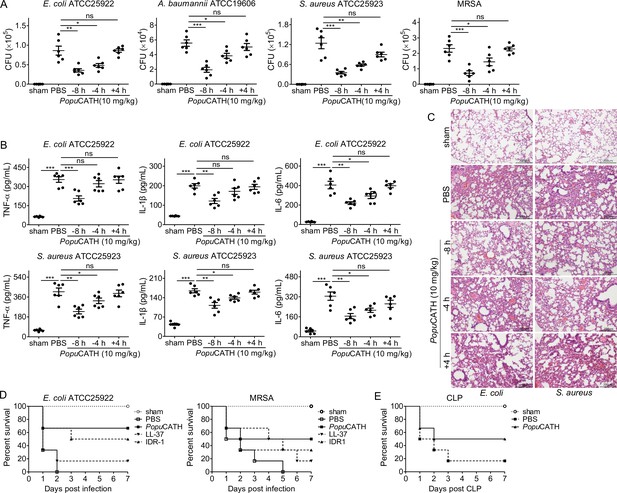
PopuCATH provides prophylactic efficacy against bacterial infection in mice.
(A–C) PopuCATH (10 mg/kg) was intraperitoneally injected into C57BL/6 mice (18–20 g, n = 6) 8 or 4 hr prior to (–8 or –4 hr), or 4 hr after ( + 4 hr) E. coli, A. baumannii, S. aureus or methicillin-resistant S. aureus (MRSA) inoculation (2 × 107 CFUs/mouse, intraperitoneal injection). At 18 hr post inoculation, peritoneal lavage was collected for bacterial load assay (A), serum was collected for cytokine assay (B), and lungs were taken for histopathological assay (C), scale bar: 200 μm. (D) C57BL/6 mice (18–20 g, n = 6) were intraperitoneally injected with PopuCATH (10 mg/kg), LL-37, or IDR-1 (control peptides) 4 hr prior to a lethal dose of E. coli (4 × 107 CFUs/mouse) or MRSA (6 × 108 CFUs/mouse, intraperitoneal injection) inoculation. The survival rates of mice were monitored for 7 days. (E) C57BL/6 mice (18–20 g, n = 6) were intraperitoneally injected with PopuCATH (10 mg/kg) at 8 and 4 hr (two times) prior to (–8 and –4 hr) CLP. At 0 hr, mice were anaesthesied with ketamine (100 mg/kg), and CLP was performed. The survival rates of mice were monitored for 7 days. *p < 0.05, **p < 0.01, ***p < 0.001, ns, not significant.
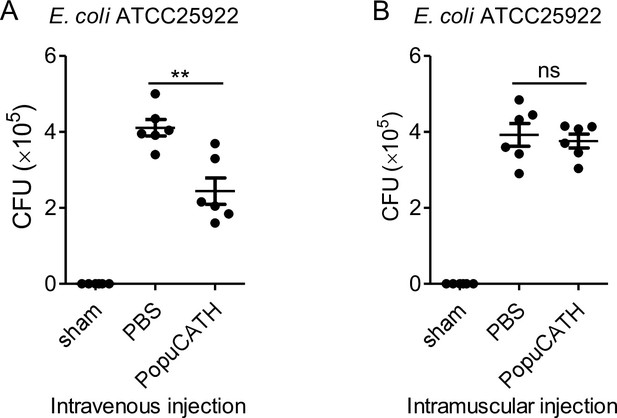
Prophylactic efficacy of PopuCATH against bacterial infection in mice by intravenous injection or intramuscular injection.
C57BL/6 mice (18–20 g, n = 6) were injected with PopuCATH (10 mg/kg) through intravenous (A) or intramuscular (B) route at 4 hr prior to (–4 hr) E. coli inoculation (2 × 107 CFUs/mouse, intraperitoneal injection). At 18 hr post inoculation, peritoneal lavage was collected for bacterial load assay. **p < 0.01, ns, not significant.
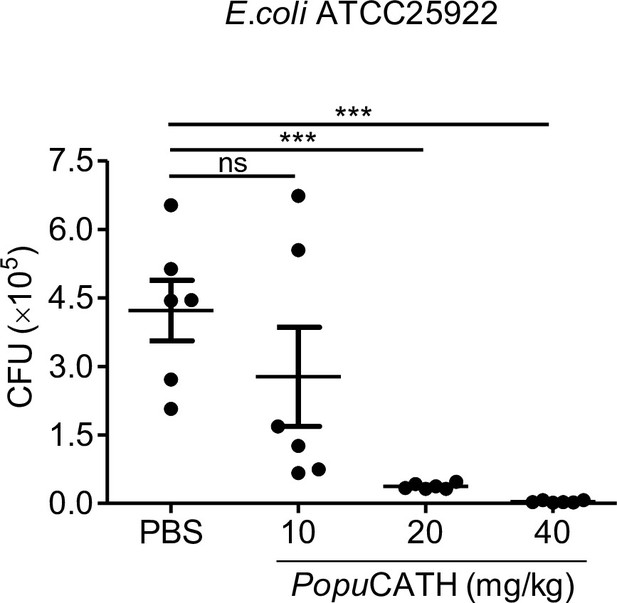
Therapeutic efficacy of different doses of PopuCATH against bacterial infection in mice.
C57BL/6 mice (18–20 g, n = 6) were injected with PopuCATH (10, 20, and 40 mg/kg) through intraperitoneal route at 4 hr after (+ 4 hr) E. coli inoculation (2 × 107 CFUs/mouse, intraperitoneal injection). At 18 hr post inoculation, peritoneal lavage was collected for bacterial load assay. ***p < 0.01, ns, not significant.
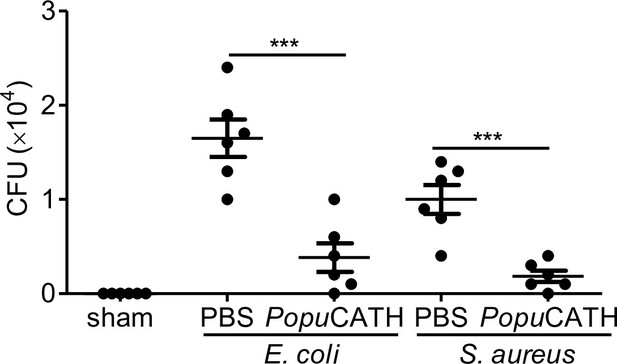
Intraperitoneal injection of PopuCATH reduces the bacterial load in peripheral blood.
PopuCATH (10 mg/kg) was intraperitoneally injected into C57BL/6 mice (18–20 g, n = 6) 4 hr prior to (–4 hr) E. coli ATCC25922 or S. aureus ATCC25923 S. aureus inoculation (2 × 107 CFUs/mouse, intraperitoneal injection). At 18 hr post inoculation, peripheral blood was collected for bacterial load assay. *** p < 0.001.
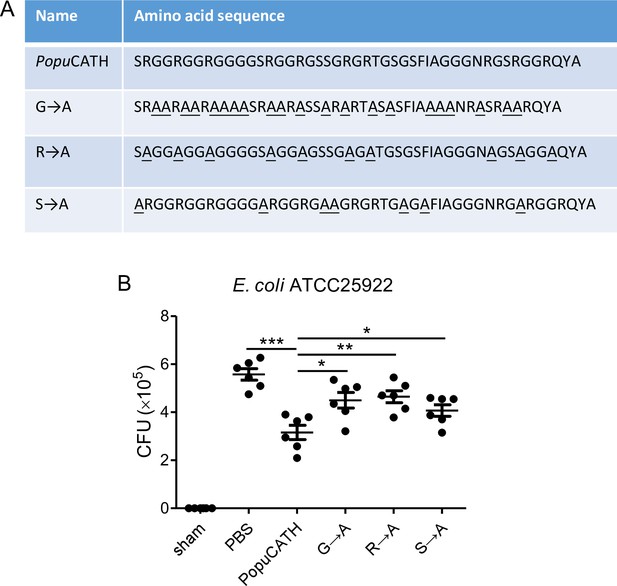
Glycine, arginine, serine residues are key structural requirements for PopuCATH-mediated prophylactic efficacy against bacterial infection.
(A) Amino acid sequence of PopuCATH and its derivatives. The glycine, arginine, serine residues were substituted with alanine residues (underlined), respectively. (B) The protective efficacy of PopuCATH and its derivatives. PopuCATH or its derivative (10 mg/kg) was intraperitoneally injected into C57BL/6 mice (18–20 g, n = 6) 4 hr prior to (–4 hr) E. coli inoculation (2 × 107 CFUs/mouse, intraperitoneal injection). At 18 hr post inoculation, peritoneal lavage was collected for bacterial load assay.
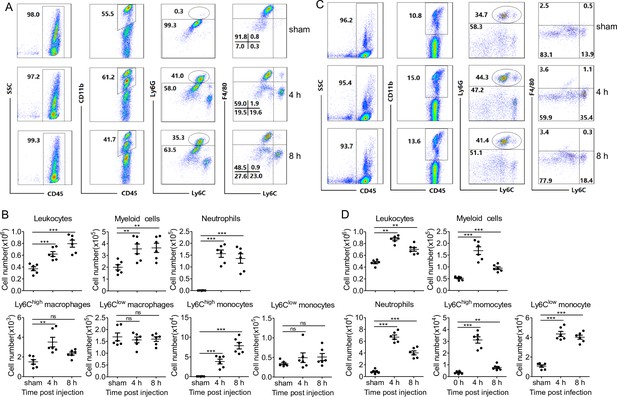
Intraperitoneal injection of PopuCATH induces leukocyte influx in mice.
(A, C) Representative flow cytometry plots and proportions of major population of myeloid cells in peritoneal lavage (A) and peripheral blood (C) are shown. (B, D) Quantitative summary of leukocytes, myeloid cells, neutrophils, monocytes/macrophages in peritoneal lavage (B) and peripheral blood (D). C57BL/6 mice (18–20 g, n = 6) were intraperitoneally injected with PopuCATH (10 mg/kg) dissolved in 0.2 mL PBS. Sham mice received the same volumes of PBS. At 4 and 8 hr post injection, cells in peritoneal lavage and peripheral blood were collected for flow cytometry assay. **p < 0.01, ***p < 0.001, ns, not significant.
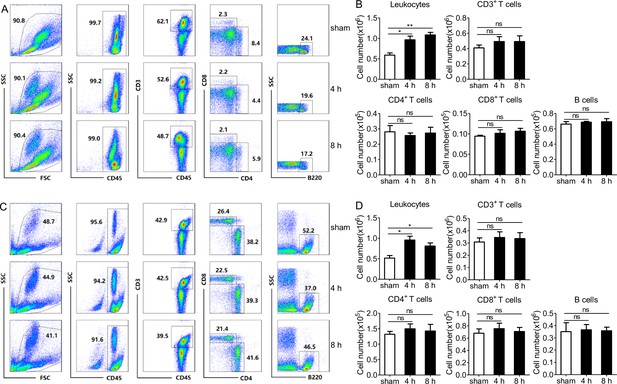
Intraperitoneal injection of PopuCATH does not induce T and B lymphocyte influx in mice.
(A, C) Representative flow cytometry plots and proportions of leukocytes, CD3+ T cells, CD4+ T cells, CD8+ T cells, and B cells in peritoneal lavage (A) and peripheral blood (C) are shown. (B, D) Quantitative summary of leukocytes, CD3+ T cells, CD4+ T cells, CD8+ T cells, and B cells in peritoneal lavage (B) and peripheral blood (D). C57BL/6 mice (18–20 g, n = 6) were intraperitoneally injected with PopuCATH (10 mg/kg) dissolved in 0.2 mL PBS. Sham mice received the same volumes of PBS. At 4 and 8 hr post injection, cells in peritoneal lavage and peripheral blood were collected for flow cytometry assay. *p < 0.05, **p < 0.01, ns, not significant.
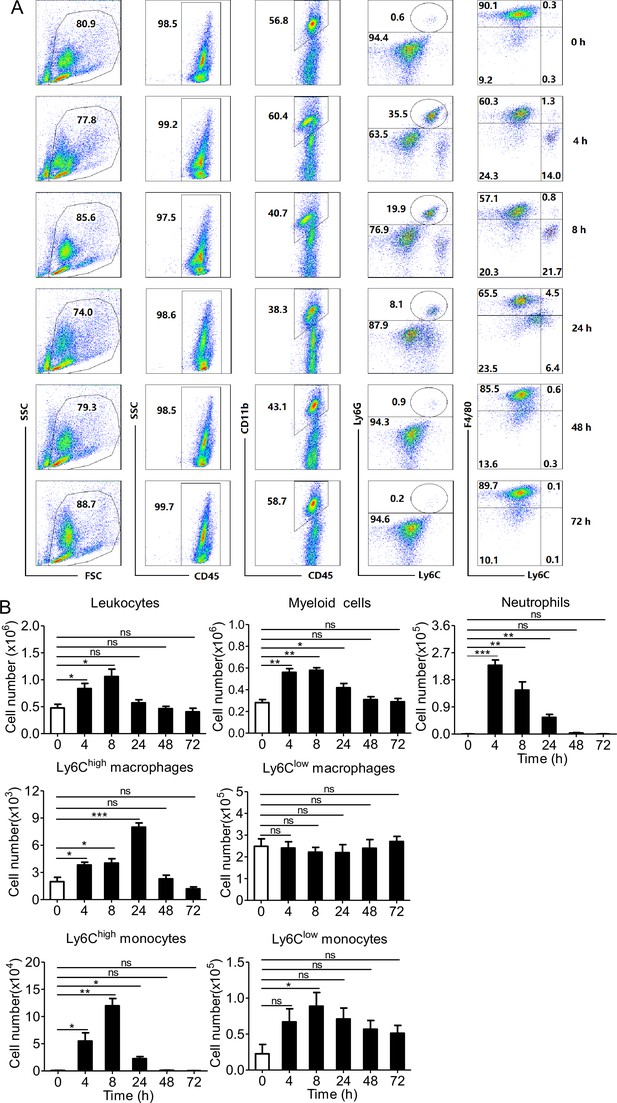
Phagocyte influx in abdominal cavity induced by PopuCATH can last for 24 hr.
(A) Representative flow cytometry plots and proportions of major population of myeloid cells in peritoneal lavage are shown. (B) Quantitative summary of leukocytes, myeloid cells, neutrophils, monocytes, and macrophages in peritoneal lavage. C57BL/6 mice (18–20 g, n = 6) were intraperitoneally injected with PopuCATH (10 mg/kg) dissolved in 0.2 mL PBS. Sham mice received the same volumes of PBS. At indicated time points post injection, cells in peritoneal lavage were collected for flow cytometry assay. *p < 0.05, **p < 0.01, *** p < 0.001, ns, not significant.
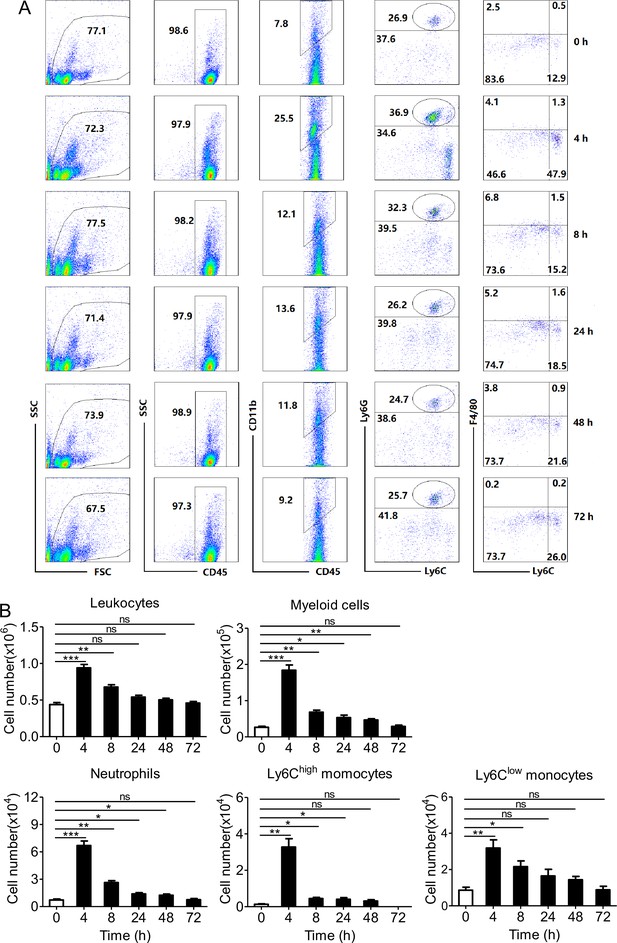
Phagocyte influx in peripheral blood induced by PopuCATH can last for 48 hr.
(A) Representative flow cytometry plots and proportions of major population of myeloid cells in peripheral blood are shown. (B) Quantitative summary of leukocytes, myeloid cells, neutrophils, and monocytes in peripheral blood. C57BL/6 mice (18–20 g, n = 6) were intraperitoneally injected with PopuCATH (10 mg/kg) dissolved in 0.2 mL PBS. Sham mice received the same volumes of PBS. At indicated time points post injection, cells in peripheral blood were collected for flow cytometry assay. *p < 0.05, **p < 0.01, *** p < 0.001, ns, not significant.
Leukocyte influx induced by intraperitoneal injection of LPS in mice.
(A, C) Representative flow cytometry plots and proportions of major population of myeloid cells in peritoneal lavage (A) and peripheral blood (C) are shown. (B, D) Quantitative summary of leukocytes, myeloid cells, neutrophils, monocytes/macrophages in peritoneal lavage (B) and peripheral blood (D). C57BL/6 mice (18–20 g, n = 6) were intraperitoneally injected with LPS (20 μg/mouse, dissolved in 0.2 mL PBS). Sham mice received the same volumes of PBS. At 4 and 8 hr post injection, cells in peritoneal lavage and peripheral blood were collected for flow cytometry assay. *p < 0.05, **p < 0.01, *** p < 0.001, ns, not significant.
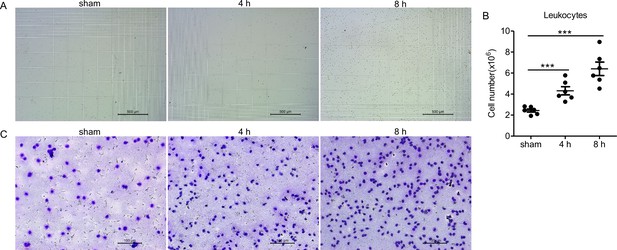
Intraperitoneal injection of PopuCATH induces leukocyte influx in tree frogs.
P. puerensis (21–30 g, n = 5) were intraperitoneally injected with PopuCATH (10 mg/kg) dissolved in 0.2 mL PBS. The same volumes of PBS served as negative control. At 4 and 8 hr post injection, PBS (5 mL) was injected into the abdominal cavity, and peritoneal lavage were collected for cell count using a hemocytometer (A, B), scale bar: 500 µm. The cells were observed under an optical microscope after Wright-Giemsa staining (C), scale bar: 100 µm.
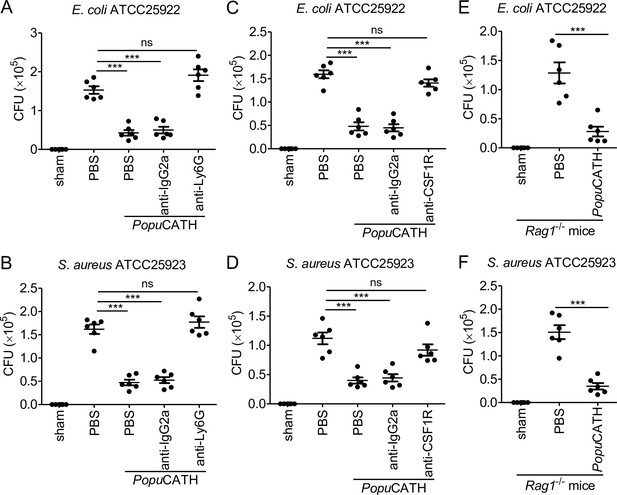
Neutrophils and monocytes/macrophages, but not T and B cells, are required for the prophylactic efficacy of PopuCATH in mice.
(A, B) Protective efficacy of PopuCATH against E. coli (A) or S. aureus (B) in neutrophil depletion mice. Anti-Ly6G antibody or rat IgG2a isotype antibody were intraperitoneally injected into C57BL/6 mice (18–20 g, n = 6) at doses of 500 µg/mouse on day 0 and day 2, respectively. (C, D) Protective efficacy of PopuCATH against E. coli (C) or S. aureus (D) in monocyte/macrophage depletion mice. Anti-CSF1R antibody or rat IgG2a isotype antibody were intraperitoneally injected into C57BL/6 mice (18–20 g, n = 6) at doses of 1 mg/mouse on day 0 followed by 0.3 mg/mouse on day 1 and day 2, respectively. (E, F) Protective efficacy of PopuCATH against E. coli (E) or S. aureus (F) in Rag1–/– mice (18–20 g, n = 6). At 4 hr before E. coli or S. aureus (2 × 107 CFUs/mouse) inoculation, PopuCATH (10 mg/kg) was intraperitoneally injected into neutrophil depletion mice (on day 3), monocyte/macrophage depletion mice (on day 3), and Rag1–/– mice. At 18 hr post bacterial inoculation, peritoneal lavage was collected for the bacterial load assay. ***p < 0.001, ns, not significant.
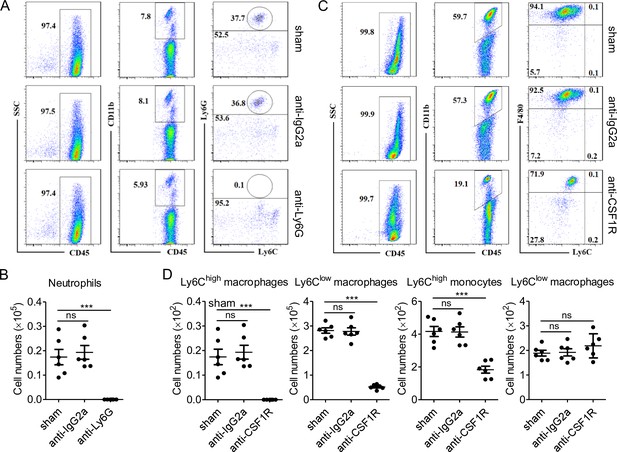
Scavenging efficiency of neutrophils and monocytes/macrophages by anti-Ly6G and anti-CSF1R antibody.
(A, B) Representative flow cytometry plots and proportions (A) and quantitative summary (B) of neutrophils in peripheral blood are shown. Anti-Ly6G antibody or rat IgG2a isotype antibody were intraperitoneally injected into C57BL/6 mice (18–20 g, n = 6) at doses of 500 µg/mouse on day 0 and day 2, respectively. At day 3, peripheral blood was collected for testing the scavenging efficiency of neutrophils by flow cytometry. (C, D) Representative flow cytometry plots and proportions (C) and quantitative summary (D) of neutrophils in peritoneal lavage are shown. Anti-CSF1R antibody or rat IgG2a isotype antibody were intraperitoneally injected into C57BL/6 mice (18–20 g, n = 6) at doses of 1 mg/mouse on day 0 followed by 0.3 mg/mouse on day 1 and day 2, respectively. At day 3, peritoneal lavage was collected for testing the scavenging efficiency of monocytes/macrophages by flow cytometry. *** p < 0.001, ns, not significant.
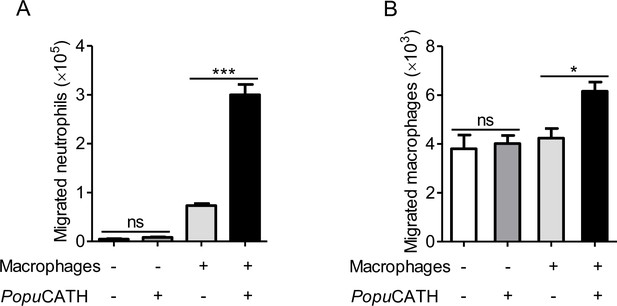
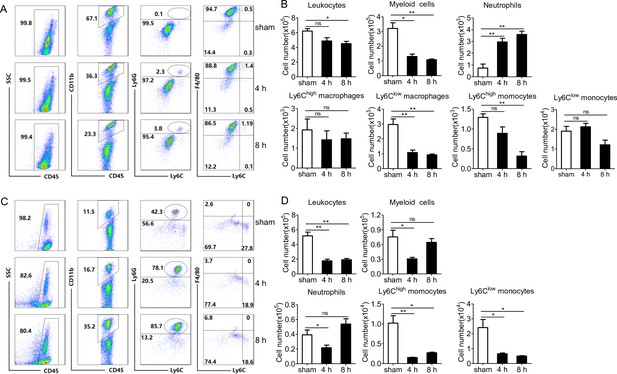
PopuCATH-induced phagocyte migration relies on its effect on macrophages.
For the direct chemotactic effect of PopuCATH to neutrophils or macrophages, 100 µL of neutrophil suspension (A) or macrophage suspension (B) (5 × 106 cells/mL) was added to the upper chamber, and 500 µL of PopuCATH (10 µM, dissolved in medium) or medium was added to the lower chamber. After neutrophils and macrophages were migrated at 37 ℃ for 8 hr, the increased cells in the lower chamber were collected and counted using a hemocytometer. For the co-cultured system, 500 µL of macrophage suspension (5 × 106 cells/mL) was seeded in the lower chamber. After macrophages were adherent to the lower chamber, 100 µL of neutrophil suspension (A) or macrophage suspension (B) (5 × 106 cells/mL) was added to the upper chamber. Then, the medium in the lower chamber was replaced with 500 µL of PopuCATH (10 µM, dissolved in medium) or fresh medium. Neutrophils and macrophages were migrated at 37 ℃ for 8 hr. The reduced cells in the upper chamber were counted using a hemocytometer. *p< 0.05, ***p < 0.001, ns, not significant.
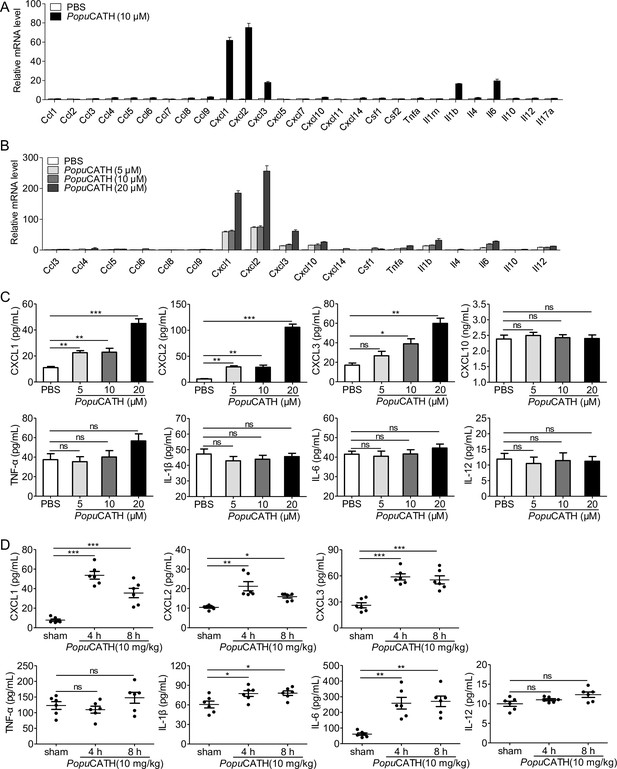
PopuCATH selectively induced the production of chemokines in macrophages and mice.
(A) The mRNA levels of chemokines/cytokines in macrophages induced by PopuCATH (10 µM). (B) Verification of the upregulated chemokines/cytokines observed in panel A by qPCR. (C) The protein levels of chemokine/cytokine production in macrophages induced by PopuCATH (10 µM). Macrophages (5 × 105 cells/well, in 2% FBS DMEM) were seeded in 24-well plates, and a single dose of PopuCATH (10 µM, dissolved in PBS) (A) or different doses of PopuCATH (5, 10, and 20 µM, dissolved in PBS) (B), or PBS was added. After incubation at 37℃ for 4 hr, cells were collected, and the mRNA levels of chemokines/cytokines were detected by qPCR analysis, the protein levels of chemokines/cytokines were quantified by ELISA (C). (D) The protein levels of chemokine/cytokines in mice induced by PopuCATH (10 mg/kg). C57BL/6 mice (18–20 g, n = 6) were intraperitoneally injected with PopuCATH (10 mg/kg) dissolved in 0.2 mL PBS. Sham mice received the same volumes of PBS. At 4 and 8 hr post injection, peritoneal lavage was collected for quantification of the protein levels of chemokines/cytokines by ELISA. *p < 0.05, **p < 0.01, ***p < 0.001, ns, not significant.
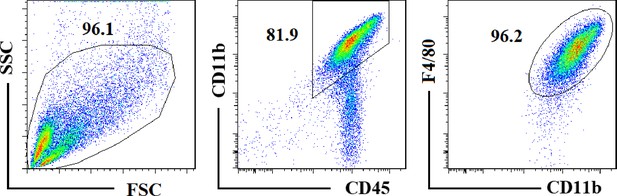
Flow cytometry analysis of mouse peritoneal macrophages.
C57BL/6 mice were intraperitoneally injected with sterile thioglycollate medium (4%, 2 mL). At 4 days post injection, the peritoneal macrophages were collected by flushing with DMEM supplemented with 10% FBS, 100 U/mL penicillin, and 100 µg/mL streptomycin. The purity of peritoneal macrophages was assayed by flow cytometry.
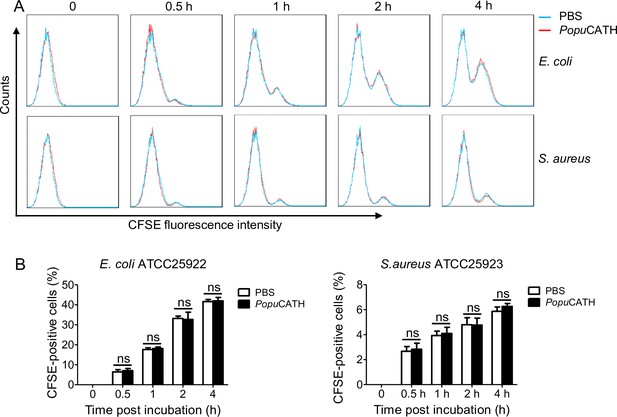
PopuCATH does not promote macrophage phagocytosis.
(A) Flow cytometry analysis of the effect of PopuCATH on macrophage phagocytosis. (B) Statistical analysis of the phagocytic uptake of the bacterial particles. Macrophages were pre-incubated with PopuCATH (10 µM) or PBS (solvent of peptide) for 1 hr, and CFSE-labelled bacterial particles were added and incubated for the indicated time points. The CFSE fluorescence were analysed by flow cytometry as a measure of the phagocytic uptake of the bacterial particles. ns, not significant.
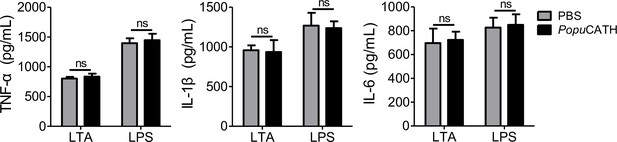
The effect of PopuCATH on LTA- or LPS-induced inflammation in macrophages.
Macrophages (5 × 105 cells/well, in 2% FBS DMEM) were seeded in 24-well plates. After the addition of LTA (1 µg/mL) or LPS (100 ng/mL), PopuCATH (10 µM), or PBS (solvent of peptide) was added and incubated at 37℃ for 6 hr. The supernatant was collected for TNF-α, IL-1β, and IL-6 quantification by ELISA. ns, not significant.
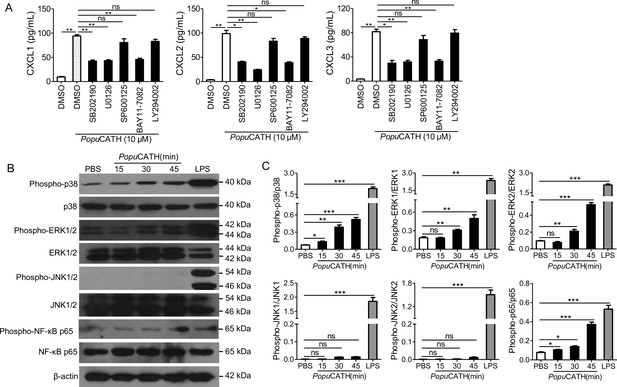
PopuCATH-induced chemokine production in macrophages were partially dependent on p38/ERK MAPKs and NF-κB signaling pathways.
(A) Effects of MAPK, PI3K, and NF-κB inhibitors on PopuCATH-induced chemokine production in macrophages. Macrophages (5 × 105 cells/well, in 2% FBS DMEM) were seeded in 24-well plates. The adherent macrophages were pre-incubated with p38 inhibitor (SB202190, 10 µM), ERK inhibitor (U0126, 10 µM), JNK inhibitor (SP600125, 10 µM), NF-κB inhibitor (BAY11-7082, 2 µM), or PI3K inhibitor (LY294002, 10 µM) for 1 hr, respectively. Then, cells were stimulated with PopuCATH (10 µM) for 4 hr. The protein levels of chemokines were quantified by ELISA. (B) Western blot analysis of the effects of PopuCATH on MAPKs and NF-κB. Macrophages (2 × 106 cells/well, in 2% FBS DMEM) were seeded in 6-well plates. PopuCATH (10 µM) was added and incubated at 37℃ for 15, 30, and 45 min, respectively. LPS (100 ng/mL, positive control) or PBS (solvent of peptide) was added and incubated for 30 min. The cells were collected for western blot analysis. (C) Ratio analysis. The ratios of phosphorylated-p38, JNK, ERK, and NF-κB p65 to total p38, JNK, ERK, and NF-κB p65 were assayed by image J, respectively. *p < 0.05, **p < 0.01, ***p < 0.001, ns, not significant.
-
Figure 9—source data 1
The original images of the unedited blots and images with the uncropped blots with the relevant bands clearly labelled.
- https://cdn.elifesciences.org/articles/72849/elife-72849-fig9-data1-v2.zip
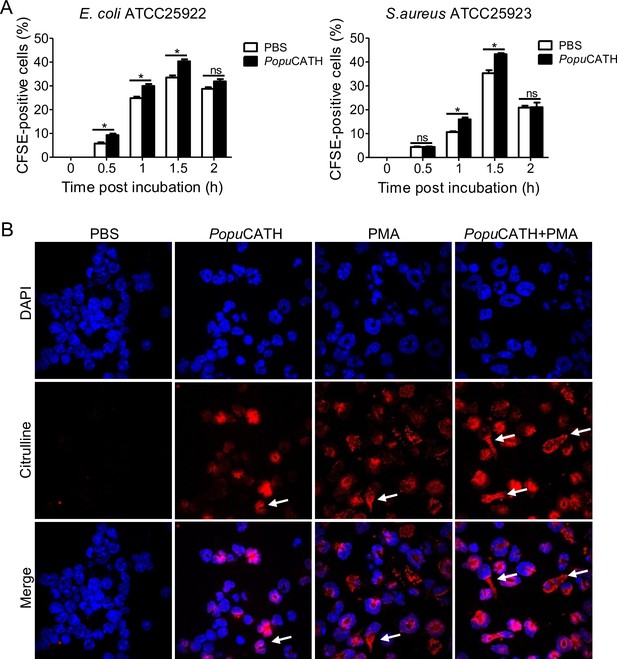
PopuCATH promoted neutrophil phagocytosis through enhancing neutrophil extracellular traps formation.
(A) Enhancement of neutrophil phagocytosis of S. aureus and E. coli by PopuCATH. Neutrophils were pre-incubated with PopuCATH (10 µM) or PBS (solvent of peptide) for 1 hr, and CFSE-labelled bacterial particles were added and incubated for indicated time points. The CFSE fluorescence were analysed by flow cytometry as a measure of the phagocytic uptake of the bacterial particles. (B) Enhancement of neutrophil extracellular formation. Neutrophils were incubated with PBS, PopuCATH (10 µM), PMA (100 nM) or PopuCATH (10 µM)+ PMA (100 nM) at 37℃ for 4 hr, respectively. Nuclei and NETs were stained with DAPI (blue) or anti-Cit-H3 (red), respectively. NETs were observed using a confocal microscope ( × 60). *p < 0.05, ns, not significant.
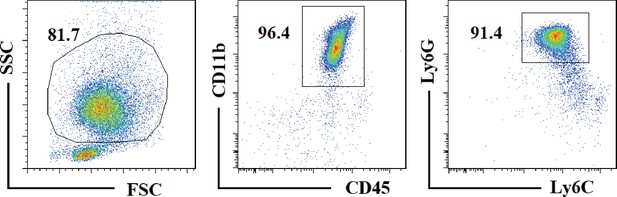
Flow cytometry analysis of mouse bone-marrow-derived neutrophils.
Bone marrow from C57BL/6 was harvested, rinsed with 5 mL PBS, filtered through a cell strainer (70 micron), and then centrifuged at 500 g for 5 min. The bone marrow-derived neutrophil pellet was re-suspended in 2 mL PBS. RPMI 1640 diluted Percoll gradient with 72%, 64%, and 54% layers was prepared, and cell suspension was over-layered onto this gradient. Percoll gradient was centrifuged at 950 g for 25 min. Neutrophils were collected from the 72%/64% interface, washed with PBS, and centrifuged at 500 g for 5 min. The purity of peritoneal macrophages was assayed by flow cytometry.
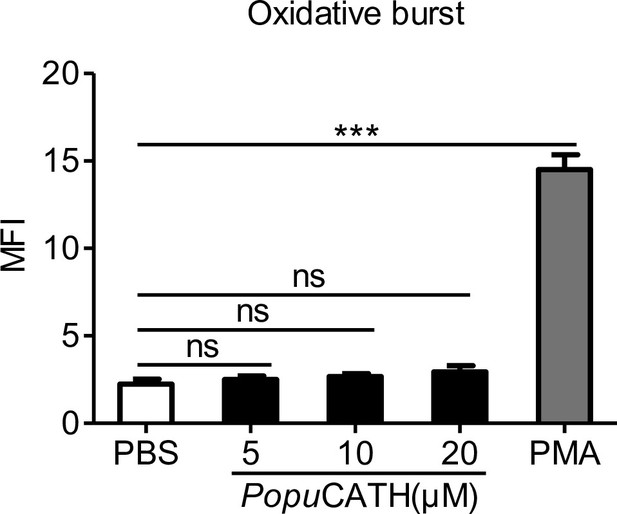
PopuCATH does not increase ROS production in neutrophils.
Neutrophils (2 × 106 cells/mL) were incubated with PopuCATH (5, 10, 20 µM), PBS (solvent of peptide) or PMA (50 nM, positive control) at 37℃ for 20 min. Then, cells were stained with 5 μM H2DCFDA (Sigma-Aldrich) for 30 min at 37℃ and washed with PBS. Fluorescence was measured by flow cytometry, and ROS levels in neutrophils was expressed as fold change of mean fluorescence intensity (MFI) normalised to the PBS treatment. ***p < 0.001, ns, not significant.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Cell line (Homo sapiens) | THP-1 | National Collection of Authenticated Cell Cultures (https://www.cellbank.org.cn/) | CSTR:19375.09.3101HUMSCSP567 | |
| Cell line (Rattus norvegicus) | RBL-2H3 | National Collection of Authenticated Cell Cultures (https://www.cellbank.org.cn/) | CSTR:19375.09.3101RATTCR7 | |
| Cell line (Mus musculus) | Macrophage | Peritoneal macrophages from C57BL/6 mice | A primary cell line identified by flow cytometry | |
| Cell line (Mus musculus) | Neutrophil | Bone marrow-derived neutrophils from C57BL/6 mice | A primary cell line identified by flow cytometry | |
| Commercial assay or kit | SMART cDNA Library Construction Kit | Clontech | Cat#: 634,901 | |
| Commercial assay or kit | Cell Counting Kit-8 | Dojindo | Cat#: CK04-500T | |
| Commercial assay or kit | Mouse C3a ELISA Kit | Wuhan Fine Biotech Co., Ltd | Cat#: EM0882 | |
| Commercial assay or kit | Alanine aminotransferase Assay Kit | Nanjing Jiancheng Bioengineering Institute | Cat#: C009-2-1 | |
| Commercial assay or kit | Creatinine Assay Kit | Nanjing Jiancheng Bioengineering Institute | Cat#: C011-2-1 | |
| Commercial assay or kit | Wright-Giemsa stain solution | Solarbio Life Sciences | Cat#: G1020 | |
| Commercial assay or kit | Trizol reagent | Life Technologies | Cat#: 15596018 | |
| Commercial assay or kit | PrimeScript RT reagent kit | Takara | Cat#: RR037A | |
| Commercial assay or kit | Mouse TNF-α ELISA Kit | eBioscience | Cat#:88-7324-88, RRID:AB_2575080 | |
| Commercial assay or kit | Mouse IL-1β ELISA Kit | eBioscience | Cat#:88-7013-88, RRID:AB_2574946 | |
| Commercial assay or kit | Mouse IL-6 ELISA Kit | eBioscience | Cat#: 88-7064-88, RRID:AB_2574990 | |
| Commercial assay or kit | Mouse IL-12 ELISA Kit | MultiSciences Biotech Co., Ltd. | Cat#:70-EK212/3-96 | |
| Commercial assay or kit | Mouse CXCL1 ELISA Kit | MultiSciences Biotech Co., Ltd. | Cat#:70-EK296/2-96 | |
| Commercial assay or kit | Mouse CXCL2 ELISA Kit | MultiSciences Biotech Co., Ltd. | Cat#:70-EK2142/2-96 | |
| Commercial assay or kit | Mouse CXCL3 ELISA Kit | Rockland | Cat#:KOA0825 | |
| Commercial assay or kit | mouse CXCL10 ELISA Kit | MultiSciences Biotech Co., Ltd. | Cat#:70-EK268/2-96 | |
| Antibody | Mouse monoclonal anti-FcγR blocking mAb | BD Biosciences | Clone: 2.4G2, Cat#: 553141, RRID:AB_394656 | FC (1: 100) |
| Antibody | Mouse monoclonal APC/Cy7 conjugated anti-CD45 | BioLegend | Clone: 30-F11, Cat#: 103116, RRID:AB_312981 | FC (1: 100) |
| Antibody | Mouse monoclonal PE conjugated anti-CD11b | BioLegend | Clone: M1/70, Cat#: 101207, RRID:AB_312790 | FC (1: 100) |
| Antibody | Mouse monoclonal PE/Cy7 conjugated anti-Ly6G | BioLegend | Clone: 1A8, Cat#: 127618, RRID:AB_1877261 | FC (1: 100) |
| Antibody | Mouse monoclonal FITC conjugated anti-Ly6C | BD Biosciences | Clone: AL-21, Cat#: 553104, RRID:AB_394628 | FC (1: 100) |
| Antibody | Mouse monoclonal APC conjugated anti-F4/80 | BioLegend | Clone: BM8, Cat#: 123116, RRID:AB_893481 | FC (1: 100) |
| Antibody | Mouse monoclonal APC conjugated anti-CD45 | BioLegend | Clone: 30-F11, Cat#: 103112, RRID:AB_312977 | FC (1: 100) |
| Antibody | Mouse monoclonal FITC conjugated anti-CD3 | BD Biosciences | Clone: 17A2, Cat#: 555274, RRID:AB_395698 | FC (1: 100) |
| Antibody | Mouse monoclonal APC conjugated anti-CD4 | BD Biosciences | Clone: H129.19, Cat#: 553650, RRID:AB_394970 | FC (1: 100) |
| Antibody | Mouse monoclonal PE/Cy7 conjugated anti-CD8 | BioLegend | Clone: 53–6.7, Cat#: 100721, RRID:AB_312760 | FC (1: 100) |
| Antibody | Mouse monoclonal PE conjugated anti-B220 | BD Biosciences | Clone: RA3-6B2, Cat#: 553090, RRID:AB_394620 | FC (1: 100) |
| Antibody | Mouse monoclonal anti-Ly6G antibody | BioXcell | Clone: 1A8, Cat#: BP0075-1, RRID:AB_1107721 | In vivo depletion of neutrophils |
| Antibody | Mouse monoclonal anti-CSF1R | BioXcell | Clone: AFS98, Cat#: BE0213, RRID:AB_2687699 | In vivo depletion of monocytes/macrophages |
| Antibody | Rat monoclonal anti-IgG2a | BioXcell | Clone: 2A3, Cat#: BE0089, RRID:AB_1107769 | Isotype control for anti-mouse Ly6G and anti-mouse CSF1R |
| Antibody | Rabbit monoclonal anti-p38 MAPK | Cell Signaling Technology | Cat#: 9,212 S, RRID: AB_330713 | WB (1: 1000) |
| Antibody | Rabbit monoclonal anti-phospho-p38 MAPK | Cell Signaling Technology | Cat#: 9,211 S, RRID:AB_331641 | WB (1: 1000) |
| Antibody | Rabbit monoclonal anti-ERK MAPK | Cell Signaling Technology | Cat#: 9,102 S, RRID:AB_330744 | WB (1: 1000) |
| Antibody | Mouse monoclonal anti-phospho-ERK MAPK | Cell Signaling Technology | Cat#: 9,106 S, RRID:AB_331768 | WB (1: 1000) |
| Antibody | Rabbit monoclonal anti-JNK MAPK Antibody | Cell Signaling Technology | Cat#: 9,252 S, RRID:AB_2250373 | WB (1: 1000) |
| Antibody | Mouse monoclonal anti-phospho-JNK MAPK | Cell Signaling Technology | Cat#: 9,255 S, RRID:AB_2307321 | WB (1: 1000) |
| Antibody | Rabbit monoclonal anti-NF-κB p65 | Cell Signaling Technology | Cat#: 8,242 S, RRID:AB_10859369 | WB (1: 1000) |
| Antibody | Rabbit monoclonal anti-phospho-NF-κB p65 | Cell Signaling Technology | Cat#: 3,033 S, RRID:AB_331284 | |
| Chemical compound, drug | Thioglycollate medium | Sigma-Aldrich | Cat#: B2551 | |
| Chemical compound, drug | EGTA | Sigma-Aldrich | Cat#: 324,626 | |
| Chemical compound, drug | Zymosan | Sigma-Aldrich | Cat#: Z4250 | |
| Chemical compound, drug | Mueller-Hinton broth | Qingdao Rishui Biotechnologies Co., Ltd | Cat#: 11,816 | |
| Chemical compound, drug | Nutrient Broth | Qingdao Rishui Biotechnologies Co., Ltd | Cat#: 10,204 | |
| Chemical compound, drug | WST-8 | Cayman | Cat#: 18,721 | |
| Chemical compound, drug | Ketamine hydrochloride | R&D Systems | Cat#: 3131/50 | |
| Chemical compound, drug | LPS | Sigma-Aldrich | Cat#: L2630 | |
| Chemical compound, drug | SB202190 | Cell Signaling Technology | Cat#: 8,158 S | |
| Chemical compound, drug | U0126 | Cell Signaling Technology | Cat#: 9,903 S | |
| Chemical compound, drug | SP600125 | Cell Signaling Technology | Cat#: 8,177 S | |
| Chemical compound, drug | BAY11-7082 | Cell Signaling Technology | Cat#: 78,679 S | |
| Chemical compound, drug | LY294002 | Cell Signaling Technology | Cat#: 9,901 S |
Additional files
-
Supplementary file 1
Physico-chemical parameters of PopuCATH.
- https://cdn.elifesciences.org/articles/72849/elife-72849-supp1-v2.docx
-
Supplementary file 2
Secondary structural components of PopuCATH in aqueous solution and membrane-mimetic solution.
- https://cdn.elifesciences.org/articles/72849/elife-72849-supp2-v2.docx
-
Supplementary file 3
MIC values of PopuCATH against Gram-negative bacteria, Gram-positive bacteria, fungi, and aquatic bacteria.
- https://cdn.elifesciences.org/articles/72849/elife-72849-supp3-v2.docx
-
Supplementary file 4
THP-1 cell line authentication report by STR profiling.
- https://cdn.elifesciences.org/articles/72849/elife-72849-supp4-v2.pdf
-
Supplementary file 5
Primers for qPCR.
- https://cdn.elifesciences.org/articles/72849/elife-72849-supp5-v2.docx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/72849/elife-72849-transrepform1-v2.docx





