Superior colliculus drives stimulus-evoked directionally biased saccades and attempted head movements in head-fixed mice
Figures
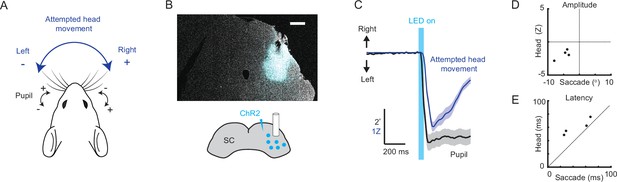
Optogenetic stimulation of the superior colliculus evokes coincident and directionally biased attempted head and eye movements.
(A) Behavioral schematic. Naive mice are head-fixed, both eyes are tracked using cameras, and attempted head rotations are measured using a strain gauge (load cell). In subsequent quantification, eye positions to the right of center (nasal for left eye, temporal for right eye) are positive, and eye positions to the left of center (temporal for left eye, nasal for right eye) are negative, with zero defined as the mean eye position. Likewise, attempted rightward head movements are positive, and leftward head movements are negative. (B) Schematic of right SC optogenetic stimulation using ChR2 and example histology for representative mouse. Scale bar, 0.5 mm. (C) Mean attempted head (blue) and eye (black) movement traces (n = 44 trials, 4 mice) in the 1 s period surrounding optogenetic stimulation. Optogenetic illumination (1 mW) was delivered for 40 ms. (D) Relationship between saccade amplitude and attempted head movement amplitude for individual mice. (E) Relationship between saccade latency and head movement latency for individual mice.
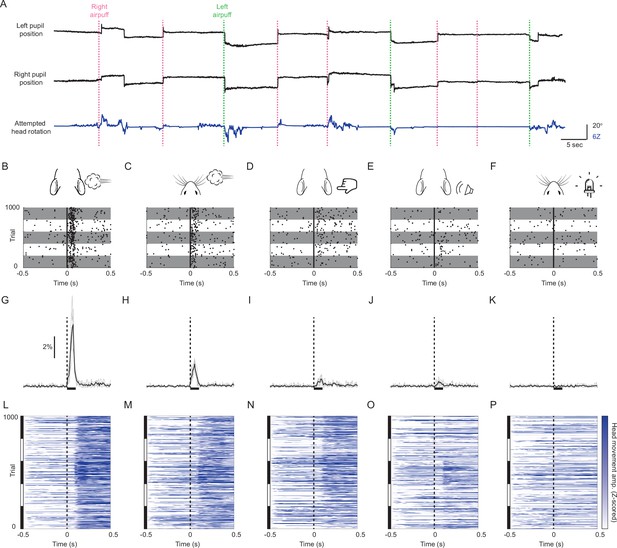
Mice innately make sound- and touch-evoked gaze shifts.
(A) Sample eye and attempted head movement traces. Dashed vertical lines indicate right (green) and left (magenta) ear airpuff delivery. (B–F) Saccade rasters for five representative mice in response to (B) ear airpuffs, (C) whisker airpuffs, (D) ear tactile stimuli, (E) auditory airpuffs, and (F) visual stimuli. Each row corresponds to a trial. Each dot indicates onset of a saccade. Vertical black lines denote time of left or right stimulus delivery. Each gray or white horizontal stripe contains data for a different mouse. n = 1000 randomly selected trials (200 /mouse). (G–K) Peri-stimulus time histograms showing instantaneous saccade probabilities in response to (G) ear airpuffs, (H) whisker airpuffs, (I) ear tactile stimuli, (J) auditory airpuffs, and (K) visual stimuli for mice from (B–F). Each light trace denotes a single animal; black traces denote population mean. Dashed lines denote time of stimulus delivery. Horizontal bar indicates the 100ms response window used in subsequent analyses. (L–P) Heatmaps of attempted head movements in response to (L) ear airpuffs, (M) whisker airpuffs, (N) ear tactile stimuli, (O) auditory airpuffs, and (P) visual stimuli for mice from (B–K). Each row corresponds to an individual trial from B-F. Black and white bars at left indicate blocks of trials corresponding to each of five different mice. Dashed line denotes stimulus delivery time.
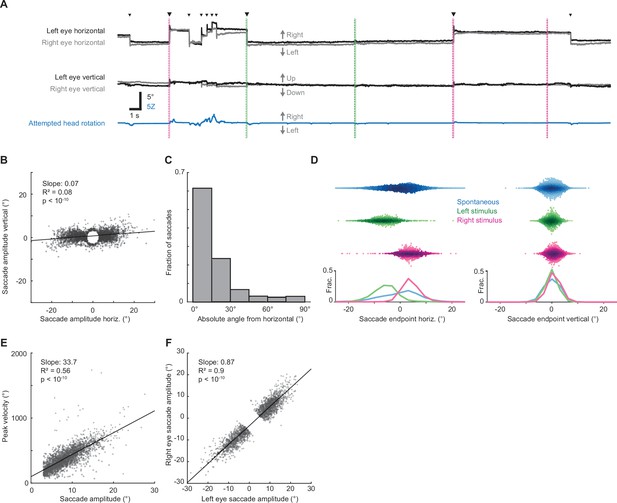
Airpuffs evoke horizontal saccades.
(A) Sample traces showing pupil azimuth (top) and elevation (middle) and attempted head rotation (bottom). Black corresponds to left eye, gray corresponds to right eye. Small arrowheads indicate spontaneous saccades. Large arrowheads indicate stimulus-evoked directionally biased saccades. Green and magenta dashed vertical lines correspond to left and right ear airpuffs, respectively. (B) Ear airpuff-evoked saccade endpoints and linear fit. n = 5 mice, 2337 trials. (C) Distribution of angles between airpuff-evoked saccade vectors and horizontal axis. Gray bars indicate population means. n = 5 mice, 2337 trials (D) Distributions of saccade endpoints in horizontal and vertical axes. n = 5 mice; trials = 16,291 (spontaneous), 1067 (left airpuff), 1270 (right airpuff). (E) Relationship between saccade amplitude and peak velocity. (F) Relationship between right and left eye saccade amplitudes. n = 4 mice, 1861 trials.
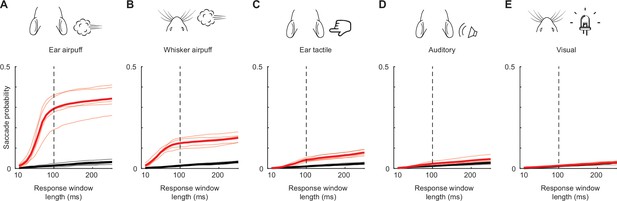
Evoked saccades occur within a narrow window after stimulus delivery.
(A–E) Cumulative probabilities of detecting evoked (red) and spontaneous (black) saccades as a function of response window length for ear airpuffs (A), whisker airpuffs (B), ear tactile stimuli (C), auditory airpuffs (D), and visual stimuli (E). Thin lines denote values for individual mice, thick lines denote population mean. Dashed vertical line indicates end of window used for analyses of evoked saccades.
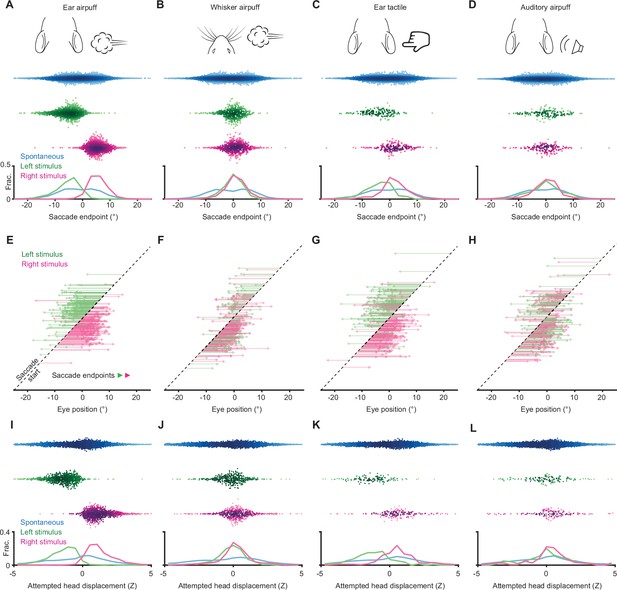
Sensory-evoked eye and attempted head movements.
(A–D) Endpoints for ear airpuff-, whisker airpuff-, ear tactile-, and auditory airpuff-evoked saccades. Top, schematics of stimuli. Middle, scatter plots showing endpoints of all saccades for all animals (n = see below, 5 animals) made spontaneously (blue) and in response to left (green) and right (magenta) stimuli. Darker shading indicates areas of higher density. Bottom, histograms of endpoint distributions for spontaneous and evoked saccades. (E–H) Trajectories of individual stimulus-evoked saccades. Each arrow denotes the trajectory of a single saccade. Saccades are sorted according to initial eye positions, which fall on the dashed diagonal line. Saccade endpoints are indicated by arrowheads. Because the probability of evoked gaze shifts differed across stimuli, data for ear and whisker airpuffs are randomly subsampled (15% and 30% of total trials, respectively) to show roughly equal numbers of trials for each condition. (I–L) Ear airpuff-, whisker airpuff-, ear tactile-, and auditory airpuff-evoked attempted head displacements associated with saccades in A-D. Top, scatter plots showing displacements of attempted head movements associated with saccades made spontaneously (blue) and in response to left (green) and right (magenta) stimuli (n = see below, 5 animals). Darker shading indicates areas of higher density. Bottom, histograms of attempted displacement distributions for spontaneous and evoked attempted head movements. Saccade numbers in A-L: ear airpuff sessions, spontaneous = 7146, left ear airpuff-evoked = 942 (141 in E), right ear airpuff-evoked = 1213 (182 in E); whisker airpuff sessions: spontaneous = 7790, left whisker airpuff-evoked = 440 (132 in F), right whisker airpuff-evoked = 606 (181 in F); ear tactile sessions, spontaneous = 6706, left ear tactile-evoked = 133, right ear tactile-evoked = 186; auditory sessions, spontaneous = 10240, left auditory-evoked = 140, right auditory-evoked = 158.
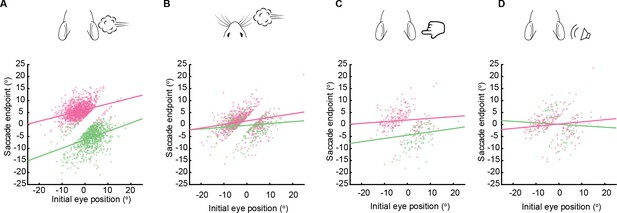
Saccade endpoints as a function of initial eye position.
(A–D) Relationship between saccade endpoint and initial eye position for left ear airpuffs (A, slope = 0.350, R2 = 0.137, p = 10–32), right ear airpuffs (A, slope = 0.241, R2 = 0.093, p = 10–28), left whisker airpuffs (B, slope = 0.076, R2 = 0.017, p = 0.004), right whisker airpuffs (B, slope = 0.149, R2 = 0.048, p = 10–8), left whisker tactile (C, slope = 0.134, R2 = 0.015, p = 0.084), right whisker tactile (C, slope = 0.070, R2 = 0.002, p = 0.236), left auditory airpuffs (D, slope = –0.062, R2 = 0.001, p = 0.356), and right auditory airpuffs (D, slope = 0.070, R2 = 0.009, p = 0.122). n = same as Figure 3.
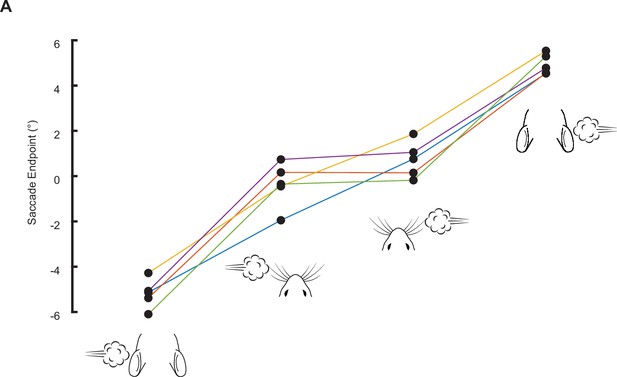
Endpoints of airpuff-evoked saccades are ordered according to site of stimulation.
Each line corresponds to a single mouse and shows mean endpoints of saccades evoked by (in order, from left to right) left ear airpuffs, left whisker airpuffs, right whisker airpuffs, and right ear airpuffs.
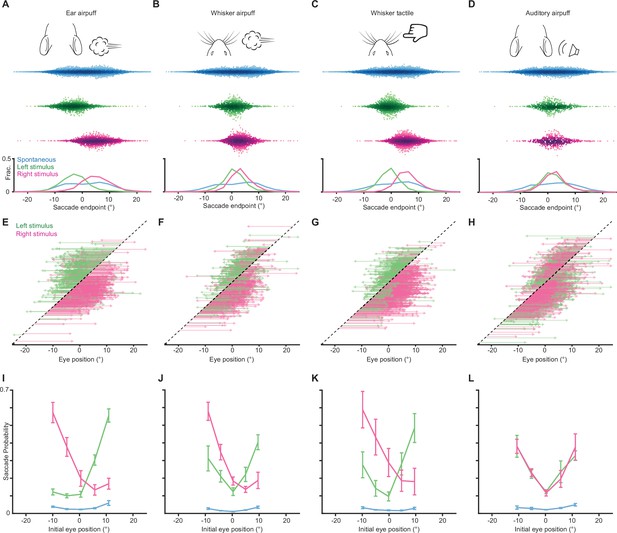
Endpoints and trajectories of sensory-evoked saccades for an additional cohort of mice.
(A–D) Endpoints for ear airpuff-, whisker airpuff-, ear tactile-, and auditory airpuff-evoked saccades. Top, schematics of stimuli. Middle, scatter plots showing endpoints of all saccades for all animals made spontaneously (blue) and in response to left (green) and right (magenta) stimuli. Darker shading indicates areas of higher density. Bottom, endpoint distributions for spontaneous and evoked saccades. (E–H) Trajectories of individual stimulus-evoked saccades. Each arrow denotes the trajectory of a single saccade. Saccades are sorted according to initial eye positions, which fall on the dashed diagonal line. Saccade endpoints are indicated by arrowheads. Because the probability of evoked gaze shifts differed across stimuli, data are randomly subsampled to show roughly equal numbers of trials for each condition. (I–L) Relationship between eye position and saccade probability. Green and magenta lines indicate population means for saccades evoked by left and right stimuli, respectively. Blue lines indicate spontaneous saccades. Error bars indicate s.e.m Saccade numbers for A-L: ear airpuff sessions, spontaneous = 14304, left ear airpuff-evoked = 1221 (244 in E), right ear airpuff-evoked = 1755 (351 in E); whisker airpuff sessions, spontaneous = 8971, left whisker airpuff-evoked = 1107 (221 in F), right whisker airpuff-evoked = 1482 (296 in F); whisker tactile sessions, spontaneous = 13242, left whisker-evoked = 1473 (294 in G), right whisker-evoked = 2408 (481 in G); auditory sessions, spontaneous = 8774, left auditory-evoked = 833 (333 in H), right auditory-evoked = 757 (302 in H).
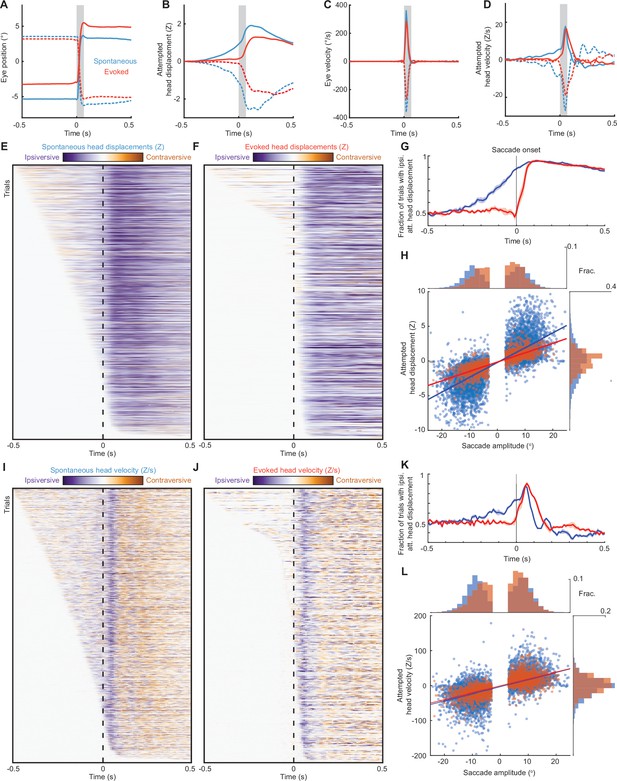
Head-eye coupling during spontaneous and touch-evoked gaze shifts.
(A) Mean trajectories of all rightward (solid traces) and leftward (dashed traces) saccades during spontaneous (blue, n = 7146) and ear airpuff-evoked (red, n = 1437) gaze shifts. Means + s.e.m. (smaller than line width). Gray bar indicates average saccade duration. (B) Mean attempted head displacement accompanying rightward (solid traces) and leftward (dashed traces) saccades during spontaneous (blue) and ear airpuff-evoked (red) gaze shifts. (C) Mean velocities of all rightward (solid traces) and leftward (dashed traces) saccades during spontaneous (blue) and ear airpuff-evoked (red) gaze shifts. (D) Mean attempted head movement velocities accompanying rightward (solid traces) and leftward (dashed traces) saccades during spontaneous (blue) and ear airpuff-evoked (red) gaze shifts. (E, F) Timing of attempted head movements relative to saccades during all spontaneous (E) and ear airpuff-evoked (F) gaze shifts. Each row corresponds to a single gaze shift. Darker shades indicate larger attempted head displacement. Purple hues denote attempted displacement in the same direction as the saccade (ipsiversive), and orange hues denote displacement in the opposite direction of the saccade (contraversive). Dashed vertical line indicates time of saccade onset. Trials are sorted by latency of attempted head movements. (G) Fraction of trials with ipsiversive attempted head displacements at different timepoints relative to saccade onset for spontaneous (blue) and evoked (red) saccades. (H). Head-eye amplitude coupling of spontaneous (blue) and ear airpuff-evoked saccades (red). Each dot corresponds to a single gaze shift. Attempted head amplitude was measured 150ms after saccade onset. Spontaneous: R2 = 0.58, slope = 0.214, p < 10–10. Evoked: R2 = 0.64, slope = 0.162, p < 10–10. Spontaneous and evoked regression slopes were significantly different (p < 10–5, permutation test). Histograms above and beside scatter plot indicate distributions of saccade and attempted head movement amplitudes. Difference in means significant (p < 10–5 for saccades, p < 10–5 for head, permutation test). (I–J) As in (E–F), but for attempted head movement velocity. (K) As in (G), but for attempted head movement velocity. (L) As in (H), but for attempted head movement velocity. Peak attempted head velocity was measured 60ms after saccade onset. Spontaneous: R2 = 0.40, slope = 2.04, p < 10–10. Evoked: R2 = 0.52, slope = 1.98, p < 10–10. Spontaneous and evoked regression slopes were not significantly different (p = 0.08, permutation test). Histograms above and beside scatter plot indicate distributions of saccade amplitudes and peak attempted head velocities. Difference in means was significant (p < 10–5 for saccades, p < 10–5 for head, permutation test).
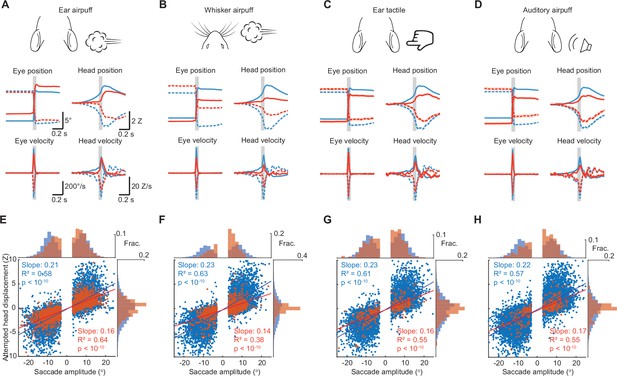
Head-eye coupling for different stimuli.
(A) Top, stimulus schematics. Middle left, mean trajectories of rightward (solid traces) and leftward (dashed traces) saccades during spontaneous (blue, n = 7146) and ear airpuff-evoked (red, n = 2151) gaze shifts. Middle right, mean attempted head displacement accompanying rightward (solid traces) and leftward (dashed traces) saccades during spontaneous (blue) and ear airpuff-evoked (red) gaze shifts. Bottom left, mean velocities of all rightward (solid traces) and leftward (dashed traces) saccades during spontaneous (blue) and ear airpuff-evoked (red) gaze shifts. Bottom right, mean attempted head movement velocities accompanying rightward (solid traces) and leftward (dashed traces) saccades during spontaneous (blue) and ear airpuff-evoked (red) gaze shifts. (B–D) As in (A) for whisker airpuffs (B), ear tactile (C) and auditory airpuffs (D). n = 5 mice. Trial numbers: whisker airpuff sessions (spontaneous = 7790, evoked = 1057), ear tactile sessions (spontaneous = 6706, evoked = 322), auditory airpuff sessions (spontaneous = 10240, evoked = 301). (E–H). Head-eye amplitude coupling of spontaneous (blue) and evoked saccades (red). Each dot corresponds to a single gaze shift. Regression statistics in figure. For every stimulus type, spontaneous and evoked regression slopes were significantly different (p < 10–5, permutation test). Histograms above and beside scatter plot indicate distributions of saccade and attempted head movement amplitudes, respectively. For each condition, distributions were significantly different between spontaneous and evoked (p < 10–5, permutation test).
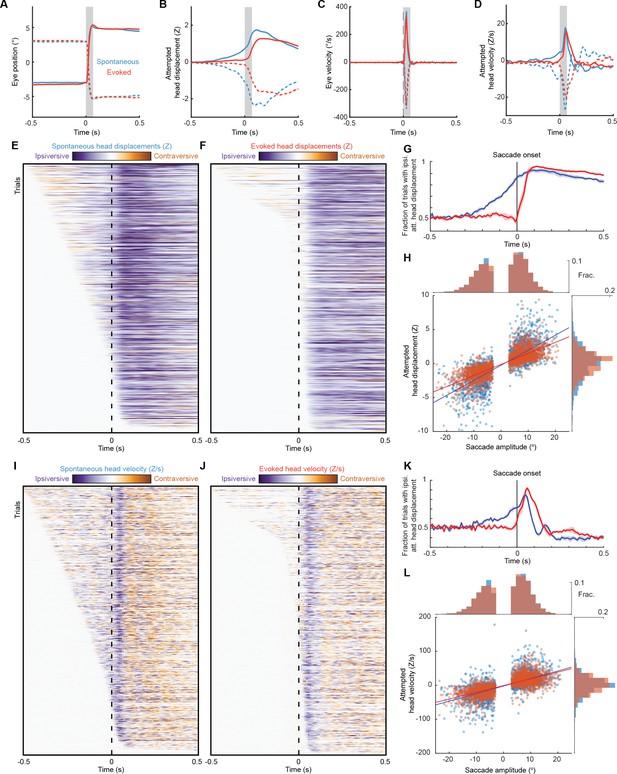
Different head-eye coupling during spontaneous and touch-evoked gaze shifts is not due to differences in saccade start and end points.
As in Figure 4, but with spontaneous and touch-evoked trials matched for initial eye position and saccade amplitude. (A) Mean trajectories of rightward (solid traces) and leftward (dashed traces) saccades during spontaneous (blue, n = 2149, 5 mice) and ear airpuff-evoked (red, n = 2149) gaze shifts. (B) Mean attempted head movement amplitudes accompanying rightward (solid traces) and leftward (dashed traces) saccades during spontaneous (blue) and ear airpuff-evoked (red) gaze shifts. (C) Mean velocities of all rightward (solid traces) and leftward (dashed traces) saccades during spontaneous (blue) and ear airpuff-evoked (red) gaze shifts. (D) Mean head movement velocities accompanying rightward (solid traces) and leftward (dashed traces) saccades during spontaneous (blue) and ear airpuff-evoked (red) gaze shifts. (E, F) Timing of attempted head movements relative to saccades during all spontaneous (E) and ear airpuff-evoked (F) gaze shifts. Each row corresponds to a single gaze shift. Darker shades indicate larger attempted head displacement. Purple hues denote attempted displacement in the same direction as the saccade (ipsiversive), and orange hues denote displacement in the opposite direction of the saccade (contraversive). Dashed vertical line indicates time of saccade onset. Trials are sorted by latency of attempted head movements. (G) Fraction of trials with ipsiversive attempted head displacements at different time points relative to saccade onset for spontaneous (blue) and evoked (red) saccades. (H). Head-eye amplitude coupling of spontaneous (blue) and ear airpuff-evoked saccades (red). Each dot corresponds to a single gaze shift. Attempted head amplitude was measured 150ms after saccade onset. Spontaneous: R2 = 0.60, slope = 0.221, p < 10–10. Evoked: R2 = 0.64, slope = 0.162, p < 10–10. Spontaneous and evoked regression slopes were significantly different (p < 10–5, permutation test). Histograms above and beside scatter plot indicate distributions of saccade and attempted head movement amplitudes. Difference in means were not significant (p = 0.46 for saccades, p = 0.19 for head, permutation test). (I–J) As in (E–F), but for attempted head movement velocity. (K) As in (G), but for attempted head movement velocity. (L) As in (H), but for attempted head movement velocity. Peak attempted head velocity was measured 60ms after saccade onset. Spontaneous: R2 = 0.42, slope = 2.22, p < 10–10. Evoked: R2 = 0.52, slope = 1.98, p < 10–10. Spontaneous and evoked regression slopes were significantly different (p < 10–5, permutation test). Histograms above and beside scatter plot indicate distributions of saccade amplitudes and peak attempted head velocities. Difference in means were not significant (p = 0.46 for saccades, p = 0.09 for head, permutation test).
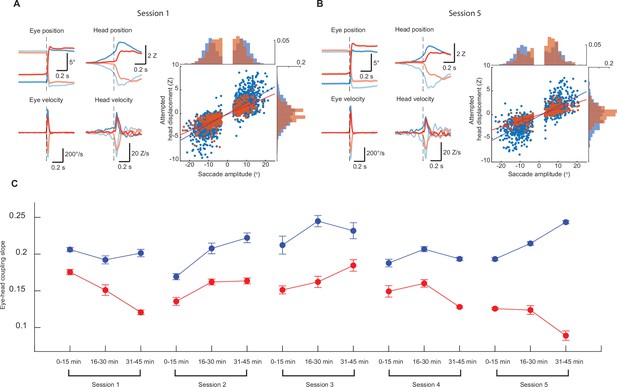
Head-eye coupling across and within sessions.
(A) Top left, mean trajectories of rightward (solid traces) and leftward (dashed traces) saccades during spontaneous (blue, n = 1631) and ear airpuff-evoked (red, n = 511) gaze shifts for first session. Top middle, mean attempted head displacements accompanying rightward (solid traces) and leftward (dashed traces) saccades during spontaneous (blue) and ear airpuff-evoked (red) gaze shifts. Bottom left, mean velocities of all rightward (solid traces) and leftward (dashed traces) saccades during spontaneous (blue) and ear airpuff-evoked (red) gaze shifts for first session. Bottom middle, mean head movement velocities accompanying rightward (solid traces) and leftward (dashed traces) saccades during spontaneous (blue) and ear airpuff-evoked (red) gaze shifts for first session. Right, head-eye amplitude coupling of spontaneous (blue) and ear airpuff-evoked saccades (red) for first session. Each dot corresponds to a single gaze shift. Attempted head movement amplitude was measured 150ms after saccade onset. Spontaneous: R2 = 0.67, slope = 0.227, p < 10–10. Evoked: R2 = 0.69, slope = 0.174, p < 10–10. Spontaneous and evoked regression slopes were significantly different (p < 10–5, permutation test). Histograms above and beside scatter plot indicate distributions of saccade and attempted head movement amplitudes. Differences in means are significant (p = 0.012 for saccades, p = 0.002 for head, permutation test). (B) Left and middle, as in (A) for spontaneous (blue, n = 884) and ear airpuff-evoked (red, n = 273) trials from fifth session. Right, head-eye amplitude coupling. Spontaneous: R2 = 0.63, slope = 0.207, p < 10–10. Evoked: R2 = 0.66, slope = 0.129, p < 10–10. Spontaneous and evoked regression slopes were significantly different (p < 10–5, permutation test). (C) Eye-head coupling slopes for sessions 1–5 subdivided into three 15-min epochs.
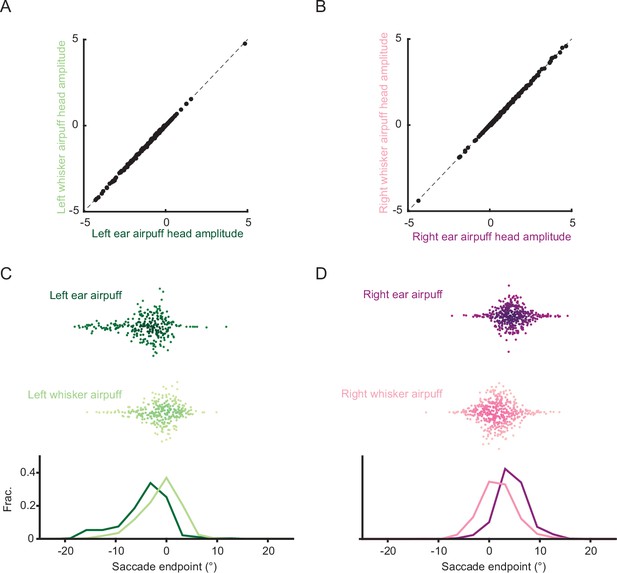
Ear and whisker airpuff trials matched for attempted head movement amplitude have different saccade endpoints.
(A–B) Amplitudes of attempted head movements for matched trials. Left ear airpuff trials were matched with left whisker airpuff trials (A, n = 281 trials per stimulus condition), and right ear airpuff trials were matched with right whisker airpuff trials (B, n = 424 trials per stimulus condition). Each point denotes the attempted head movement amplitudes of a direction- and amplitude-matched pair of trials from the two datasets. Dashed line denotes unity line. (C–D) Distributions of saccade endpoints for matched trials shown in A and B.
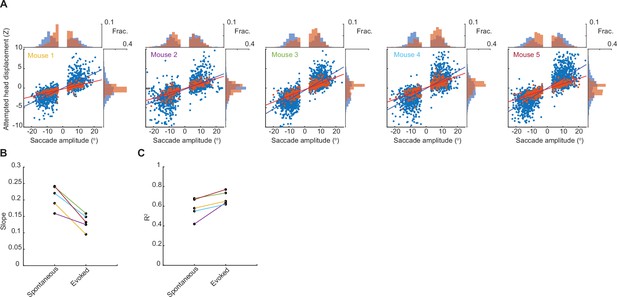
Gain of head-eye coupling variability across mice.
(A) Head-eye amplitude coupling of spontaneous (blue) and ear airpuff-evoked saccades (red) for five individual mice. Each dot corresponds to a single gaze shift. The spontaneous and evoked regression slopes were significantly different for all mice (p < 10–5, permutation test). Histograms above and beside scatter plot indicate distributions of saccade and attempted head movement amplitudes.(B, C) Slope and R2 for linear fits to spontaneous and evoked gaze shifts for each mouse. Colored lines indicate values for the different mice in A.
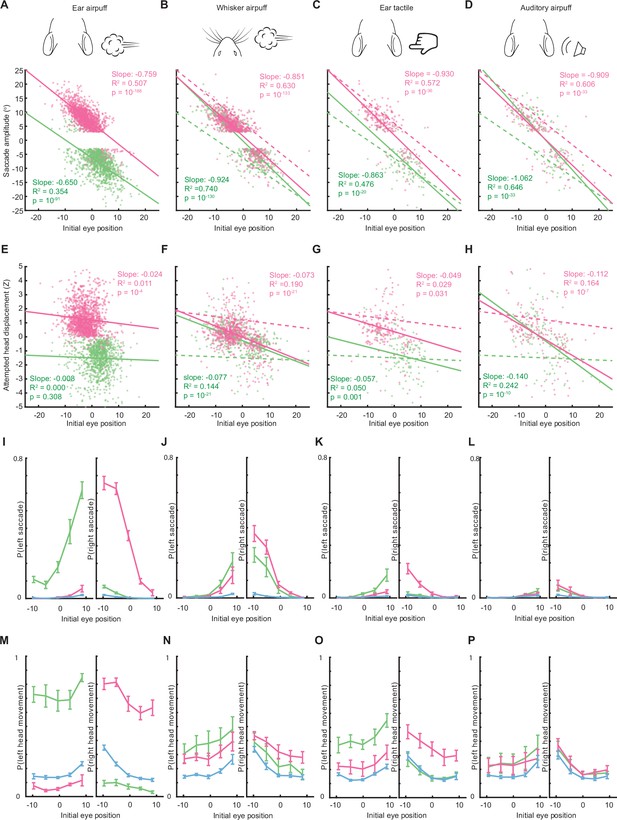
Saccade and head movement direction, amplitude, and probability depend on initial eye position.
(A–D) Relationship between saccade amplitude and eye position for ear airpuffs (A), whisker airpuffs (B), ear tactile (C), and auditory airpuffs (D). Dotted lines in B-D are lines of best fit from A for comparison. (E–H) Relationship between attempted head displacement and eye position for ear airpuffs (E), whisker airpuffs (F), ear tactile (G), and auditory airpuffs (H). Dotted lines in F-H are lines of best fit from E. The trials in A-H are the same as those in Figure 2. (I–L) Relationship between initial eye position and saccade probability for left and right saccades. (M–P) Relationship between initial eye position and attempted head movement probability for left and right attempted head movements. Green and magenta lines in I-P indicate population means for movements evoked by left and right stimuli, respectively. Blue lines indicate spontaneous saccades or head movements. Error bars indicate s.e.m. Total trial numbers for I-P: ear airpuff sessions, spontaneous = 13,384, left ear airpuff = 3506, right ear airpuff = 3497; whisker airpuff sessions, spontaneous = 14,511, left whisker airpuff = 3926, right whisker airpuff = 4026; tactile ear sessions, spontaneous = 13,529, left tactile ear = 3646, right tactile ear; = 3695; auditory airpuff, spontaneous = 13,404, left auditory airpuff = 6362, right auditory airpuff = 6385.
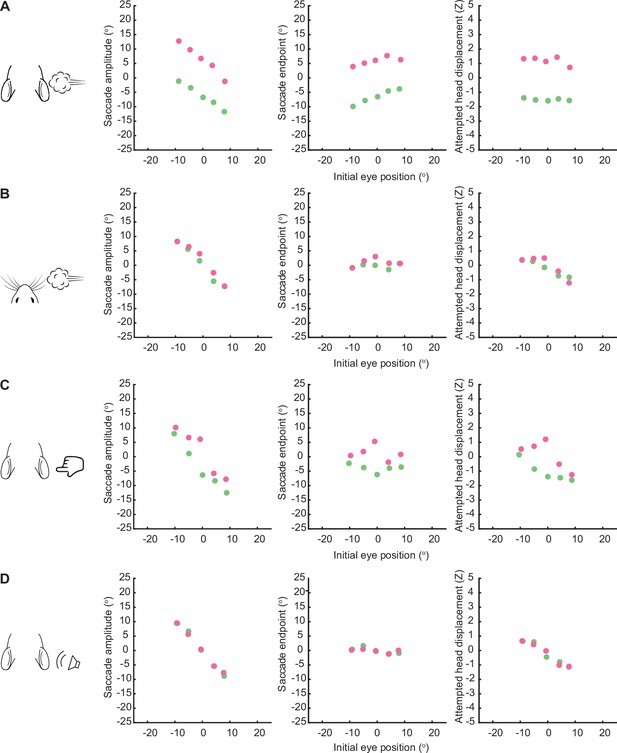
Saccade amplitude, saccade endpoint, and attempted head displacement binned by initial eye position.
(A) Left, mean saccade amplitudes binned by initial eye position for ear airpuff. Middle, mean saccade endpoints binned by initial eye position. Right, mean attempted head displacement binned by initial eye position. (B) As in (A) for whisker airpuff. (C) As in (A) for ear tactile. (D) As in (A) for auditory airpuff.
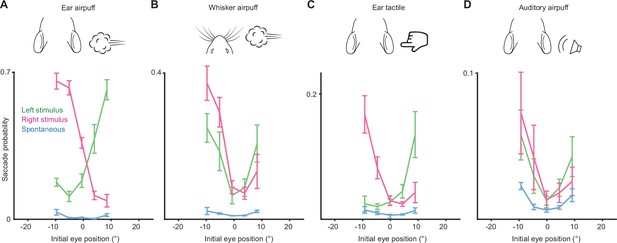
Relationship between initial eye position and saccade probability.
Green and magenta lines indicate population means (n = 5 mice) for saccades evoked by left and right stimuli, respectively. Blue lines indicate spontaneous saccades. Error bars indicate s.e.m. Saccade numbers: ear airpuff sessions, spontaneous = 7146, left ear airpuff-evoked = 942, right ear airpuff-evoked = 1213; whisker airpuff sessions: spontaneous = 7790, left whisker airpuff-evoked = 440, right whisker airpuff-evoked = 606; ear tactile sessions, spontaneous = 6706, left ear tactile-evoked = 133, right ear tactile-evoked = 186; auditory sessions, spontaneous = 10,240, left auditory-evoked = 140, right auditory-evoked = 158.
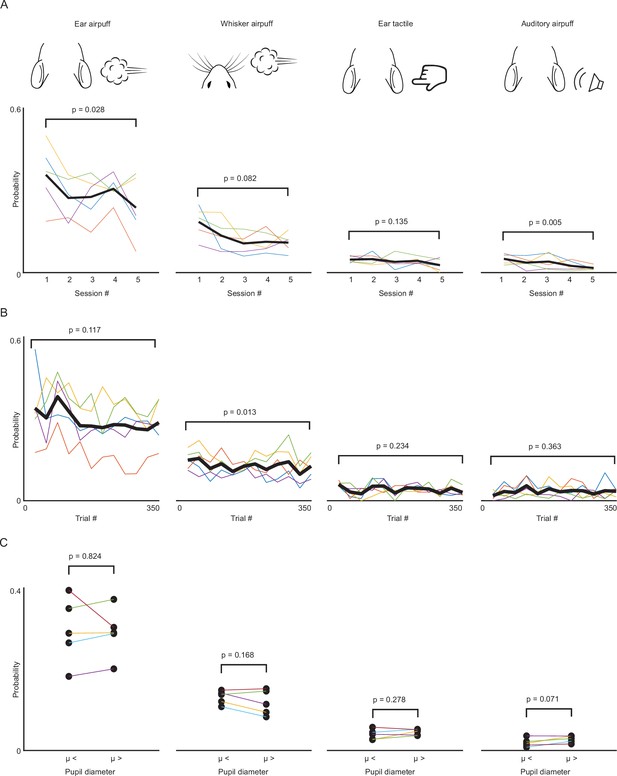
Effects of sensory history and arousal on saccade generation.
(A) Overall gaze shift probability across five sessions for ear airpuffs, whisker airpuffs, ear tactile, and auditory airpuffs stimuli. Each thin colored line corresponds to an individual mouse. Black line corresponds to mean (B) As in (A) but for gaze shift probability within sessions. (C) Effects of arousal on saccade probability. μ denotes mean pupil diameter. Statistical significance assessed using paired Student’s t-test.
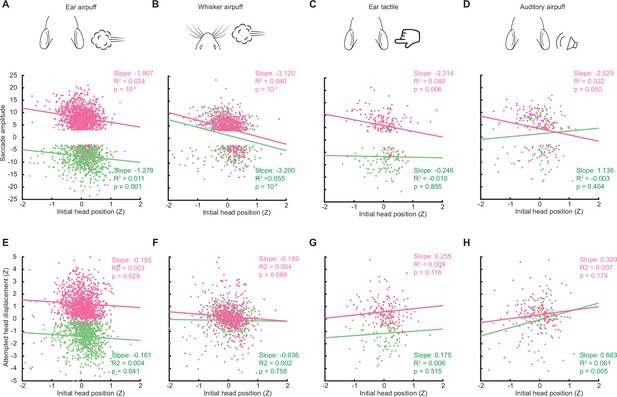
Relationship between initial head position and saccade and head movement amplitude.
(A–D) Relationship between saccade amplitude and initial head position for ear airpuffs (A), whisker airpuffs (B), whisker tactile (C), and auditory airpuffs (D). (E–H) Relationship between attempted head displacement and initial head position for ear airpuffs (E), whisker airpuffs (F), whisker tactile (G), and auditory airpuffs (H). Only trials in which animals maintained a stable head position in the 50ms preceding stimulus onset were analyzed. Total trial numbers for A-H: ear airpuff sessions, left ear airpuff = 862, right ear airpuff = 1140; whisker airpuff sessions, left whisker airpuff = 380, right whisker airpuff = 537; tactile ear sessions, left tactile ear = 103, right tactile ear; = 159; auditory airpuff, left auditory airpuff = 112, right auditory airpuff = 132. R2 values for initial eye position vs. saccade amplitude and initial eye position vs. head movement amplitude for identical trials were similar to those listed in Figure 5A–H.
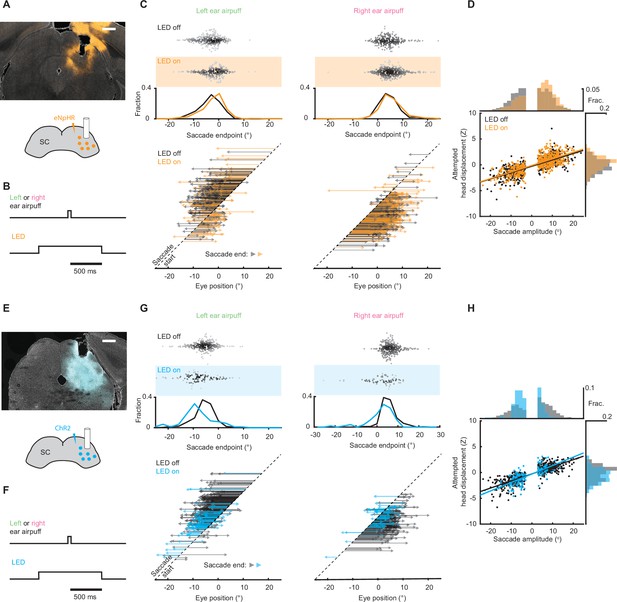
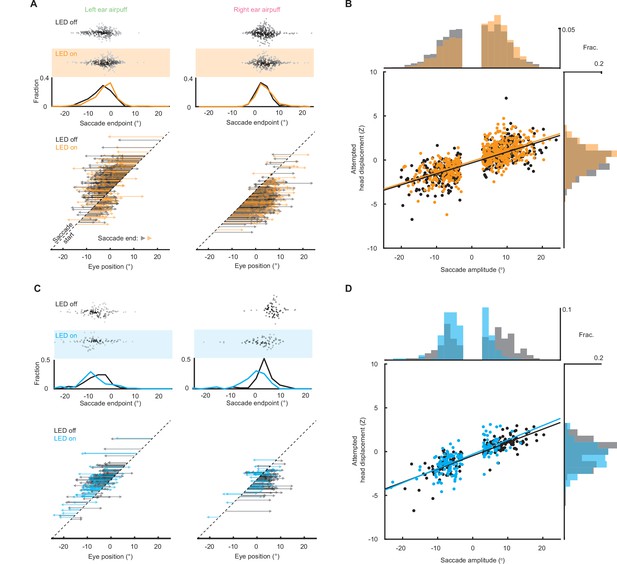
Superior colliculus controls touch-evoked gaze shifts.
(A) Schematic of right SC optogenetic inhibition using eNpHR3.0 and example histology for representative mouse. Scale bar, 0.5 mm. The lack of fluorescence immediately surrounding fiber tip is due to photobleaching by high photostimulation intensity (12 mW, as opposed to 50–120 μW for ChR2 experiments in (E–H)). (B) Trial structure. Optogenetic illumination is provided for a 1 s period centered around airpuff delivery. (C) Effects of SC optogenetic inhibition on saccade endpoints. Top, scatter plots and histograms of endpoints for control (white background, n = 296) and LED on (orange background, n = 235) trials. Middle, endpoint histograms for control (black) and LED on (orange) trials. Bottom, saccade vectors for control (black) and LED on (orange) trials. (D) Head-eye amplitude coupling during ear airpuff-evoked gaze shifts for control (black) and LED on (orange) trials. Each dot represents an individual gaze shift. Control: R2 = 0.56, slope = 0.123, p < 10–10. LED on: R2 = 0.53, slope = 0.127, p < 10–10. Control and LED on regression slopes were significantly different (p = 0.01, permutation test) due to differences in eye positions from which gaze shifts were generated, because controlling for initial eye position eliminated this difference (Figure 6—figure supplement 1). Histograms above and beside scatter plot show distributions of saccade amplitudes and attempted head displacements, respectively. Distribution means were significantly different (p < 10–5, permutation test). (E) Schematic of right SC optogenetic subthreshold stimulation using ChR2 and example histology for representative mouse. Scale bar, 0.5 mm. (F) Trial structure. Optogenetic illumination is provided for a 1 s period centered around airpuff delivery. (G) Effects of weak SC optogenetic stimulation on saccade endpoints. Top, scatter plots and histograms of endpoints for control (white background, n = 547) and LED on (blue background, n = 157) trials. We observed fewer trials in the LED-on condition because SC stimulation increased the probability of spontaneous saccades prior to stimulus onset, and trials with saccades in the 500ms before stimulus delivery were excluded from analysis. Middle, histograms of endpoints for control (black) and LED on (blue) trials. Bottom saccade vectors for control (black) and LED on (blue) trials. (H) Head-eye amplitude coupling during ear airpuff-evoked gaze shifts for control (black) and LED on (blue) trials. Each dot represents an individual gaze shift. Attempted head amplitude was measured 150ms after saccade onset. Control: R2 = 0.69, slope = 0.137, p < 10–10. LED on: R2 = 0.52, slope = 0.164, p < 10–10. Control and LED-on regression slopes were significantly different (p < 10–5, permutation test) due to difference in eye positions from which gaze shifts were generated, because controlling for initial eye position eliminated this difference (Figure 6—figure supplement 1). Histograms above and beside scatter plot show distributions of saccade amplitudes and attempted head displacements, respectively. Distribution means were significantly different (p < 10–5, permutation test).
Controlling for the effects of initial eye position on superior colliculus manipulations.
(A) Effects of SC optogenetic inhibition on saccade endpoints for trials matched for initial eye position. Top, scatter plots and histograms of endpoints for control (white background) and LED on (orange background) trials. Middle, endpoint histograms for control (black) and LED on (orange) trials. Bottom, saccade vectors for control (black) and LED on (orange) trials. (B) Head-eye amplitude coupling during ear airpuff-evoked gaze shifts for control (black) and LED on (orange) trials matched for initial eye position. Each dot represents an individual gaze shift. Control: R2 = 0.57, slope = 0.124, p < 10–10. LED on: R2 = 0.53, slope = 0.125, p < 10–10. Control and LED-on regression slopes were not significantly different (p = 0.41, permutation test) Histograms above and beside scatter plot show distributions of saccade amplitude and head displacement, respectively. Distribution means were significantly different (p = 0.002 for saccades, p < 10–5 for attempted head movements, permutation test). (C) Effects of weak SC optogenetic stimulation on saccade endpoints for trials matched for initial eye position. Top, scatter plots and histograms of endpoints for control (white background) and LED on (blue background) trials. Middle, histograms of endpoints for control (black) and LED on (blue) trials. Bottom, saccade vectors for control (black) and LED on (blue) trials. (D) Head-eye amplitude coupling during ear airpuff-evoked gaze shifts for control (black) and LED on (blue) trials matched for initial eye position. Each dot represents an individual gaze shift. Control: R2 = 0.74, slope = 0.15, p < 10–10. LED on: R2 = 0.52, slope = 0.164, p < 10–10. Control and LED-on regression slopes were not significantly different (p = 0.35, permutation test) Histograms above and beside scatter plot show distributions of saccade amplitude and head displacement, respectively. Means were significantly different (p < 10–5 for saccades, p = 0.002 for attempted head movements, permutation test).
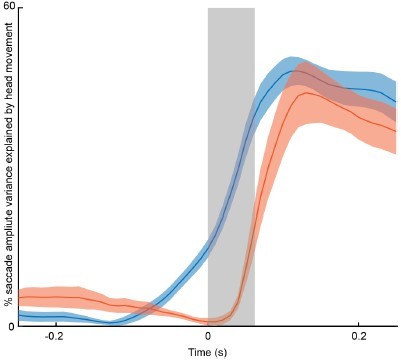
Performance of linear regression models for saccade amplitude and head position.
Performance of linear models trained separately for spontaneous (blue) and puff-evoked (red) gaze shifts at different times relative to saccade onset. Each model was trained on distributions of trials matched for initial eye position and amplitude (6 mice, 20 sessions, 1920 matched saccades). Traces denote population mean R2 values +/- s.e.m. Shaded gray area indicates mean saccade duration.

Relationship between saccade amplitude and initial eye position for ear airpuff- and auditory-evoked saccades.
Each point denotes a single saccade. Brighter areas indicate higher point density. Lines are fits of linear regression model. Dashed horizontal line indicates saccade amplitude of 0°. Dashed vertical line indicates initial eye position at which linear fit intersects with dashed horizontal line, i.e., the eye position at which predicted saccade amplitude is 0°. For left and right ear airpuffs, these intercepts are at -6.35° +/- 0.15 and 6.20° +/- 0.10 (mean +/- s.e.m) and R2 values are 0.352 and 0.506, respectively. For left and right auditory airpuffs, intercepts are at 0.14° (+/- 0.45) and 0.19° (+/- 0.43) and R2 values are 0.648 and 0.615, respectively.

Ear airpuff-evoked attempted head movement latencies using matched, raw strain gauge measurements for individual mice.
(A) Left, distributions of raw attempted head movement amplitude-matched trials accompanying spontaneous (blue) and ear airpuff-evoked (red) gaze shifts for a single animal. Trials are matched for raw attempted head movement magnitude at 150 ms after saccade onset as used for amplitude analyses in Figure 4. Middle, mean attempted head movement traces for raw magnitude-matched head movements accompanying spontaneous (blue) and ear airpuff-evoked (red) gaze shifts. Saccade onset is at 0 ms. Right, head movement latencies for magnitude-matched head movements accompanying spontaneous (blue) and ear airpuff-evoked (red) gaze shifts. Dashed vertical line indicates saccade onset. (B-E) As in A for four other animals. Medians indicated by blue and red vertical lines. Spontaneous and evoked medians are significantly different for all animals (p< 10-5, permutation test)
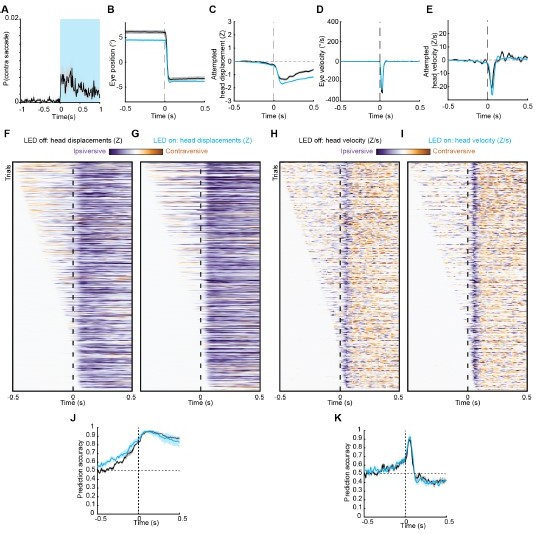
Effect of subthreshold ChR2 stimulation on spontaneous saccades.
(A) Saccade probability before and during (blue shading) a 1 second subthreshold ChR2 stimulus. (B) Mean trajectories of spontaneous saccades occurring during LED off (black) and LED on (blue) periods. (C) Mean attempted head movement amplitudes accompanying spontaneous saccades occurring during LED off (black) and LED on (blue) periods. (D) Mean velocities of spontaneous saccades occurring during LED off (black) and LED on (blue) periods. (E) Mean head movement velocities accompanying spontaneous saccades occurring during LED off (black) and LED on (blue) periods. (F, G) Timing of attempted head movements relative to spontaneous saccades occurring during LED off and LED on periods. Each row corresponds to a single gaze shift. Darker shades indicate larger attempted head displacement. Purple hues denote attempted displacement in the same direction as the saccade (ipsiversive; note that this is contraversive relative to stimulated SC), and orange hues denote displacement in the opposite direction of the saccade (contraversive). Dashed vertical line indicates time of saccade onset. Trials are sorted by latency of attempted head movements. (H, I) As in (F, G) but for attempted head movement velocity. (J) Instantaneous fraction of LED off spontaneous saccades with ipsiversive attempted head displacements. (K) As in (J) but for attempted head velocity.
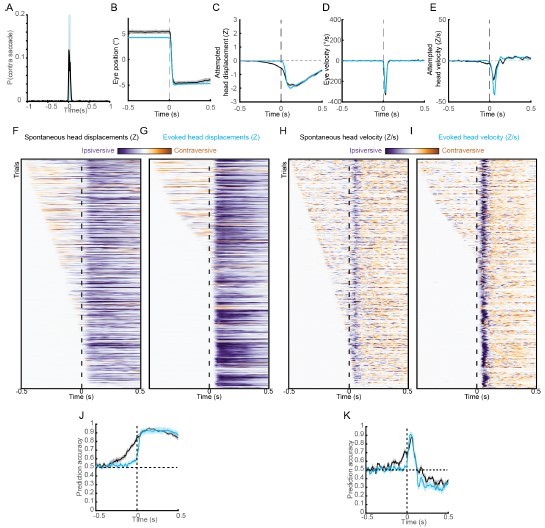
Effect of suprathreshold ChR2 stimulation on head-eye coupling. (A) Saccade probability during a 40 ms suprathreshold ChR2 stimulus. (B) Mean trajectories of saccades during spontaneous (black) and opto-evoked (blue) gaze shifts. (C) Mean attempted head movement amplitudes accompanying saccades during spontaneous (black) and opto-evoked (blue) gaze shifts. (D) Mean velocities of saccades during spontaneous (black) and opto-evoked (blue) gaze shifts. (E) Mean head movement velocities accompanying saccades during spontaneous (black) and opto-evoked (blue) gaze shifts. (F, G) Timing of attempted head movements relative to saccades occurring spontaneous (black) and opto-evoked (blue) gaze shifts. Each row corresponds to a single gaze shift. Darker shades indicate larger attempted head displacement. Purple hues denote attempted displacement in the same direction as the saccade (ipsiversive, which is contraversive to stimulated SC), and orange hues denote displacement in the opposite direction of the saccade (contraversive). Dashed vertical line indicates time of saccade onset. Trials are sorted by latency of attempted head movements. (H, I) As in (F, G) but for attempted head movement velocity. (J) Instantaneous fraction of trials with ipsiversive attempted head displacements relative to saccade onset for spontaneous (black) and opto-evoked (blue) gaze shifts. (K) As in (J) but for attempted head velocity.
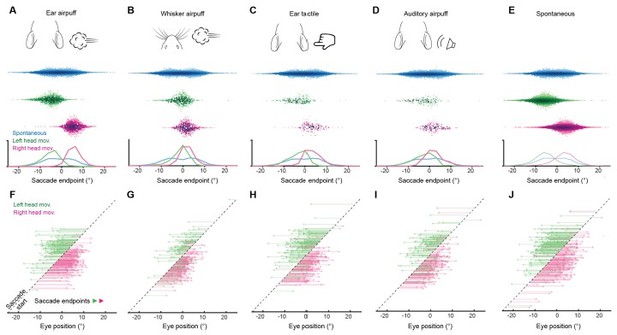
Endpoints and trajectories of sensory-evoked saccades organized by head movement direction. (A-D) Endpoints for ear airpuff-, whisker airpuff-, ear tactile-, and auditory airpuff-evoked saccade organized by head movement direction. Top, schematics of stimuli. Middle, scatter plots showing endpoints of all saccades for all animals (n = see below, 5 animals) made spontaneously (blue) and during left (green) and right (magenta) attempted head movements. Darker shading indicates areas of higher density. Bottom, histograms of endpoint distributions for spontaneous and evoked saccades. (E) As in (A-D) but with endpoints for spontaneous saccades organized by head movement direction. (F-J) Trajectories of individual stimulus-evoked saccades. Each arrow denotes the trajectory of a single saccade. Saccades are sorted according to initial eye positions, which fall on the dashed diagonal line. Saccade endpoints are indicated by arrowheads. Because the probability of evoked gaze shifts differed across stimuli, data for ear and whisker airpuffs are randomly subsampled (15% and 30% of total trials, respectively) to show roughly equal numbers of trials for each condition. Saccade numbers in A-J: ear airpuff sessions, spontaneous = 7146, evoked left head movement = 951 (143 in E), evoked right head movement = 1204 (181 in E); whisker airpuff sessions: spontaneous = 7790, evoked left head movement = 486 (146 in F), evoked right head movement = 560 (168 in F); ear tactile sessions, spontaneous = 6706, evoked left head movement = 167 evoked right head movement = 152; auditory sessions, spontaneous = 10240 evoked left head movement = 134, evoked right head movement = 164; spontaneous = 7146, spontaneous left head movement = 3565 (171 in J), spontaneous right head movements = 3581 (168 in J).
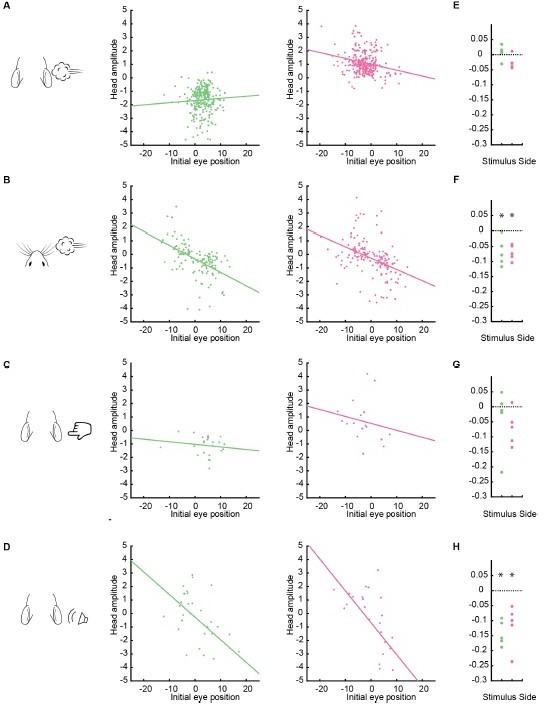
Evoked attempted head movement amplitude varies according to initial eye position across individual mice.
(A-D) Attempted head movement amplitude as a function of initial eye position for left and right stimulus-evoked gaze shifts for an example animal. Each dot corresponds to a single gaze shift. (E-F) Summary of the slopes of the lines of best fit for initial eye position vs. attempted head movement data for 5 mice after removing outlier head movements. Asterisks indicate p < 0.05 using Student’s t-test.





