Phox2b mutation mediated by Atoh1 expression impaired respiratory rhythm and ventilatory responses to hypoxia and hypercapnia
Figures
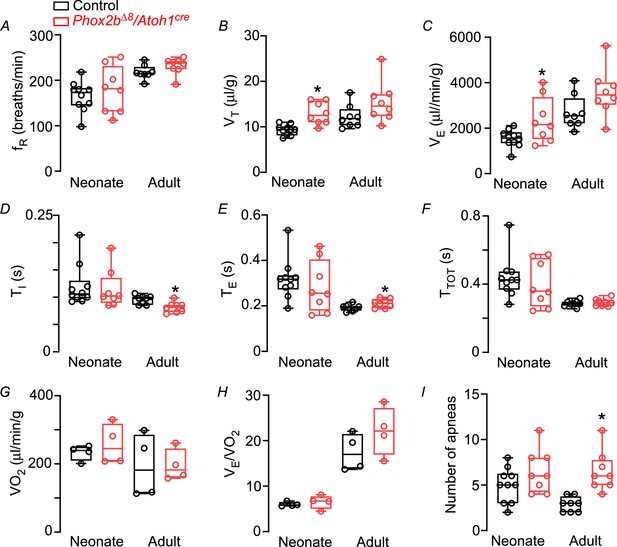
Functional respiratory changes observed in the Phox2b∆8 mutation in Atoh1cre-expressing cells.
Changes in (A) respiratory frequency (fR; breaths/min), (B) tidal volume (VT; μL/g), (C) minute ventilation (VE; μL/min/g), (D) inspiratory time (TI; s), (E) expiratory time (TE; s), (F) total cycle duration (TTOT; s), (G) oxygen consumption (VO2, μL/min/g), (H) air convection requirements VE/VO2 (a.u.), and (I) number of apneas in control and mutant (Phox2b∆8, Atoh1cre) mice during neonatal and adult phase. Values are expressed as scatter dot plot with means ± SEM. Neonate (N=8–10/group); adult (N=8/group). *p<0.05 vs. control from Mann-Whitney U test.
-
Figure 1—source data 1
Raw respiratory parameters of control and Phox2bdelta8/Atoh1-cre mice.
- https://cdn.elifesciences.org/articles/73130/elife-73130-fig1-data1-v2.xlsx
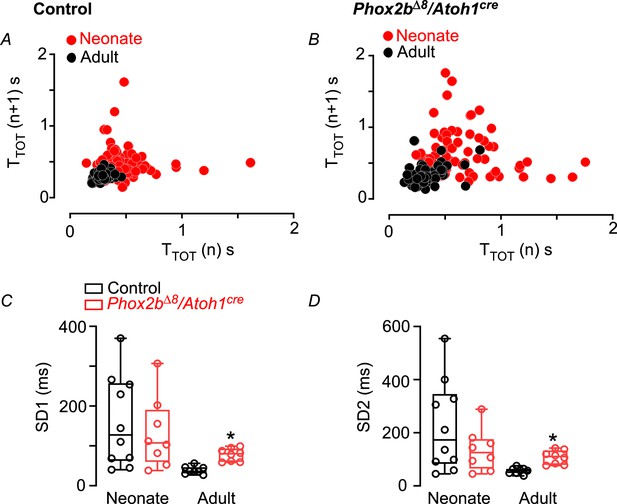
Breath variability increased in adult mice.
Typical examples of Poincare plot graphs showing SD1 and SD2 from breath duration (TTOT) vs. duration of the subsequent breath (TTOT n+1) in (A) control and (B) mutant mice (Phox2b∆8, Atoh1cre) in neonatal (P3; red circles) and adult (P45; closed circles) phase. (C) Mean ± SEM of SD1 and (D) SD2 during neonatal and adult phases. Neonate (N=8–10/group); adult (N=8/group). *p<0.05 from Mann-Whitney U test.
-
Figure 2—source data 1
Raw breath variability of control and Phox2bdelta8/Atoh1-cre mice.
- https://cdn.elifesciences.org/articles/73130/elife-73130-fig2-data1-v2.xlsx
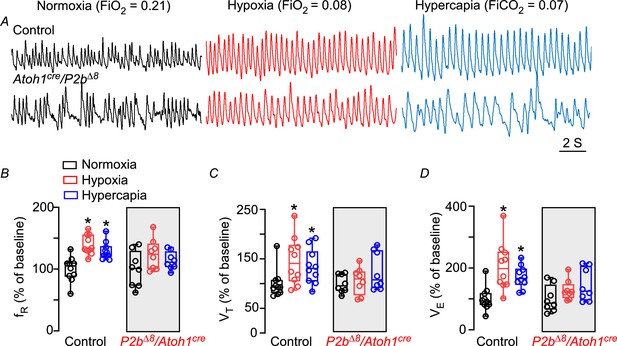
Phox2bΔ8 in Atoh1cre cells impaired ventilatory responses to hypoxia and hypercapnia in neonates.
(A) Representative plethysmograph breathing traces in a control (top traces) and mutant (Phox2b∆8, Atoh1cre; bottom traces) neonate (P3) mice while ventilated with room air (normoxia; FiO2=0.21); hypoxia (FiO2=0.08); and hypercapnia (FiCO2=0.07). Percentage changes produced by hypoxia or hypercapnia in neonate control and mutant mice. (B) Respiratory frequency (fR; interaction: F(2,32)=0.8, p=0.455; effect of mutation F(1,16)=4.3, p=0.052; effect of hypoxia and hypercapnia: F(2,32)=10.5, p=0.0008). (C) Tidal volume (VT; interaction: F(2,32)=1.92, p=0.162; effect of mutation F(1,16)=2.44, p=0.138); effect of hypoxia and hypercapnia: F(2,32)=4.50, p=0.019. (D) Minute ventilation (VE; interaction: F(2,32)=3.32, p=0.048; effect of mutation F(1,16)=4.48, p=0.0503; effect of hypoxia and hypercapnia: F(2,32)=11.6, p=0.0002). Values are expressed as scatter dot plot with means ± SEM. N=8–10/group. ANOVA two-way Dunnett’s multiple comparisons test.
-
Figure 3—source data 1
Raw respiratory parameters of control and Phox2bdelta8/Atoh1-cre neonate mice under hypoxia and hypercapnia.
- https://cdn.elifesciences.org/articles/73130/elife-73130-fig3-data1-v2.xlsx
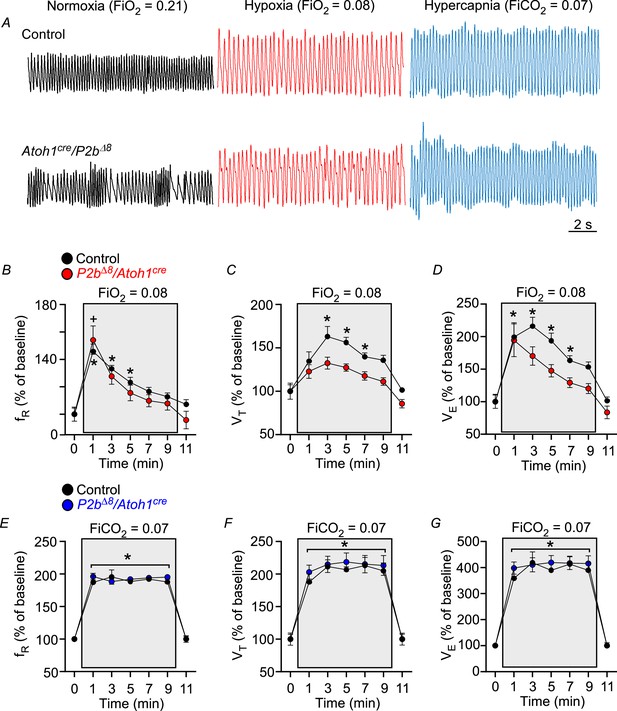
Phox2bΔ8 in Atoh1cre cells impaired ventilatory responses to hypoxia in adult.
(A) Representative plethysmograph breathing traces in a control and mutant (Phox2b∆8, Atoh1cre) adult (P45) mice while ventilated with room air (normoxia; FiO2=0.21); hypoxia (FiO2=0.08); and hypercapnia (FiCO2=0.07). Percentage changes produced by hypoxia or hypercapnia in adult control and mutant mice in (B) respiratory frequency (fR; interaction: F(6,84) = 0.97, p=0.448; effect of mutation F(1,14)=0.86, p=0.368; effect of time of hypoxia: F(6,84) = 29.32, p<0.0001); (C) tidal volume (VT, interaction: F(6,84) = 1.26, p=0.285; effect of mutation F(1,14)=20.76, p=0.0004; effect of time of hypoxia: F(6,84) = 17.49, p<0.0001); (D) minute ventilation (VE, interaction: F(6,84) = 1.20, p=0.316; effect of mutation F(1,14)=8.22, p=0.012; effect of time of hypoxia: F(6,84) = 23.92, p<0.0001). N=8/group. *p<0.05 vs. 21% O2 in controls. +p < 0.05 vs. 21% O2 in mutants. ANOVA two-way Dunnett’s multiple comparisons test. (E) Respiratory frequency (fR; interaction: F(6,84) = 0.56, p=0.763; effect of mutation F(1,14)=0.41, p=0.532; effect of time of hypercapnia: F(6,84) = 155.48, p<0.0001); (F) tidal volume (VT, interaction: F(6,84) = 0.22, p=0.968; effect of mutation F(1,14)=0.31, p=0.585; effect of time of hypercapnia: F(6,84) = 69.77, p<0.0001); (G) minute ventilation (VE, interaction: F (6,84)=0.34, p=0.914; effect of mutation F(1,14)=0.38, p=0.547; effect of time of hypercapnia: F(6,84) = 86.85, p<0.0001). N=8/group. *p<0.05 vs. 21% O2 for both control and mutation group. ANOVA two-way Dunnett’s multiple comparisons test.
-
Figure 4—source data 1
Raw respiratory parameters of control and Phox2bdelta8/Atoh1-cre adult mice under hypoxia and hypercapnia.
- https://cdn.elifesciences.org/articles/73130/elife-73130-fig4-data1-v2.xlsx
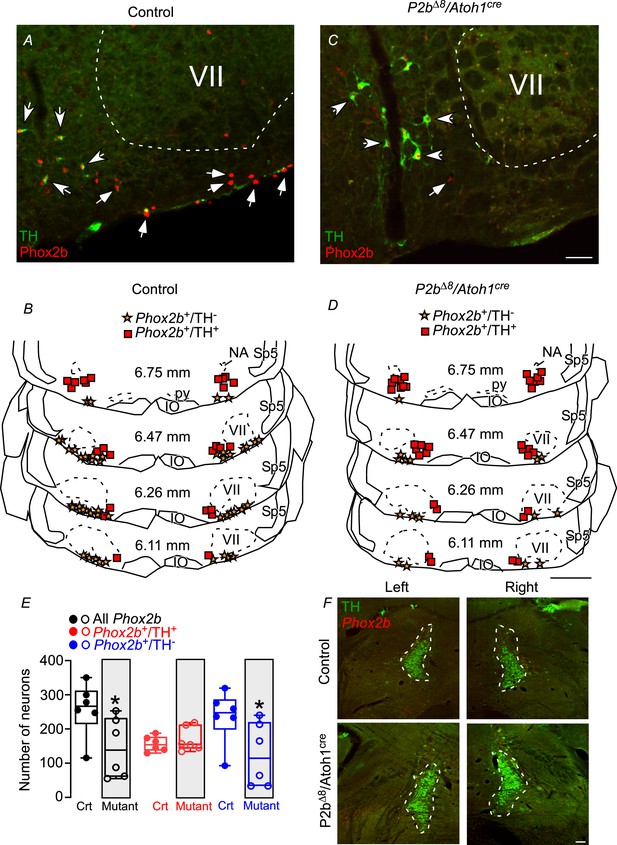
Adult mutant mice reduced Phox2b expression in the parafacial/retrotrapezoid nucleus (RTN) region.
Photomicrographs of ventrolateral medulla from (A) control and (C) mutant (Phox2b∆8, Atoh1cre) adult mice. Schematic drawings represent examples of coronal sections of ventrolateral medulla in (B) control and (D) Phox2b∆8, Atoh1cre mutant mice. Each square represents immunoreactivity for Phox2b and tyrosine hydroxylase (Phox2b+/TH+). The stars represent immunoreactivity for Phox2b and absence of TH (Phox2b+/TH-). The numbers in the middle of each section refer to the location caudal to the Bregma level (in mm) according to the Mouse Brain Atlas of Franklin and Paxinos, 2015. (E) Total number of cells that expressed Phox2b and TH immunoreactivity in the ventrolateral medulla (parafacial/RTN and C1 region) in control and Phox2b∆8, Atoh1cre (N=6/group). (F) Photomicrographs showing locus coeruleus and subcoeruleus region from control and mutant (Phox2b∆8, Atoh1cre) mice. *p<0.05 vs. control, unpaired t-test. Abbreviations: IO, inferior olive; NA, nucleus ambiguous; py, pyramid tract; Sp5, spinal trigeminal tract; VII, facial motor nucleus. Scale bar: C=50 μm applied to A; D=1 mm applied to B; F=100 μm.
-
Figure 5—source data 1
Raw numbers of neuronal profiles (Phox2b and TH) of control and Phox2bdelta8/Atoh1-cre adult mice.
- https://cdn.elifesciences.org/articles/73130/elife-73130-fig5-data1-v2.xlsx
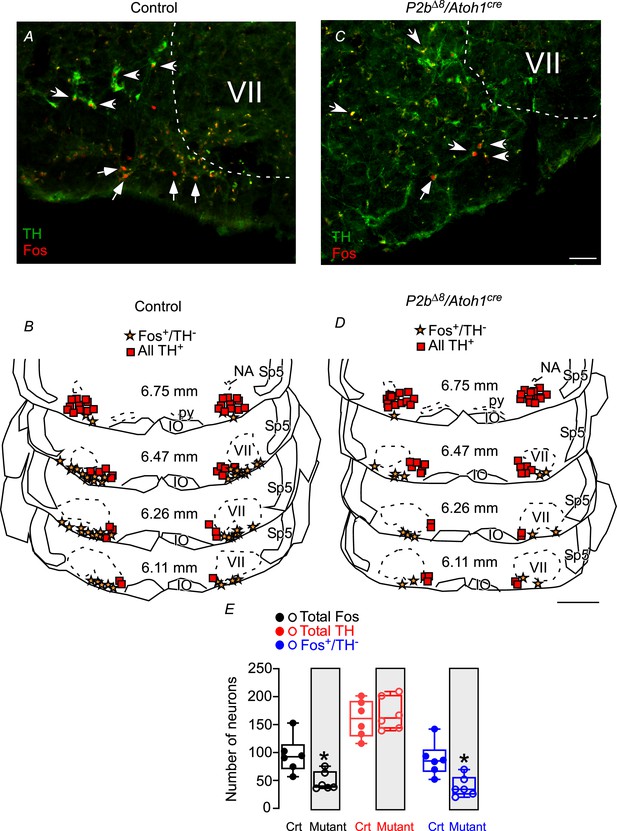
Fos-activated neurons in the parafacial/retrotrapezoid nucleus (RTN) region in response to hypercapnia are reduced in mutant mice.
Photomicrographs of ventrolateral medulla from (A) control and (C) mutant (Phox2b∆8, Atoh1cre) mice exposed to hypercapnia (FiCO2=0.07). Schematic drawings represent coronal sections of ventrolateral medulla in (B) control and (D) Phox2b∆8, Atoh1cre mutant mice. Each square represents tyrosine hydroxylase immunoreactivity (TH+). The stars represent fos and the absence of TH (fos+/TH-). The numbers in the middle of the sections refer to the location caudal to the Bregma level (in mm) according to the Mouse Brain Atlas of Franklin and Paxinos, 2015. (E) Total number of cells that expressed fos and TH immunoreactivity in the ventrolateral medulla (respiratory parafacial/RTN region) in control and Phox2b∆8, Atoh1cre mice (N=6/group). *p<0.05 vs. control; unpaired t-test. Abbreviations: IO, inferior olive; NA, nucleus ambiguous; py, pyramid tract; Sp5, spinal trigeminal tract; VII, facial motor nucleus. Scale bar: C=50 μm applied to A; D=1 mm applied to B.
-
Figure 6—source data 1
Raw numbers of neuronal profiles (fos and TH) of control and Phox2bdelta8/Atoh1-cre adult mice under hypercapnia.
- https://cdn.elifesciences.org/articles/73130/elife-73130-fig6-data1-v2.xlsx
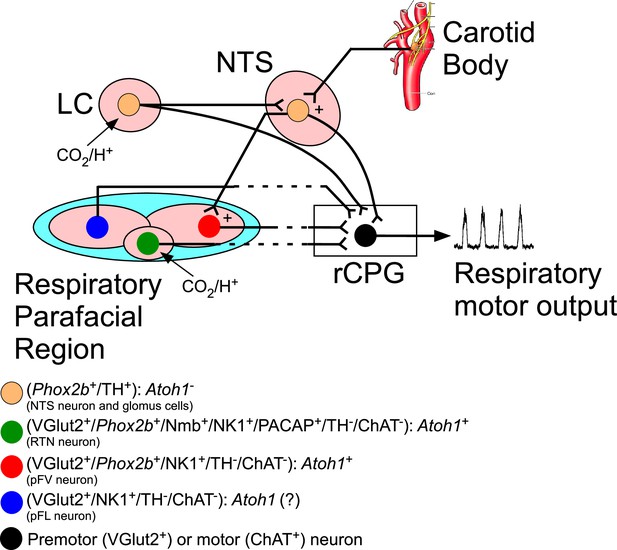
Schematic view of the mouse hindbrain control of breathing and the role of transcriptions factors and neuromodulators.
The respiratory parafacial region (pF) contains neurons involved in breathing regulation. Within the ventral aspect of the pF, retrotrapezoid nucleus (RTN) could be defined as a cluster of neurons positive for Phox2b, neuromedin (Nmb), NK1, glutamatergic (VGlut2), pituitary adenylate cyclase-activating peptide (PACAP) and the absence of tyrosine hydroxylase (TH), choline acetyltransferase (ChAT), serotonin, GABA, and glycine. These neurons are activated by CO2 via their intrinsic pH sensitivity and via inputs from the carotid bodies. The RTN of mice has a distinctive developmental lineage that relies on transcription factors Egr2, Phox2b, Lbx1, and Atoh1. Phox2b is the only one that remains expressed in adulthood. RTN progenitors originate from the dB2 domain of rhombomere 5. These progenitors are Phox2b-positive, switch on Lbx1 at the postmitotic stage, migrate ventrally, and activate Atoh-1 expression once they reach the region of the facial motor nucleus. In the respiratory pF also have distinct functional subgroup of neurons, that is, pF ventral neurons (non-RTN) and pF lateral neurons (expiratory oscillators). RTN neurons target various components of the respiratory central pattern generator (rCPG) and are presumed to play a key role in breathing automaticity during anesthesia, sleep, and quiet waking. The carotid body may also influence the activity of the rCPG neurons through connections that bypass the RTN (Stornetta et al., 2006; Takakura et al., 2006). The ventilatory response to CO2 also has a contribution of the catecholaminergic neurons located in the locus coeruleus (LC). Here, we showed that breathing dysfunction of the humanized NPARM Phox2bΔ8 mutation in Atoh1-expressing cells is presumably mediated by loss of cells in the ventral parafacial region. Abbreviations: Atoh1, atonal homolog 1; ChAT, choline acetyltransferase; LC, locus coeruleus; Nmb, neuromedin B; NTS, nucleus of the solitary tract; NK1, tachykinin 1; PACAP, pituitary adenylate cyclase-activating peptide; Phox2b, paired like homeobox 2B; rCPG, respiratory central pattern generator; TH, tyrosine hydroxylase; VGlut2 (Slc17a6), vesicular glutamate transporter 2.
Tables
Genotyping primers.
| Mouse line | Strain name | Strain # | Obtained from | Primers | Band sizes |
|---|---|---|---|---|---|
| Atoh1Cre | B6.Cg-Tg(Atoh1-cre)1Bfri/J | Jax: 011104 | Jackson Laboratories | Tg FWD 5'-CCG GCA GAG TTT ACA GAA GC-3' | Tg = 450 bp |
| Tg REV 5'-ATG TTT AGC TGG CCC AAA TG-3' | CTR = 324 bp | ||||
| CTR FWD 5'-CTA GGC CAC AGA ATT GAA AGA TCT-3' | |||||
| CTR REV 5'-GTA GGT GGA AAT TCT AGC ATC ATC C-3' | |||||
| Phox2bΔ8 | B6.129(Cg)-Phox2btm1Rth/J | Jax: 025436 | David Rowitch, UCSF | FWD 5'-GCC CAC AGT GCC TCT TAA CTC-3' | Mutant = 450 bp |
| REV 5'-CGT ACT CTT AAA CGG GCG TCT C-3' | Wild type = 334 bp |





