An epithelial signalling centre in sharks supports homology of tooth morphogenesis in vertebrates
Figures
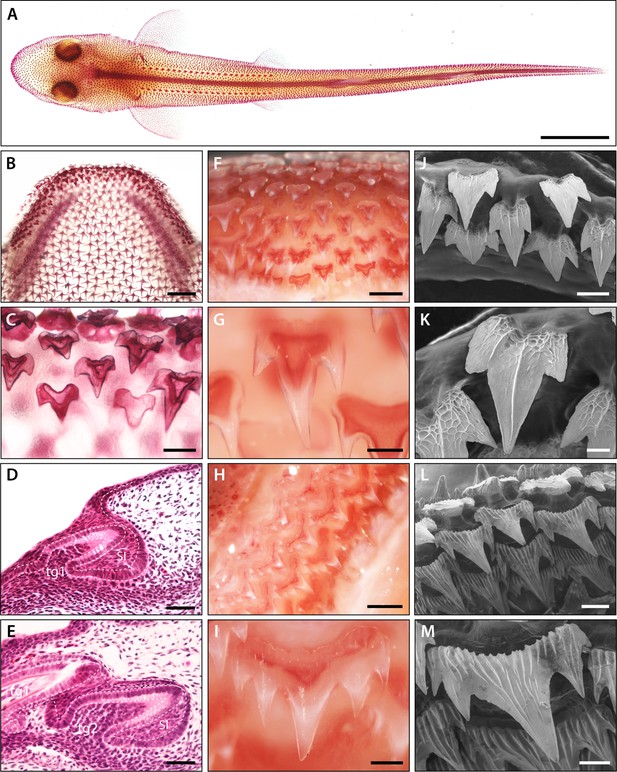
Pattern and morphology of the catshark dentition.
Images of catshark samples cleared and stained with alizarin red, reveal pattern and morphology of the dentition in the lower jaw (A–C and F–I). (A) Dorsal view of hatchling stage catshark (stage 34) revealing denticle on the body surface. (B) Dorsal view of hatchling stage (stage 34) lower jaw. (C) Magnification of B, showing staggered pattern of adjacent tooth families. Histological staining with haematoxylin and eosin on sagittal cross sections through early stage 32 (D) and late stage 32 (E) lower jaws shows the growth of the DL during dental development. The first dental generation (tg1) develops relatively superficially at the oral surface, before full invagination and elongation of the DL (D). The DL then grows deep into the underlying mesenchyme, with the second (tg2) and subsequent dental generations initiated at the SL (E). The addition of numerous successional dental generations can be seen in the adult jaw (F and H). At the jaw symphysis (F), teeth often remain tricuspid (G). However, in lateral regions (H), teeth develop 5–7 cusps (I). Scanning electron microscope (SEM) images reveal tricuspid teeth in the embryo (stage 33) (J–K) and pentacuspid teeth in the adult (L and M). Scale bars are 10 mm in A; 1 mm in B, F, and H; 250 μm in C, G, I, and M; 50 μm in D, E, and K; 125 μm in J, and 500 μm in L. ora; oral, abo; aboral, lin; lingual, lab; labial.
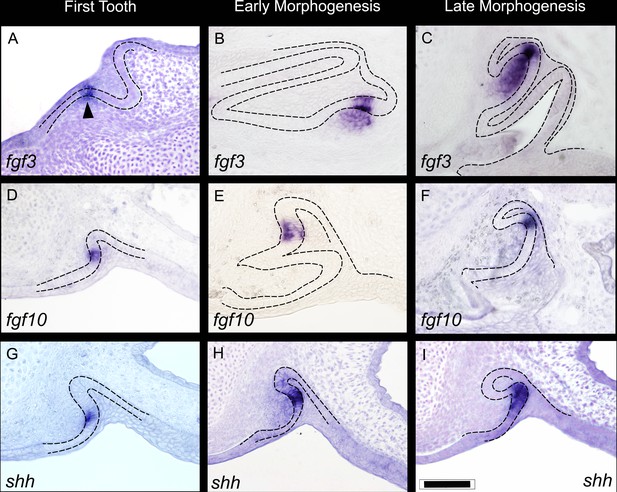
Expression of fgf3, fgf10, and shh during three key stages of shark tooth morphogenesis.
First tooth initiation (A,D,G); early morphogenesis (B, E, H); and late morphogenesis (C,F,I). First tooth initiation is superficial with expression of the fgf3, fgf10, and shh in a restricted compartment of the dental epithelium; fgf3 expression extends into the underlying papilla mesenchyme (arrowhead). During early morphogenesis, both fgf3 and fgf10 continue to show restricted expression to the site of the EK-like unit (although the underlying mesenchymal expression of fgf3 does expand further throughout the mesenchymal papilla at later stages). shh expression at this stage, however, starts to expand away from the EK-like unit of the dental epithelium (H). At late morphogenesis (C,F,I), fgf3 has an even more expanded expression pattern in the mesenchymal papilla, yet the epithelial expression is still very restricted the apex of the tooth cusp, C. fgf10 expression is always confined to the apical tip (F), and shh expression expands further throughout the inner dental epithelium in the labial cusp sites (I). Scale bar in I=100 μm.
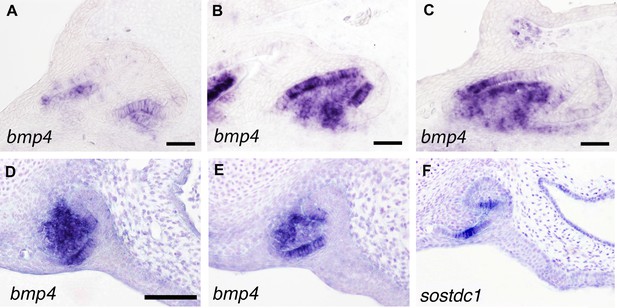
Expression of bmp4 and sostdc1 in the shark dentition.
Expression of bmp4 across three stages of tooth morphogenesis in the developing lower jaw dentition (A–C): early replacement tooth. (A) Expression is present in both the inner dental epithelium (IDE) and underlying mesenchymal papilla cells. (B and C) As tooth morphogenesis progresses, the expression of bmp4 extends throughout the IDE and dental papilla, however expression is downregulated in the epithelial apex (B; enamel knot [EK]-like unit), and this continues to be downregulated further in the IDE away from the apex (C). (D and E) Expression of bmp4 in the upper jaw teeth during morphogenesis showing equivalent expression pattern to (B and C). (F) sostdc1 expression showing a complementary expression pattern to bmp4 in the IDE, similarly no expression is noted within the apex epithelium at this stage. Scale bar in D is 100 μm; in A–C scale bars are 50 μm.
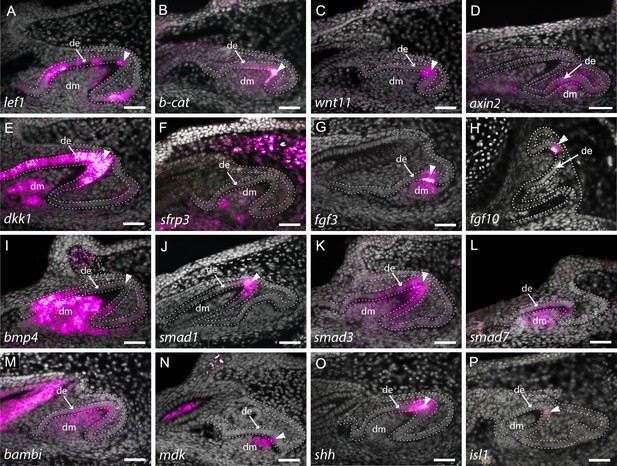
Expression of enamel knot (EK) markers during catshark dental morphogenesis.
In situ hybridisation assay on sagittal paraffin sections of late stage 32 catshark jaws reveals the expression of markers involved in major developmental pathways, including canonical Wnt signalling (A–F), fibroblast growth factor (Fgf) signalling (G and H) and bone morphogenetic protein (Bmp) signalling (I–L). Wnt markers, lef1 (A), β-catenin, (B) and wnt11 (C) are all expressed within the apical tip of the developing tooth during cap stage. Axin2 is expressed in the early dental epithelium, not specifically associated with the apical tip (D). Weak expression can also be seen within the dental mesenchyme and surrounding dental epithelium in lef1 (A) and β-catenin (B). Weak expression of the Wnt inhibitor, sfrp3 (F), is found within the dental mesenchyme during cap stage, whereas dkk1 is also found within the dental epithelium later in morphogenesis (E). The expression of Fgf markers fgf3 (G) and fgf10 (H) is highly specific to the EK during late bud to early cap stage, with fgf3 weakly expressed in the dental mesenchyme, below the EK. bmp4 (I) expression is absent from the EK during late morphogenesis, but is strongly expressed within both the rest of the dental epithelium and dental papilla. smad1 (J) is found within the apical tip of the dental epithelium, whilst smad3 (K) is also expressed in the dental mesenchyme during late cap stage. Bmp inhibitors smad7 (L) and bambi (M) are both expressed throughout both epithelium and mesenchyme of developing teeth. mdk (N) and isl1 (P) expression is restricted to a few cells of the EK, with mdk also expressed within the underlying dental mesenchyme. In contrast, shh (O) expression is extensive and broad, but is still restricted to the apical tip of the dental epithelium. Gene expression is false coloured in magenta. White arrowhead points to expression within the apical tip of developing teeth and putative EK. White dotted lines depict the columnar basal epithelial cells of the dental lamina and dental epithelium. DAPI nuclear stain is false coloured in grey. All images are of lower jaws, except H, which is of the upper jaw. Scale bars are 50 μm. de, dental epithelium; dm, dental mesenchyme.
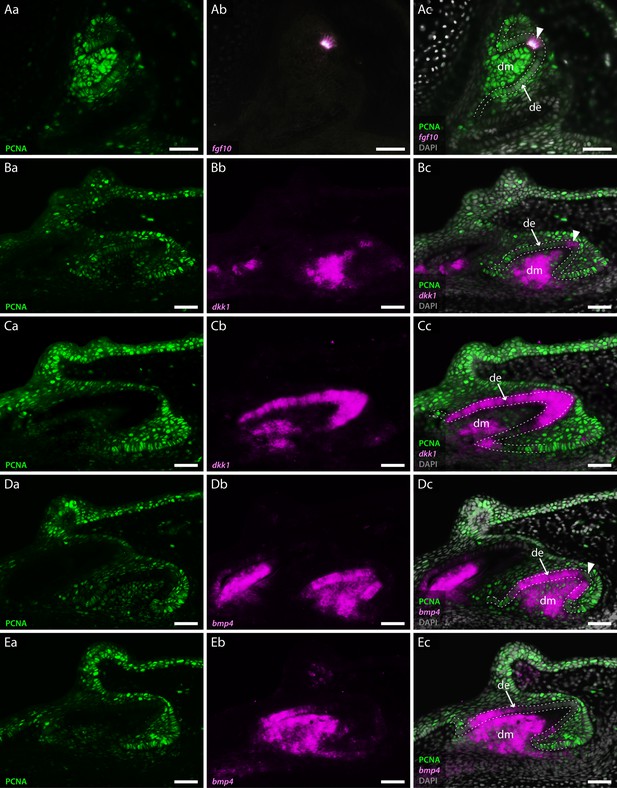
Co-expression of odontogenic markers regulating dental morphogenesis with proliferative cell nuclear antigen (PCNA).
Double section in situ hybridisation/immunohistochemistry on catshark jaws reveals co-expression of markers involved in enamel knot (EK) signalling and PCNA, within teeth undergoing cap stage (A, B, and D) and late morphogenesis (C and E). Images Aa–Ea reveal expression of PCNA. Images Ab–Eb reveal expression of in situ hybridisation markers. Images Ac–Ec reveal co-expression of PCNA and in situ hybridisation markers. PCNA expression is absent from the tip of the dental epithelium, corresponding to the EK in cap stage teeth (Aa, Ba, and Da). The extent of PCNA expression within developing teeth decreases as teeth undergo morphogenesis (Ca and Ea). fgf10 is expressed within a small subset of dental epithelial cells corresponding to the EK (Ab). Its expression is inversely complementary to the expression of PCNA (Ac). dkk1 expression is initially upregulated in the dental mesenchyme during cap stage (Bb), with restricted epithelial expression present only within non-proliferative cells of the EK (Bc). During late morphogenesis dkk1 expression weakens within the dental mesenchyme, with observable upregulation of its expression throughout the entire dental epithelium (Cb and Cc). Unlike dkk1 and fgf10, bmp4 is absent from the apical tip of the dental epithelium throughout dental morphogenesis (Db and Eb). fgf10 (A) is shown in the upper jaw; dkk1 (B and C) and bmp4 (D and E) are shown in the lower jaw. Gene expression is shown in magenta, PCNA protein expression is in green, and DAPI nuclear stain in grey. White dotted lines depict the outer dental epithelium of the developing tooth. Scale bars are 50 μm. de, dental epithelium; dm, dental mesenchyme.
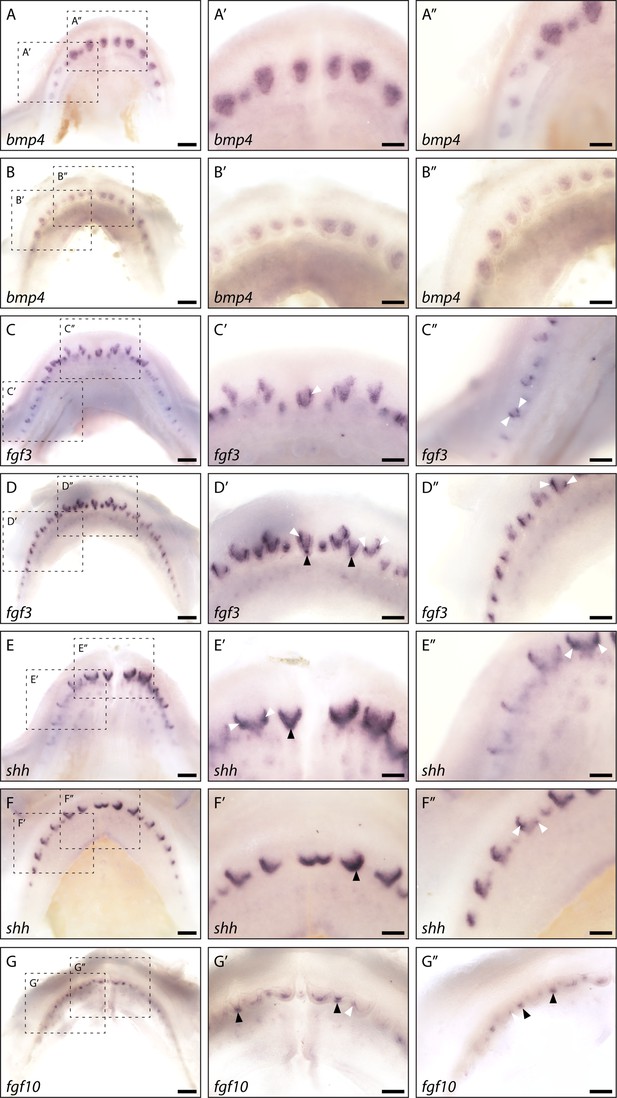
Whole mount in situ hybridisation reveals enamel knot (EK)-specific gene expression.
Whole mount in situ hybridisation on catshark lower (A, C, and E) and upper (B, D, F, and G) jaws highlights the expression of bmp4 (A and B), fgf3 (C and D), shh (E and F), and fgf10 (G) across the jaw. Images are of early stage 32 (~90 days post fertilisation [dpf]) samples, with developing first-generation teeth visible on the jaw margin. Images A–G are low magnification images. Images A’–G’ are magnified images of the central teeth along the jaw. Images A’’–G’’ are magnified images of the left lateral side of the jaw. bmp4 expression is visible throughout the dental papilla, but appears absent from the dental epithelium (A’, A’’, B’, and B’’). fgf3 expression is strongly upregulated within both primary EKs (D’: black arrowheads) and secondary EKs (C’, C’’, D’, and D’’: white arrowheads). Weaker fgf3 expression is also noted within the dental mesenchyme (C’, C’’, D’, and D’’). shh expression is absent from the dental mesenchyme. However, its expression can be seen throughout the dental epithelium at the leading edge of the developing tooth (E’, E’’, F’, and F’’). Although its expression is not restricted to the EK throughout morphogenesis, clear shh expression present within both the primary EKs (E’ and F’: black arrowheads) and secondary EKs (E’, E’’, and F’’: white arrowheads). As with fgf3, fgf10 expression is also restricted to the future cusp forming primary (G’ and G’’: black arrowheads) and secondary EKs (G’ and G’’: white arrowhead), however its expression does not extend into the dental mesenchyme. Dotted black boxes in A–G depict a magnified region in A’–G’ and A’’–G’’. Scale bars are 250 μm in A–G and 125 μm in A’–G’ and A’’–G’’.
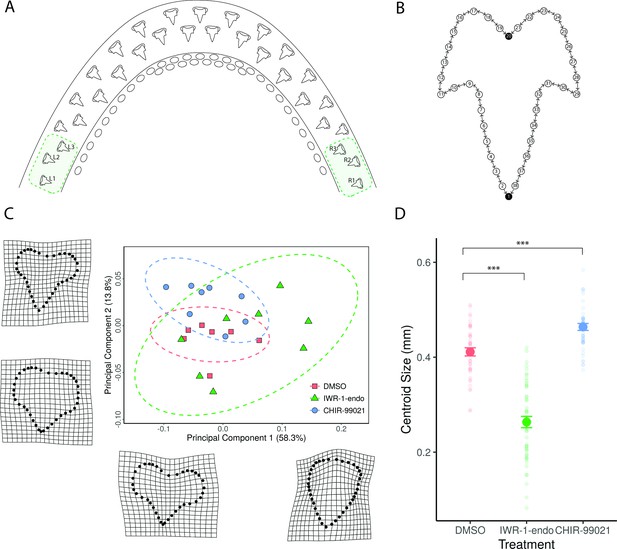
Geometric morphometric analysis reveals change in shape and size following canonical Wnt manipulation.
Small molecule treatments consisted of a 2-week treatment with 0.1% DMSO (control), 1 μM IWR-1-endo, and 2 μM CHIR99021 and a subsequent 4-week recovery. The three most lateral teeth at both left and right lower jaw margins (A) were included in the geometric morphometric analysis. A total of 38 landmarks were used to label tooth shape (B). Two fixed landmarks were placed at the tip of the primary cusp and at the base of the tooth, represented by black circles. Eighteen sliding semi-landmarks were placed on either side of the tooth (white circles), which were allowed to move relative to adjacent landmarks. The direction of movement is depicted by directional arrows in the schematic (B). Following Procrustes alignment of landmark coordinates and principal component analysis of the resulting shapes, the average PC1 and PC2 scores for each sample were plotted in order to depict the position of treated samples within a given shape space (C). PC1 accounted for 58.3% of the variation observed between samples, whereas PC2 accounted for 13.8%. Warpgrids shown in C reveal representative shapes at maximum and minimum PC1 and PC2 values. There is a significant effect of treatment on overall tooth shape (Procrustes ANOVA: R2=0.09174, F2,115=8.2771, p<0.001), whilst controlling for variation within samples. There is also a significant effect of sample on shape (Procrustes ANOVA: R2=0.27092, F20,115=2.4442, p<0.001). Aside from shape, centroid size was also measured following treatment (D). There is a significant effect of treatment on centroid size (ANOVA: F2,115=235.5886, p<0.001), whilst controlling for variation within samples. There is also a significant effect of sample on centroid size (ANOVA: F20,115=7.2957, p<0.001). Faint circular points are plots of each individual data point, revealing the distribution of the data. Error bars represent standard error.
-
Figure 6—source data 1
Principal component analysis (PCA) data.
- https://cdn.elifesciences.org/articles/73173/elife-73173-fig6-data1-v2.txt
-
Figure 6—source data 2
Procrustes shape data.
- https://cdn.elifesciences.org/articles/73173/elife-73173-fig6-data2-v2.txt
-
Figure 6—source data 3
Principal component analysis (PCA) landmarks/scale.
- https://cdn.elifesciences.org/articles/73173/elife-73173-fig6-data3-v2.txt
-
Figure 6—source data 4
Csize by treatment.
- https://cdn.elifesciences.org/articles/73173/elife-73173-fig6-data4-v2.txt
-
Figure 6—source data 5
GMM metadata.
- https://cdn.elifesciences.org/articles/73173/elife-73173-fig6-data5-v2.csv
-
Figure 6—source data 6
CurveSlide.
- https://cdn.elifesciences.org/articles/73173/elife-73173-fig6-data6-v2.csv
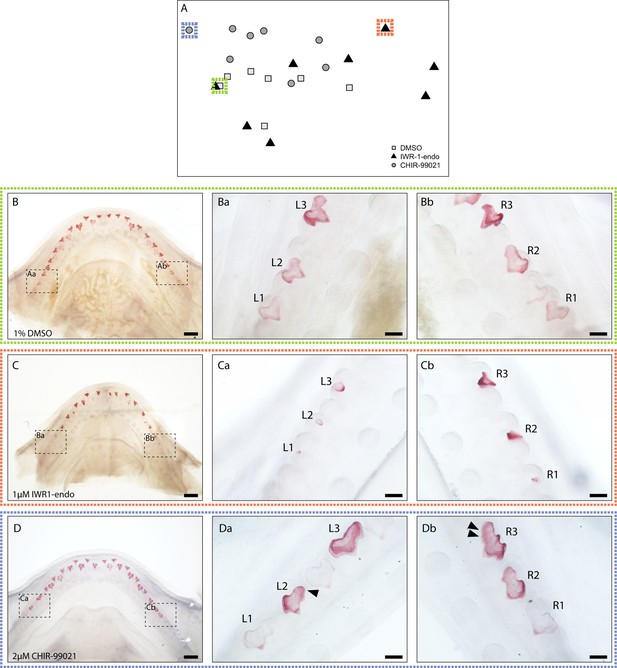
Representative experimental images from geometric morphometric principal component analysis.
(A) Principal component analysis from Figure 6C, highlighting one sample from each treatment depicted in B–D. Representative images of 0.1% DMSO (control) (B), 1 μM IWR-1-endo (C), and 2 μM CHIR9902 (D) treated lower jaws following 2-week treatment and 4-week recovery. Samples have been cleared and stained. Ba–Da and Bb–Db are magnified images of left and right lateral jaw regions, respectively. A tricuspid dentition is visible in 0.1% DMSO-treated teeth (Ba and Bb). A shift to a unicuspid morphology takes place following treatment with 1 μM IWR-1-endo (Ca and Cb). In contrast, a widening of the teeth is observed following 2 μM CHIR9902 (Da and Dc). Black arrowheads represent the addition of putative supernumerary cusps. Scale bars are 500 μm in B–D and 100 μm in Ba–Da and Bb–Db.
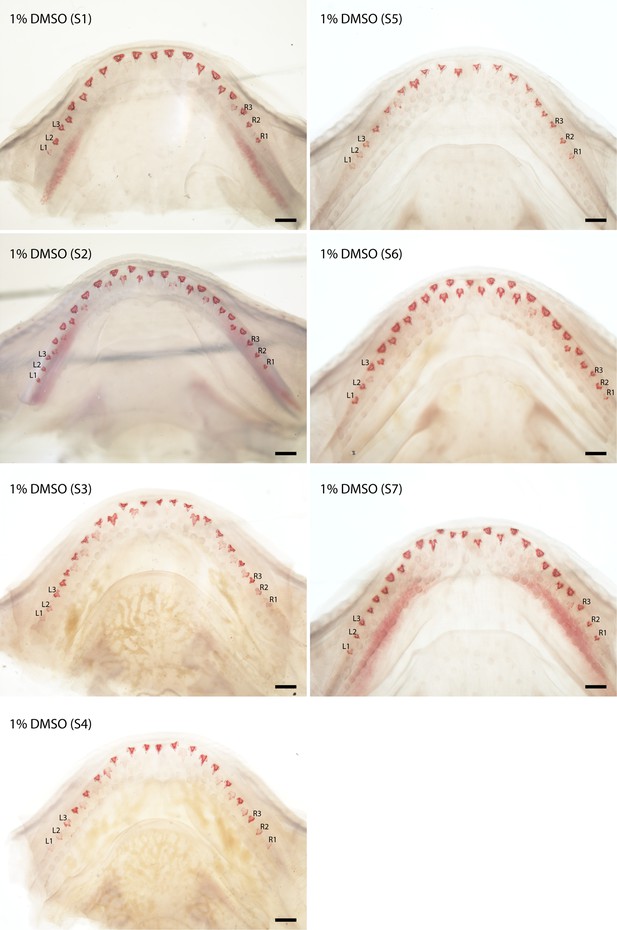
Canonical Wnt signalling control experiments: cleared and stained (Alizarin Red S) lower jaws (dorsal views) of Scyliorhinus canicula, DMSO controls.
A set of DMSO 1% (delivery solution without chemical/pharmacological agent) controls in seven specimens (S1–7). Labelled teeth (left (L)1–3 and right (R) 1–3) were used in the morphometric analysis (in Figure 6). Scale bars are all 500 μm.
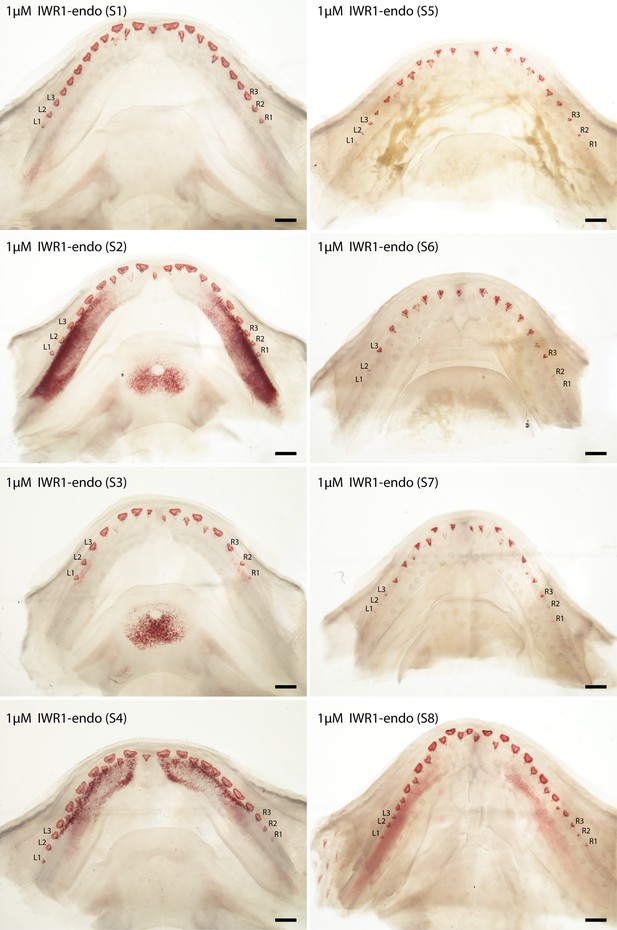
Canonical Wnt signalling inhibition experiments: cleared and stained (Alizarin Red S) lower jaws (dorsal views) of Scyliorhinus canicula, treated with 1 μM IWR-1-endo (Wnt pathway inhibitor).
A set of eight specimens treated with 1 μM IWR-1-endo 1 (S1–8). Labelled teeth (left (L)1–3 and right (R) 1–3) were used in the morphometric analysis (in Figure 6). Scale bars are all 500 μm.
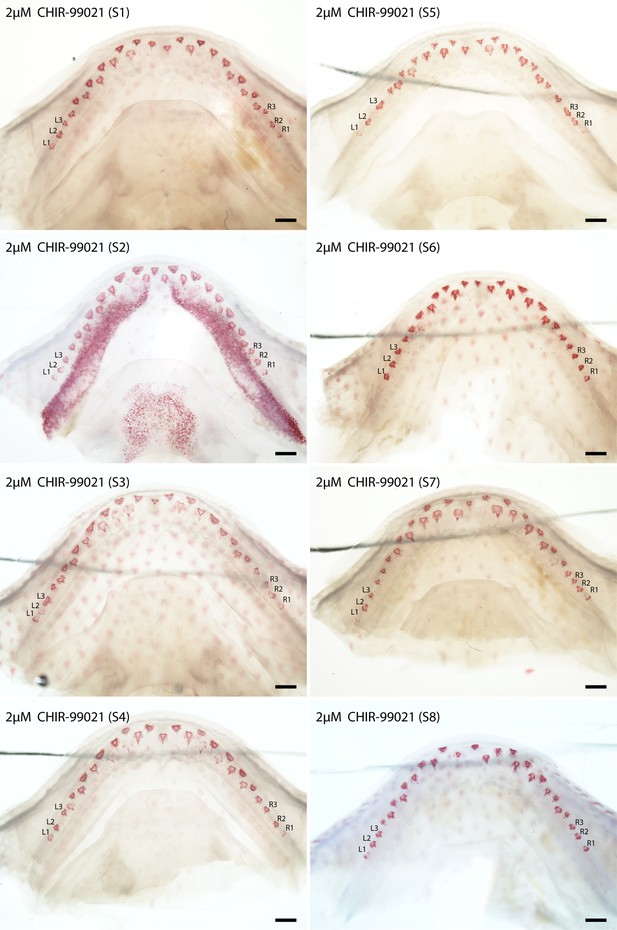
Canonical Wnt signalling activation experiments: cleared and stained (Alizarin Red S) lower jaws (dorsal views) of Scyliorhinus canicula, treated with 2 μM CHIR-99021 (small molecule Wnt pathway activator; GSK3 inhibitor).
A set of eight specimens treated with 2 μM CHIR-99021 (S1–8). Labelled teeth (left (L)1–3 and right (R) 1–3) were used in the morphometric analysis (in Figure 6). Scale bars are all 500 μm.
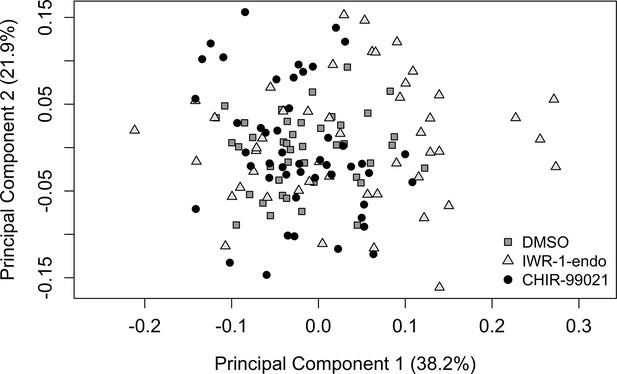
Scatter plot illustrating all individual tooth data points from principal component analysis illustrated in Figure 6C.
There is an increase in the variation of dental shapes across the PC1 axis following Wnt downregulation with IWR-1-endo relative to DMSO control teeth (pairwise F-test: F41,47 = 0.277, p<0.001).
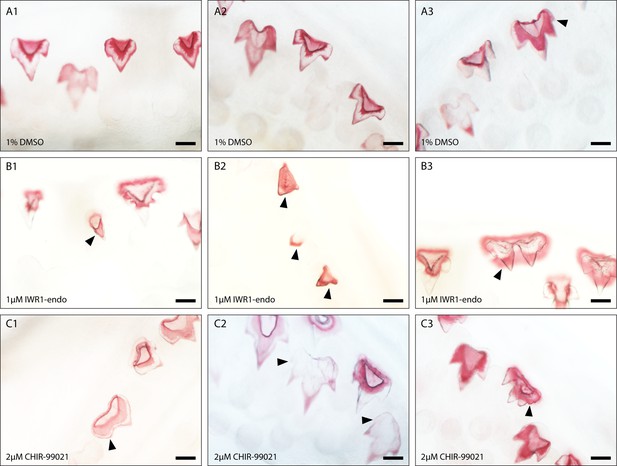
Dental diversity following canonical Wnt manipulation.
Selected images depicting dental diversity following 0.1% DMSO (control) (A1–A3), 1 μM IWR-1-endo (B1–B3), and 2 μM CHIR9902 (C1–C3) treated lower jaws following 2-week treatment and 4-week recovery. 0.1% DMSO are relatively similar in shape, although the presence of 4 cusps is observed in a small subset of teeth (A3). Following 1 μM IWR-1-endo treatment, there are numerous mineralised unicuspid and stunted teeth (B1 and B2). There are also teeth which appear duplicated in nature, but which are connected to a single root (B3). In contrast, 2 μM CHIR9902 treatment leads to a widening of the teeth and defects in the position of cups (C1) and the development of supernumerary cusps (C2 and C3). Black arrowheads point to cusp defects and/or shifts from the typical tricuspid dental morphology. Scale bars are 100 μm.
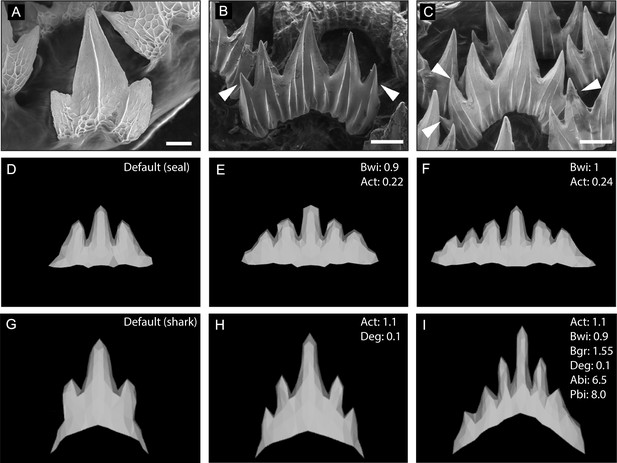
In silico modelling of the catshark dentition.
Wild-type scanning electron microscope (SEM) images of embryonic (A) and juvenile (B and C) catshark samples show a shift in cusp number from 3 to 6 cusps during ontogeny. The computational model ‘ToothMaker’ (Salazar-Ciudad and Jernvall, 2010) was used to generate in silico models of the dentition using baseline seal parameters (D–F) and parameters refined to produce the characteristic shark dental morphology (G–I). Modification to the default seal (D; Supplementary file 1: seal) and default shark (G; Supplementary file 1: shark) parameters to generate 5-cusped (E and H) or 6-cusped (H–I) teeth are displayed in the individual panels. 11,000 iterations of the model were run when modelling dentition. White arrowheads represent the addition of extra cusps during ontogeny. Scale bars are 50 μm, and 200 μm in B and C.
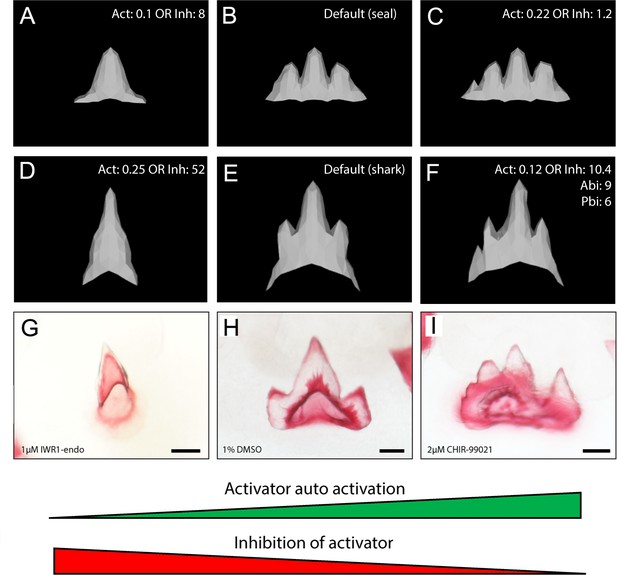
In silico modelling recreates catshark tooth phenotypes comparable to canonical Wnt manipulation.
In silico modelling using baseline seal parameters (A–C) and parameters refined to produce the characteristic shark dental morphology (D–F) is also capable of reproducing dental morphologies observed following chemical treatment (G, I). 1 μM IWR-1-endo resulted in unicuspid teeth (G), whereas 2 μM CHIR9902 (I) resulted in the development of supernumerary cusps. DMSO controls exhibit a normal tricuspid dentition (H). Either an increase in activator auto-activation (Act) or decrease in inhibition of activator (Inh) is sufficient to shift teeth from a unicuspid (A,D) to tricuspid (B,E) and quadricuspid (C) morphology. Modification to the default seal (B; Supplementary file 1: seal) and default shark (E; Supplementary file 1: shark) are displayed in the individual panels. 11,000 iterations of the model were run when modelling the effect of small molecule treatment. Scale bars are 50 μm in D–F.
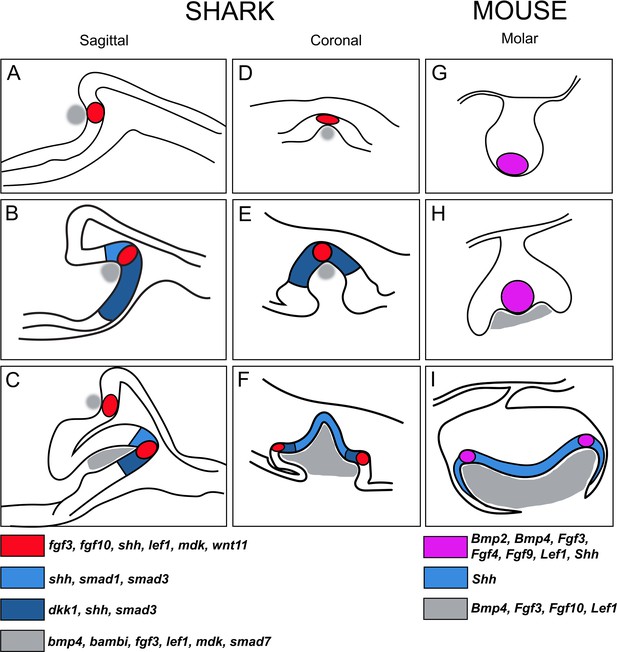
Schematic summary of the progression of enamel knot (EK)-like signalling centres in shark teeth versus the EKs during mouse molar development.
(A–C) Representative sagittal images (based on histological sections) through stages of tooth morphogenesis in the shark (Scyliorhinus canicula). (A) Initial expression within cells of the pEK-like structure (red; fgf3, fgf10, shh, lef1, mdk, wnt11) in the first, superficial upper jaw tooth position. (B) Expression of shh expands in the inner dental epithelium beyond the restriction of the EK-like unit (red in A), together with expression of other markers of the IDE (blue), for example, smad1, smad3, and dkk1. Other markers of the apical cells remain restricted, for example, fgf3, fgf10. (C) Further apical signalling occurs as new replacement teeth emerge within the successional lamina. Mesenchymal expression is shown in grey (bmp4, bambi, fgf3, lef1, mdk, and smad7). (D–F) Comparative coronal sections through stages of shark tooth morphogenesis, with (F) additional secondary EK-like signalling centres (sEK) associated with accessory cusps (2 and 3) in the shark tricuspid tooth (red); (F) expanded shh expression observed in the primary cusp (blue) during later morphogenesis. (G–I) Documented expression of genes in the pEK (G and H; although these two signalling centres in G and H represent different structures in time and place as described by Prochazka et al., 2010; Mogollón et al., 2021; Sadier et al., 2019) and sEK (I) during mammalian molar morphogenesis, showing somewhat equivalent expression and expanded Shh expression in the inner dental epithelial cells (IDE; I). EK and EK-like units in mouse and shark, respectively, both are restrictive and non-proliferative cell clusters, however, mouse EKs (magenta) are also apoptotic, a character not yet defined in the shark EK-like unit.
Additional files
-
Supplementary file 1
ToothMaker parameters.
Default ToothMaker parameters set for baseline seal tricuspid tooth and baseline shark tricuspid tooth in Figures 8 and 9 (Savriama et al., 2018). Shifted parameters in red.
- https://cdn.elifesciences.org/articles/73173/elife-73173-supp1-v2.docx
-
Supplementary file 2
Primer sequences.
Primer sequences used to generate the RNA probes for in situ hybridisation in Figures 2—5 (and Figure 2—figure supplement 1).
- https://cdn.elifesciences.org/articles/73173/elife-73173-supp2-v2.docx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/73173/elife-73173-transrepform1-v2.docx





