A gene regulatory network for neural induction
Figures
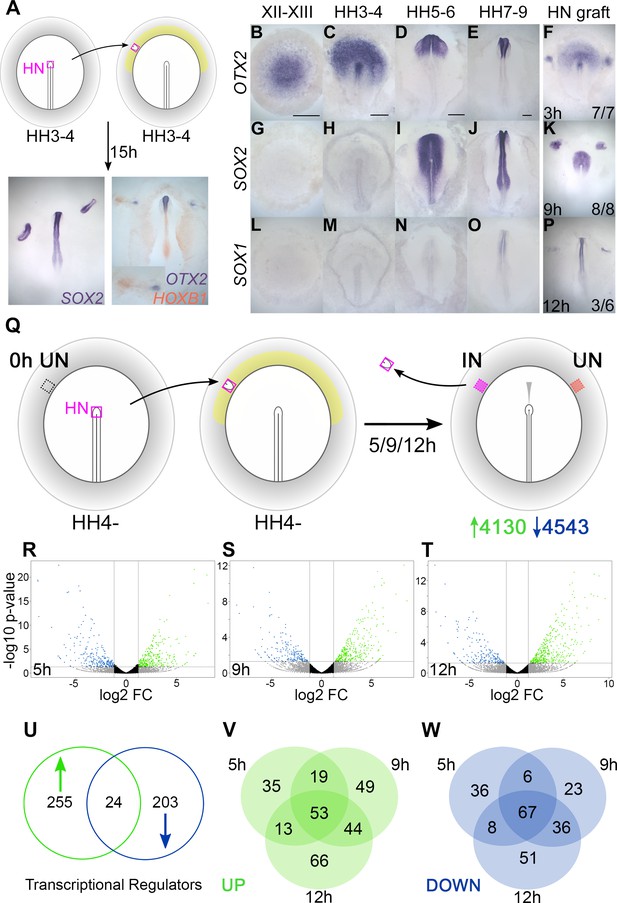
Transcriptional profiling identifies responses to neural induction in time course.
(A) Hensen’s node (HN) was grafted from HH3-4 donors to the inner area opaca (yellow) of hosts at the same stage. An ectopic neural tube expressing SOX2, OTX2, and HOXB1 is induced after 15 hr of culture. (B–F) Expression of neural markers compared to their time course of induction by a node graft: OTX2; first expressed in pre-streak epiblast (EGKXII-XIII) and induced by grafts after 3 hr. (G–K) SOX2; first expressed in the neural plate at HH5-6 and induced after 9 hr. (L–P) SOX1; first expressed in the forming neural tube at HH7-8 and induced after 12 hr. (Q) Identifying transcriptional responses to a node graft. HN was grafted from HH4- donors to HH4- hosts. The HN graft was removed and underlying ‘induced’ (IN) and contralateral ‘uninduced’ (UN) ectoderm isolated after 5, 9, or 12 hr. Uninduced ‘0 hr’ ectoderm from HH4- embryos was also dissected. Samples were analysed by RNA-sequencing (RNAseq). (R–T) Induced and corresponding uninduced tissues were compared at each time point to identify differentially expressed transcripts. Volcano plots show upregulated (green) and downregulated (blue) transcripts. (U–W) Venn diagrams of 482 genes encoding transcriptional regulators (U) that are upregulated (V) or downregulated (W) at different time points. Scale bars: B: 1mm; C: 250 μm; D: 250 μm; E: 250 μm. These scale bars apply to all other figures with embryos at equivalent stages throughout the paper.
-
Figure 1—source data 1
RNAseq galGal3 analysis key and differentially expressed transcripts.
- https://cdn.elifesciences.org/articles/73189/elife-73189-fig1-data1-v2.xlsx
-
Figure 1—source data 2
RNAseq galGal4 analysis key and differentially expressed transcripts.
- https://cdn.elifesciences.org/articles/73189/elife-73189-fig1-data2-v2.xlsx
-
Figure 1—source data 3
Nanostring probe codeset.
- https://cdn.elifesciences.org/articles/73189/elife-73189-fig1-data3-v2.xlsx
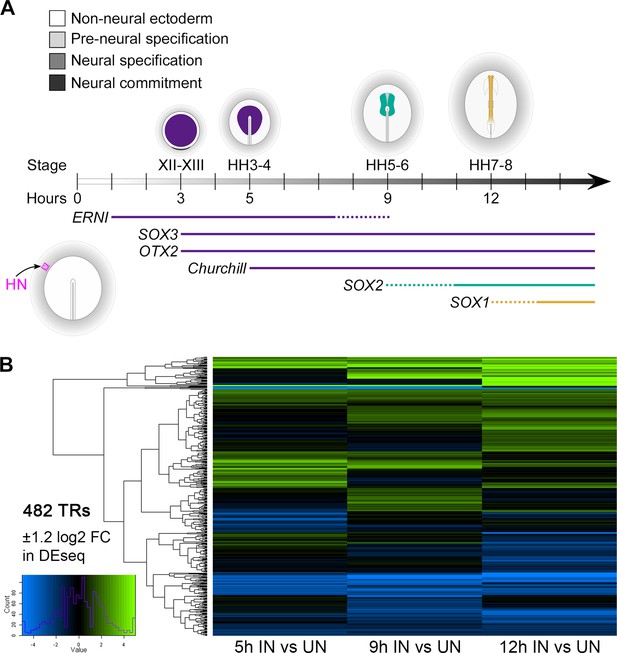
Further transcriptional responses to neural induction in time course.
(A) Neural induction occurs as a sequence of events. Extraembryonic ‘non-neural’ ectoderm does not normally contribute to the nervous system. Three hours after a graft of Hensen’s node (HN), this tissue expresses the ‘pre-neural’ markers ERNI, SOX3, and OTX2. The neural plate marker SOX2 is induced after 9 hr. Neural commitment is associated with the induction of SOX1 expression after 12 hr. The relative timing of the induction of these genes is equivalent to the order in which they are expressed during normal neural development in the embryo. Pre-neural genes are expressed in the pre-streak epiblast at EGKXII-XIII, whereas SOX2 and SOX1 expression begins later, respectively, in the neural plate at HH5-6 and neural tube at HH7-8 (updated from Pinho et al., 2011). (B) Heat map representing 482 genes encoding transcriptional regulators that are differentially expressed in response to 5, 9, or 12 hr of a node graft. (Genes were selected with threshold ±1.2 log2 fold change between induced and uninduced at each time point.)
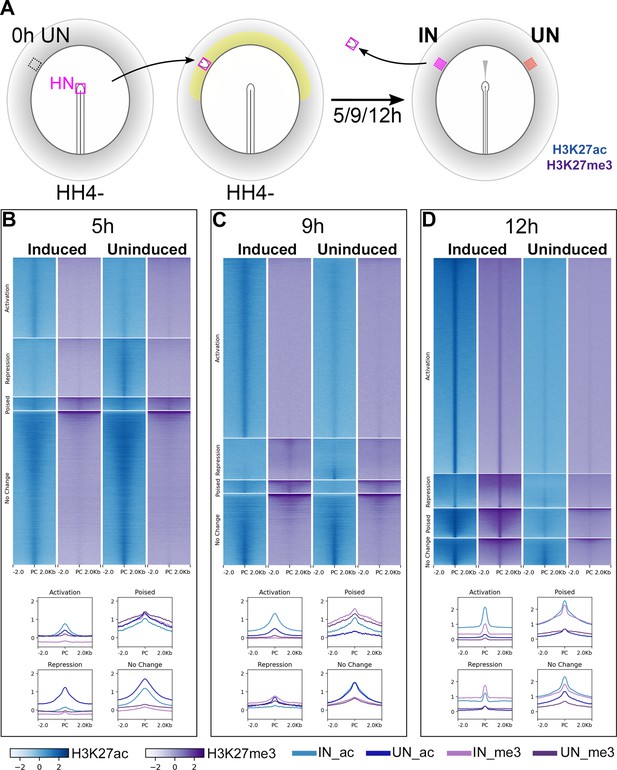
Epigenetic changes identify chromatin elements associated with neural induction.
(A) Hensen’s node (HN at HH4-) was grafted to hosts of the same stage. Embryos were cultured for 5, 9, or 12 hr before HN was removed and induced (IN) and contralateral uninduced (UN) ectoderm collected. ChIPseq was performed for H3K27ac and H3K27me3. (B–D) Putative regulatory sites were predicted according to the H3K27 profiles of IN and UN tissues at each time point (see Figure 2—figure supplement 1). They include sites undergoing ‘activation’ or ‘repression’, being ‘poised’, or showing no change. Heat maps illustrate the enrichment of H3K27ac (blue) and H3K27me3 (purple) in IN and UN tissues within ±2.0 kb from the peak centre (PC) for each annotated group. Graphs plot the distributions of H3K27ac and H3K27me3 enrichment for each group.
-
Figure 2—source data 1
ChIPseq tissue dissociation details.
- https://cdn.elifesciences.org/articles/73189/elife-73189-fig2-data1-v2.xlsx
-
Figure 2—source data 2
ATACseq indices.
- https://cdn.elifesciences.org/articles/73189/elife-73189-fig2-data2-v2.xlsx
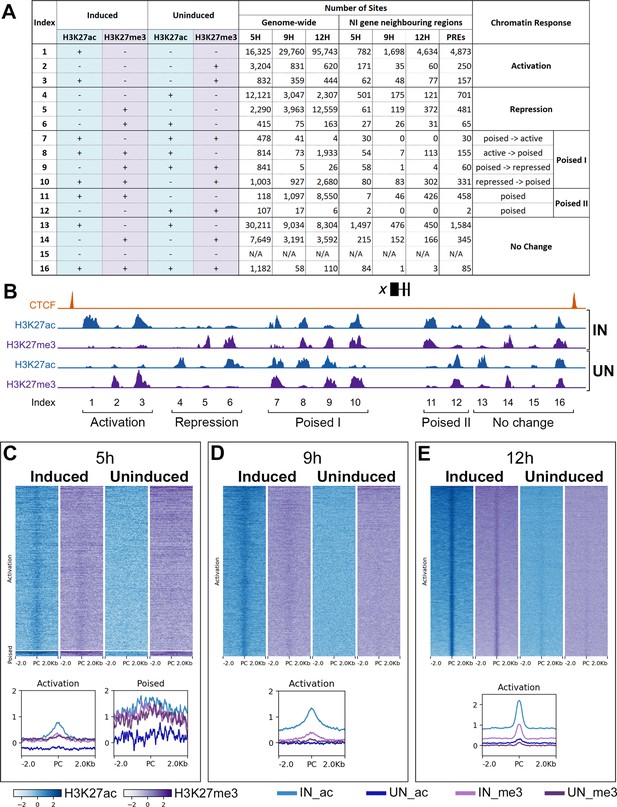
Further chromatin profiles during neural induction.
(A) Genomic regions can be categorized into 16 indices based on the different combinations of H3K27ac and H3K27me3 marks across induced and contralateral uninduced ectoderm at each time point. The number of sites belonging to each index is listed genome-wide. The numbers of sites which fall within the regulatory loci of neural induction associated genes (see B) are also listed. These correspond to a defined number of non-overlapping putative regulatory elements (PREs) across all time points. (B) Sites with changing chromatin marks were identified within loci of 213 transcriptional regulators. Loci of up to 500 kb were predicted using CTCF ChIPseq data for constitutive CTCF binding sites. Within these, chromatin sites were indexed according to the their H3K27 profiles. (C–E) Chromatin sites belonging to Indices 1, 2, 3, and 7 were selected from the regions neighbouring 213 genes. These are represented by heat maps which plot their H3K27ac (blue) and H3K27me3 (purple) profiles across induced and uninduced tissues ±2.0 kb of the peak centre (PC). Graphs plot the mean H3K27ac and H3K27me3 distribution profiles for marks for each group of responses.
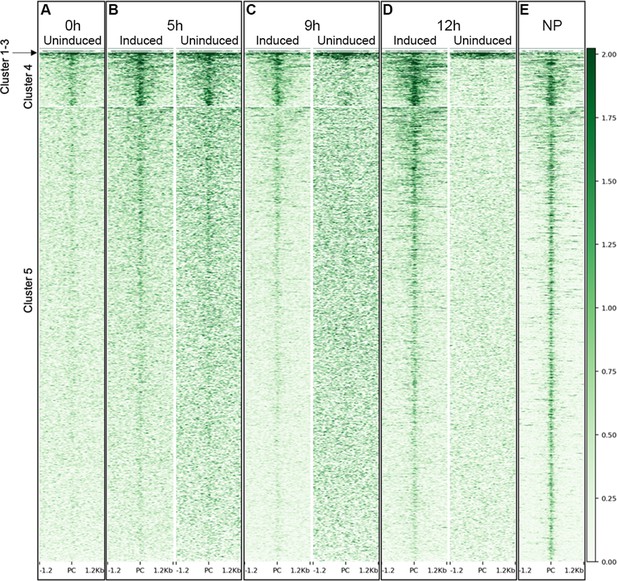
ATACseq identifies open chromatin during neural induction.
(A–E) Heat maps illustrate the chromatin accessibility of putative regulatory sites ±1.5 kb from the peak centre (PC) in induced (IN) and uninduced (UN) tissues for each annotated group defined in Figure 2—figure supplement 1A.
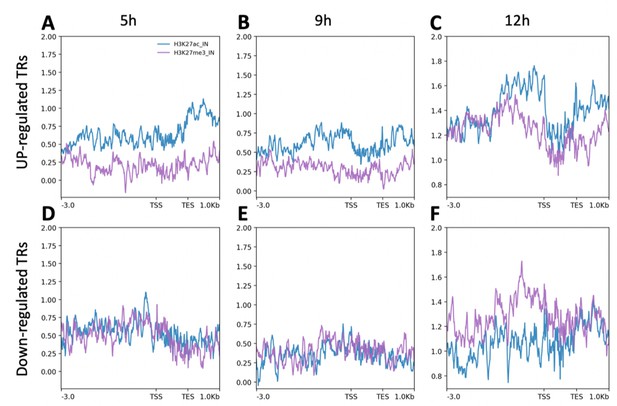
H3K27 histone marks flanking transcriptional regulators that are differentially expressed during neural induction.
(A–F) Enrichment of H3K27ac and H3K27me3 at the flanking regions (3.0 kb upstream of transcription start sites [TSS] and 1 kb downstream of transcription end sites [TES]) of transcriptional regulators that are upregulated (A–C) or downregulated (D–F) at 5, 9, or 12 hr.
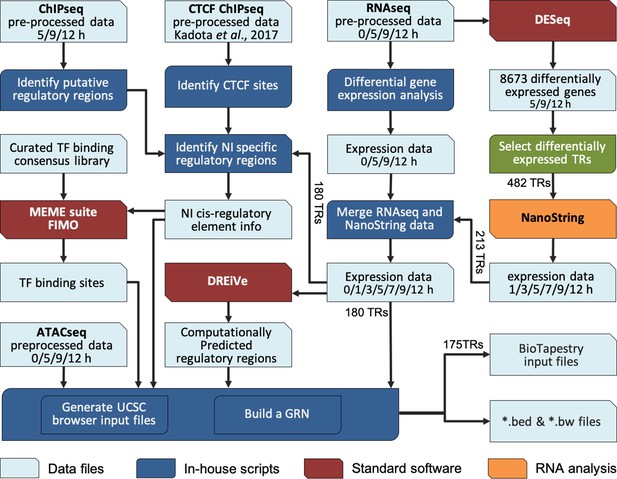
Constructing a BioTapestry model for regulatory gene interactions: computational workflow.
Computational pipeline to combine transcriptomic and epigenetic time course data, to build a gene regulatory network (GRN) for neural induction. The output data are available to view in the UCSC browser.
-
Figure 3—source data 1
Integrated GRN gene expression profile.
- https://cdn.elifesciences.org/articles/73189/elife-73189-fig3-data1-v2.xlsx
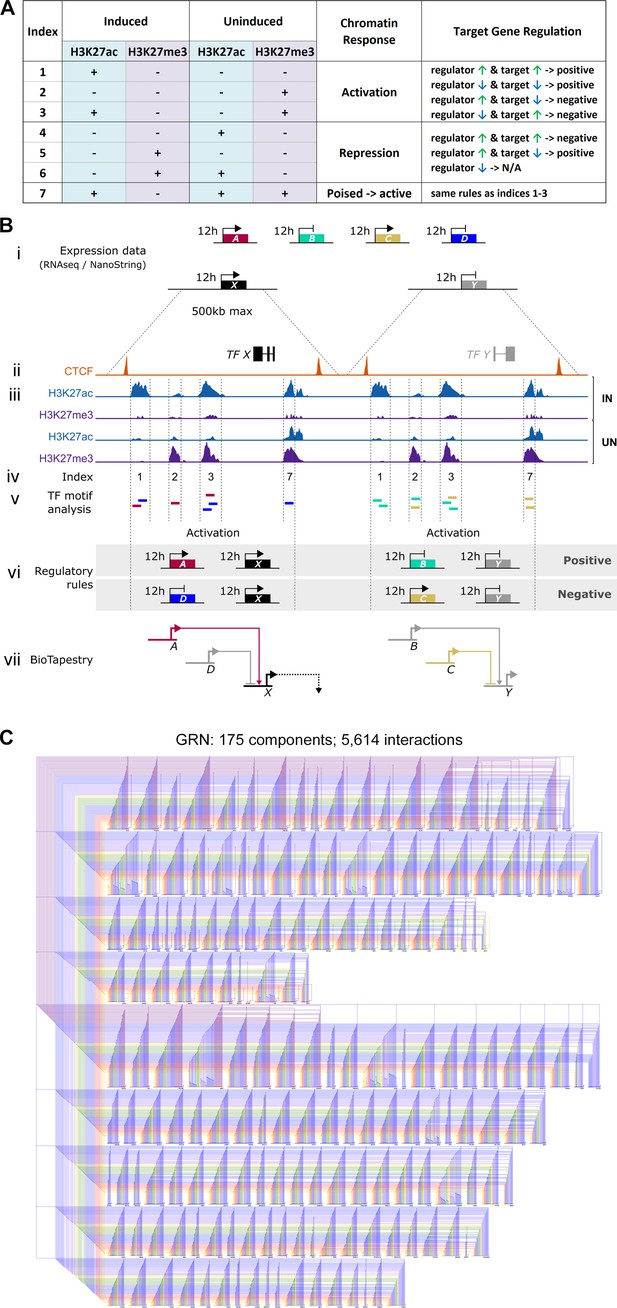
Constructing a BioTapestry model for regulatory gene interactions: the regulatory logic.
(A) Network interactions were modelled using chromatin sites that belong to Indices 1–7 and the defined rules between regulators and their potential targets. At sites that become active in induced tissues (Indices 1, 2, 3, and 7): positive interactions are depicted when a putative input and its target are co-regulated and negative (inhibitory) interactions are modelled when an input and its target have opposing expression profiles. These rules are reversed at sites that undergo repression in induced tissues (Indices 4, 5, and 6) except that interactions are not predicted when the potential regulators of these repressed sites are not expressed. (B) Schematic depicting how expression and chromatin profiles were combined to predict interactions between gene regulatory network (GRN) components. (i) RNA-sequencing (RNAseq) and NanoString expression data provide a list of 213 transcriptional regulators that are upregulated (genes A, C, and X) or downregulated (genes B, D, and Y) after 0, 1, 3, 5, 7, 9, or 12 hr of a node graft. (ii) For these candidate GRN components, CTCF-ChIPseq data was used to predict neighbouring CTCF-bound domains of up to 500 kb. (iii) Within these, sites that are enriched for H3K27ac or H3K27me3 were identified by ChIPseq performed on induced and uninduced tissues at 5, 9, or 12 hr. (iv) Chromatin sites were categorized (according to Figure 2—figure supplement 1A) and those belonging to Indices 1, 2, 3, and 7 were selected to build a network. (v) These were screened for transcription factor binding motifs that correspond to other GRN components. This identifies genes A and D as potential regulators of target X and genes B and C as potential regulators of target Y. (vi) At each time point, the consequence of interactions was predicted according to the regulatory rules in A. Positive interactions are depicted when a putative input and its target are co-regulated. Negative (inhibitory) interactions are modelled when an input and its target have opposing expression profiles. (vii) Predicted interactions are modelled in BioTapestry using arrows for positive interactions and blunt ends for inhibitory interactions. Components are shown in colour at time points when they are expressed or shaded grey when they are not. (C) A GRN comprising 5614 predicted interactions between 175 components is visualized using BioTapestry. Each component is coloured by the time point when it first starts to express at 0 hr (purple), 1 hr (grey-blue), 3 hr (blue), 5 hr (green), 7 hr (yellow), 9 hr (orange), and 12 hr (red).
-
Figure 4—source data 1
List of GRN transcription factor binding sites within regulatory sites at 5h, 9h and 12h following a node graft.
- https://cdn.elifesciences.org/articles/73189/elife-73189-fig4-data1-v2.xlsx
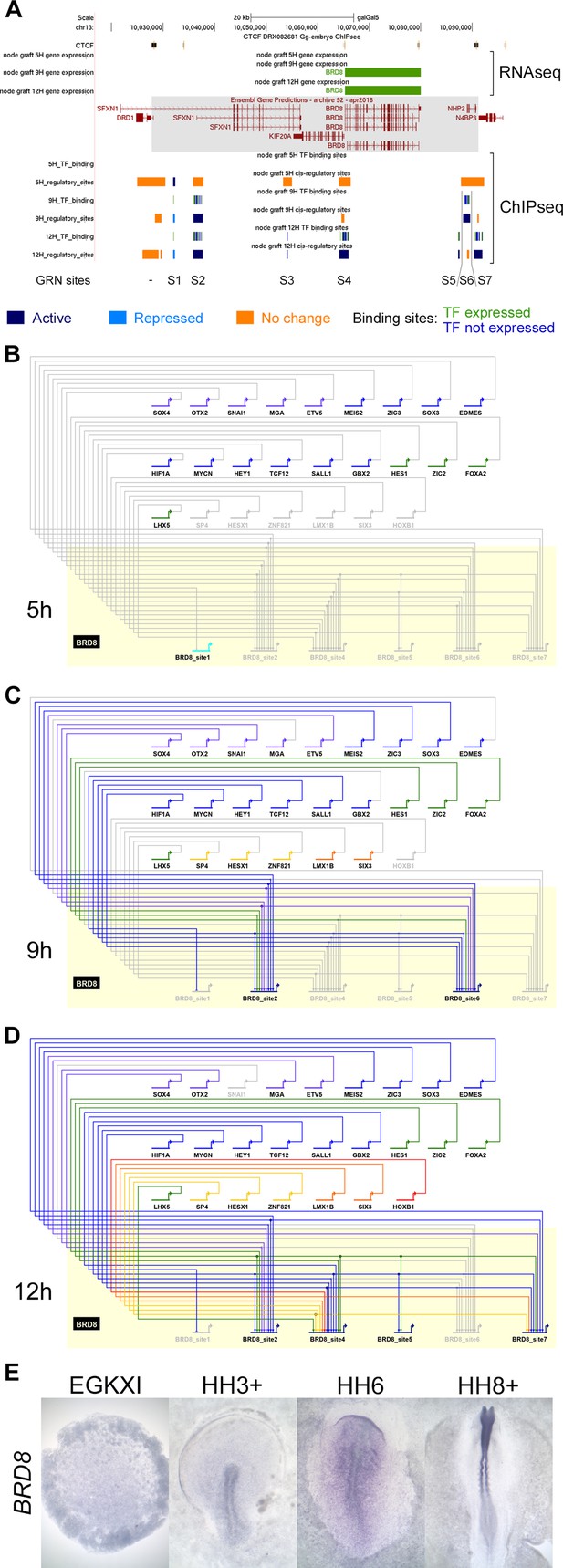
A subnetwork incorporating individual regulatory sites and their dynamics.
(A) UCSC browser view of RNA-sequencing (RNAseq) and ChIPseq tracks associated with BRD8. BRD8 is upregulated after 9 and 12 hr of neural induction (green bars). The BRD8 regulatory locus (grey box; chr13:10028086–10091079) is defined by flanking CTCF-bound sites. Seven putative regulatory sites (S1-7) within this domain were predicted based on the ChIPseq H3K27 profiles. This includes sites that undergo activation (Indices 1–3, 7, coloured in dark blue), repression (Indices 4–6, coloured in cyan), or show no change (coloured in orange). Transcription factor binding sites by network components are shown; green for components that are expressed at the same time point and blue for those that are not. A BioTapestry subnetwork was generated from these predicted binding sites and expression profiles. Site 3, active at 12 hr, is not shown in the subnetwork as there is no TF that is expressed at 12 hr and predicted to bind to it. (B) BRD8 regulation during neural induction: BRD8 is initially not expressed; site 1 is active but is not predicted to be bound by other gene regulatory network (GRN) components. Each GRN component is coloured according to the time point when it first starts to express at 0 hr (purple), 1 hr (grey-blue), 3 hr (blue), 5 hr (green), 7 hr (yellow), 9 hr (orange), and 12 hr (red). (C) BRD8 is upregulated after 9 hr; sites 2 and 6 undergo activation and could be bound by various transcription factors that are also expressed. Site 1 undergoes repression. (D) BRD8 expression is maintained at 12 hr; regulators potentially bind to sites 2, 4, 5, and 7. Site 6 is no longer predicted to be active. (E) In situ hybridization detects BRD8 transcripts in the neural plate at HH6 and neural tube at HH8+, but not at earlier stages, EGKXI and HH3+.
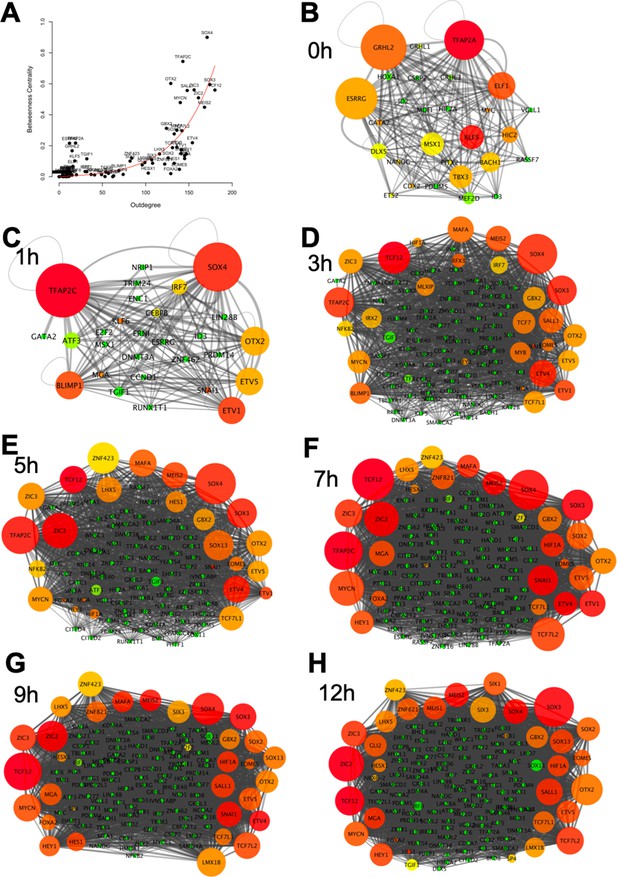
Identifying core regulatory factors in the neural induction gene regulatory network (GRN).
(A) Correlation between the number of regulatory output (outdegree) and the degree of centrality of genes. (B–H) Network interactions at 0, 1, 3, 5, 7, 9, and 12 hr. The size of a node indicates the degree of the centrality of a gene. The degree of regulatory outputs is represented with the following colour scheme: red, orange, yellow, and green indicate high, medium, to low degree of regulatory outputs.
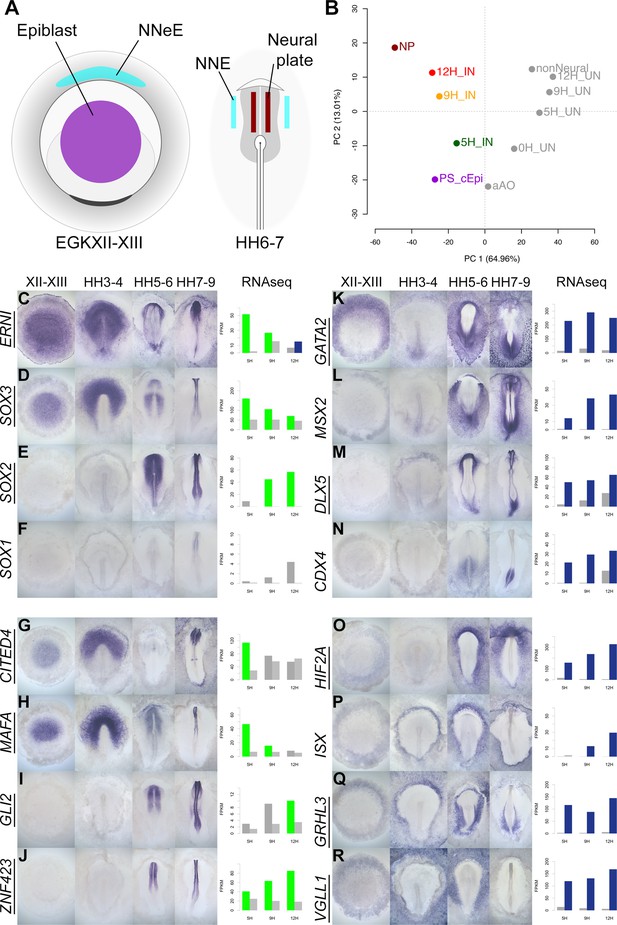
A spatiotemporal map of the normal embryo for transcripts differentially expressed during neural induction.
(A) Central epiblast from pre-primitive-streak stage embryos (PS_cEpi) at EGKXII-XIII, neural plate (NP) at HH6-7, and corresponding non-neural extraembryonic ectoderm were dissected and processed by RNA-sequencing (RNAseq). (B) Principal component analysis comparing prospective neural and non-neural tissues from the normal embryo to induced (IN) and uninduced (UN) ectoderm 5, 9, or 12 hr after a node graft. (C–R) In situ hybridization of genes encoding transcriptional regulators at four stages of embryonic development (EGKXII-XIII, HH3-4, HH5-6, and HH7-9) compared to their RNAseq expression after 5, 9, and 12 hr of a node graft. Bar charts plotted RNAseq values (FPKM) in induced and uninduced tissues. Bars are shaded green when genes are upregulated in induced tissue (FPKM >10 in induced tissue and fold change (FC) >1.5 compared to uninduced). Bars are shaded blue when genes are downregulated (FPKM >10 in uninduced tissue and FC <0.5 compared to uninduced). When a gene is neither upregulated nor downregulated, the bars are coloured in dark grey in induced and light grey in uninduced. Genes represented within the neural induction GRN are underlined.
-
Figure 7—source data 1
Sumary of changes for upregulated transcriptional regulators based on in situ expression patterns.
- https://cdn.elifesciences.org/articles/73189/elife-73189-fig7-data1-v2.xlsx
-
Figure 7—source data 2
Summary of changes in downregulated transcriptional regulators based on in situ expression patterns.
- https://cdn.elifesciences.org/articles/73189/elife-73189-fig7-data2-v2.xlsx
-
Figure 7—source data 3
Details of probes generated by digestion of cDNAs that were used for in situ hybridisation.
- https://cdn.elifesciences.org/articles/73189/elife-73189-fig7-data3-v2.xlsx
-
Figure 7—source data 4
Details of probes generated by PCR that were used for in situ hybridisation.
- https://cdn.elifesciences.org/articles/73189/elife-73189-fig7-data4-v2.xlsx
-
Figure 7—source data 5
PCR primer sequences.
- https://cdn.elifesciences.org/articles/73189/elife-73189-fig7-data5-v2.xlsx
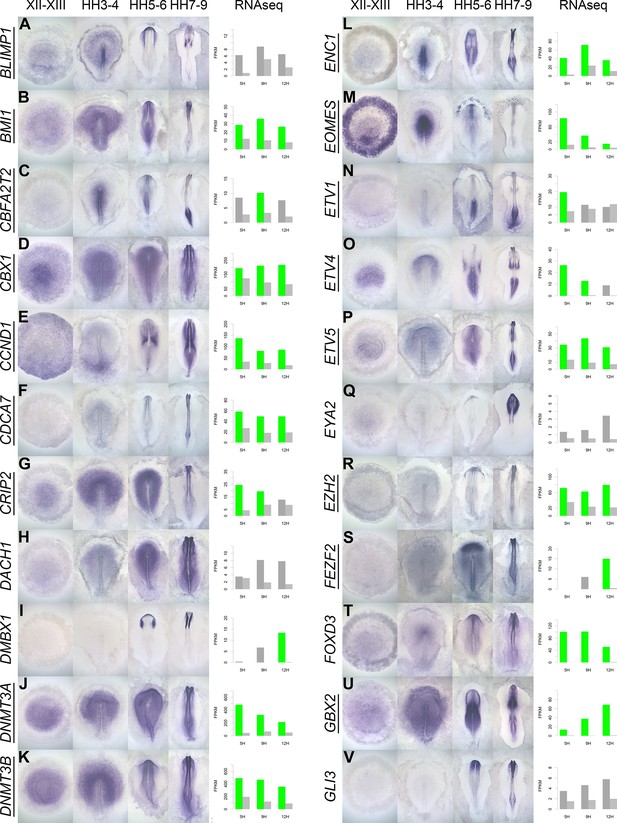
A spatiotemporal map in the normal embryo of transcripts that are differentially expressed during neural induction.
(A–V) In situ hybridization of genes encoding transcriptional regulators at four stages of embryonic development (EGKXII-XIII, HH3-4, HH5-6, and HH7-9) compared to their RNA-sequencing (RNAseq) expression after 5, 9, and 12 hr of a node graft. Induced values are plotted in dark grey and uninduced in light grey. Columns are shaded green when induced FPKM values are upregulated (1.5 fold change compared to uninduced) and >10. Columns are shaded blue when genes are downregulated (0.5 fold change compared to uninduced) and uninduced FPKM values are >10. Genes that are represented within the neural induction gene regulatory network (GRN) are underlined.
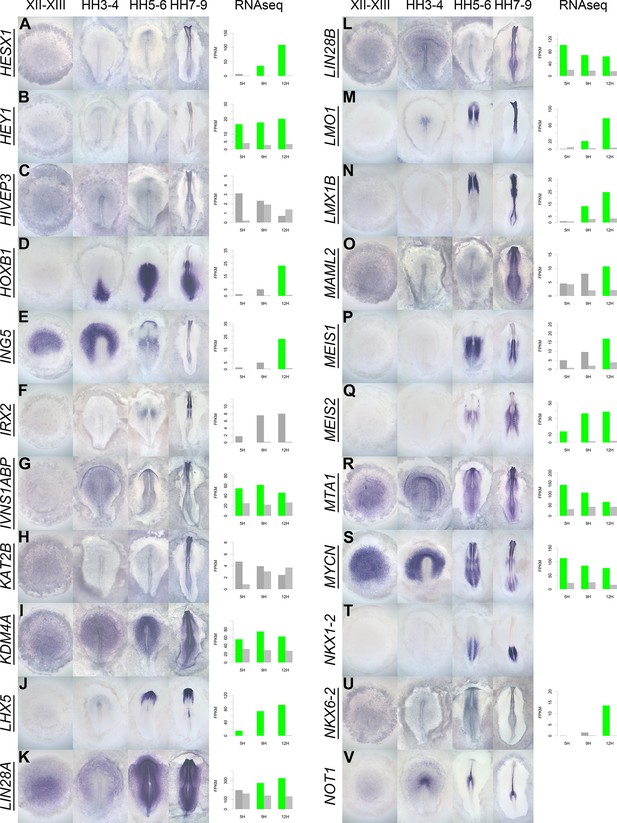
A spatiotemporal map in the normal embryo of transcripts that are differentially expressed during neural induction.
(A–V) In situ hybridization of genes encoding transcriptional regulators at four stages of embryonic development (EGKXII-XIII, HH3-4, HH5-6, and HH7-9) compared to their RNA-sequencing (RNAseq) expression after 5, 9, and 12 hr of a node graft. Induced values are plotted in dark grey and uninduced in light grey. Columns are shaded green when induced FPKM values are upregulated (1.5 fold change compared to uninduced) and >10. Columns are shaded blue when genes are downregulated (0.5 fold change compared to uninduced) and uninduced FPKM values are >10. Genes that are represented within the neural induction gene regulatory network (GRN) are underlined.
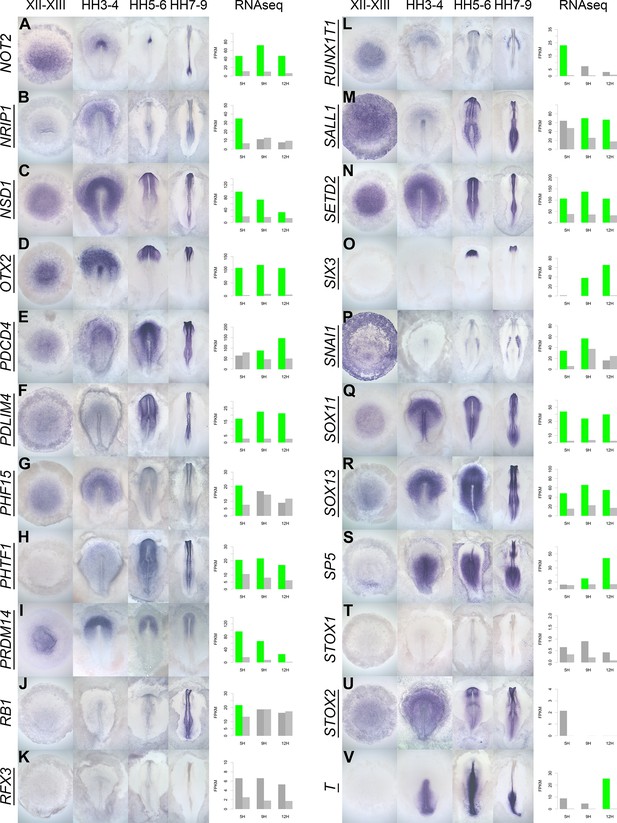
A spatiotemporal map in the normal embryo of transcripts that are differentially expressed during neural induction.
(A–V) In situ hybridization of genes encoding transcriptional regulators at four stages of embryonic development (EGKXII-XIII, HH3-4, HH5-6, and HH7-9) compared to their RNA-sequencing (RNAseq) expression after 5, 9, and 12 hr of a node graft. Induced values are plotted in dark grey and uninduced in light grey. Columns are shaded green when induced FPKM values are upregulated (1.5 fold change compared to uninduced) and >10. Columns are shaded blue when genes are downregulated (0.5 fold change compared to uninduced) and uninduced FPKM values are >10. Genes that are represented within the neural induction gene regulatory network (GRN) are underlined.
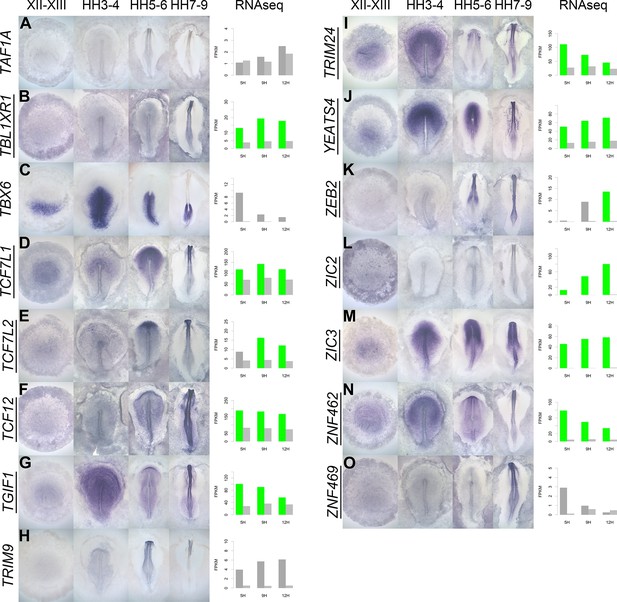
A spatiotemporal map in the normal embryo of transcripts that are differentially expressed during neural induction.
(A–O) In situ hybridization of genes encoding transcriptional regulators at four stages of embryonic development (EGKXII-XIII, HH3-4, HH5-6, and HH7-9) compared to their RNA-sequencing (RNAseq) expression after 5, 9, and 12 hr of a node graft. Induced values are plotted in dark grey and uninduced in light grey. Columns are shaded green when induced FPKM values are upregulated (1.5 fold change compared to uninduced) and >10. Columns are shaded blue when genes are downregulated (0.5 fold change compared to uninduced) and uninduced FPKM values are >10. Genes that are represented within the neural induction gene regulatory network (GRN) are underlined.
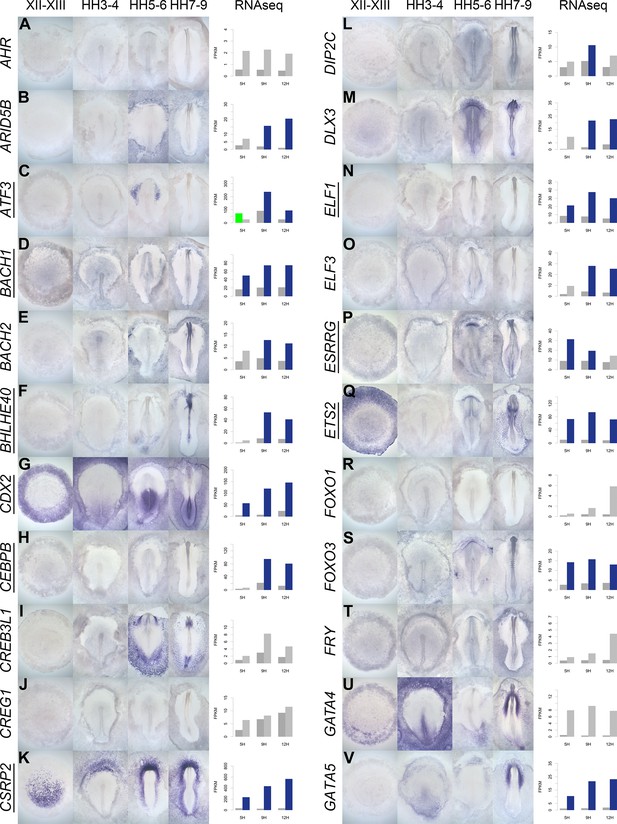
A spatiotemporal map in the normal embryo of transcripts that are differentially expressed during neural induction.
(A–V) In situ hybridization of genes encoding transcriptional regulators at four stages of embryonic development (EGKXII-XIII, HH3-4, HH5-6, and HH7-9) compared to their RNA-sequencing (RNAseq) expression after 5, 9, and 12 hr of a node graft. Induced values are plotted in dark grey and uninduced in light grey. Columns are shaded green when induced FPKM values are upregulated (1.5 fold change compared to uninduced) and >10. Columns are shaded blue when genes are downregulated (0.5 fold change compared to uninduced) and uninduced FPKM values are >10. Genes that are represented within the neural induction gene regulatory network (GRN) are underlined.
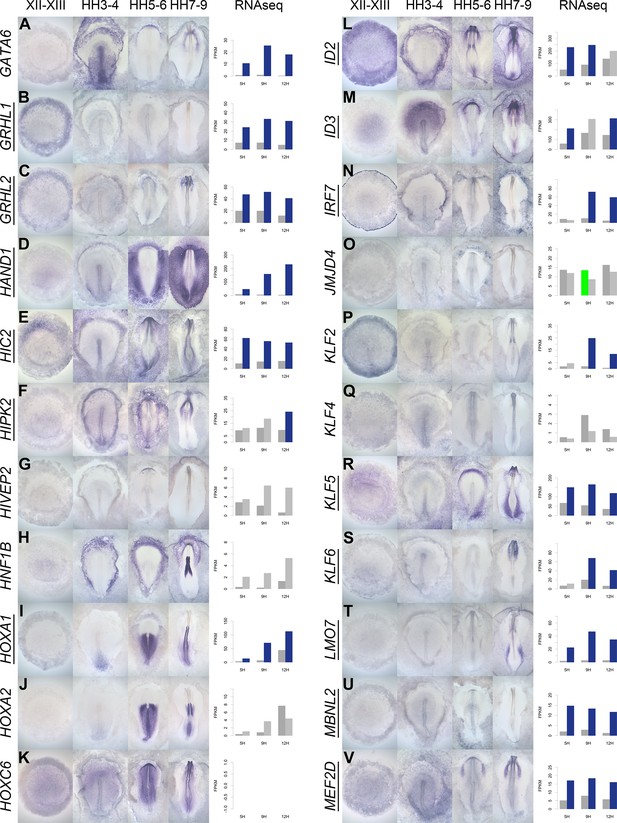
A spatiotemporal map in the normal embryo of transcripts that are differentially expressed during neural induction.
(A–V) In situ hybridization of genes encoding transcriptional regulators at four stages of embryonic development (EGKXII-XIII, HH3-4, HH5-6, and HH7-9) compared to their RNA-sequencing (RNAseq) expression after 5, 9, and 12 hr of a node graft. Induced values are plotted in dark grey and uninduced in light grey. Columns are shaded green when induced FPKM values are upregulated (1.5 fold change compared to uninduced) and >10. Columns are shaded blue when genes are downregulated (0.5 fold change compared to uninduced) and uninduced FPKM values are >10. Genes that are represented within the neural induction gene regulatory network (GRN) are underlined.
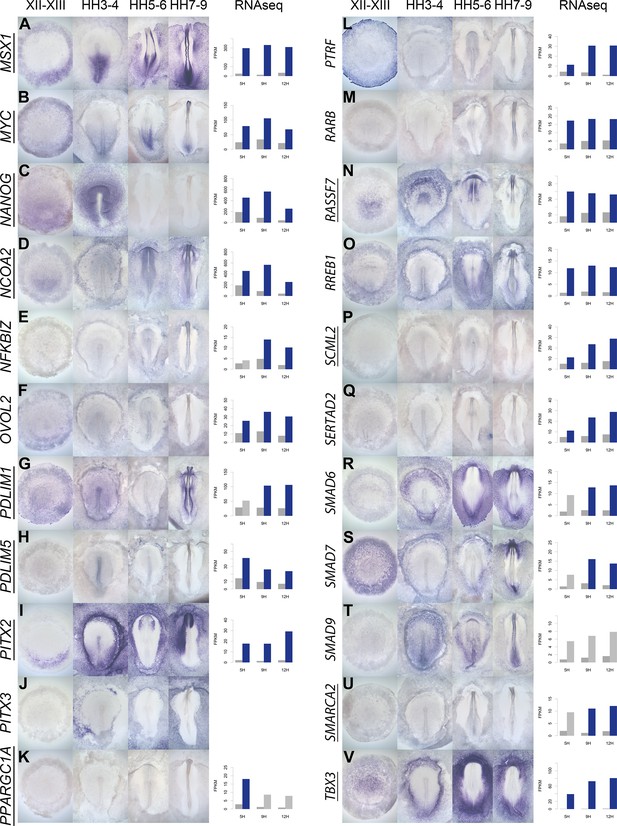
A spatiotemporal map in the normal embryo of transcripts that are differentially expressed during neural induction.
(A–V) In situ hybridization of genes encoding transcriptional regulators at four stages of embryonic development (EGKXII-XIII, HH3-4, HH5-6, and HH7-9) compared to their RNA-sequencing (RNAseq) expression after 5, 9, and 12 hr of a node graft. Induced values are plotted in dark grey and uninduced in light grey. Columns are shaded green when induced FPKM values are upregulated (1.5 fold change compared to uninduced) and >10. Columns are shaded blue when genes are downregulated (0.5 fold change compared to uninduced) and uninduced FPKM values are >10. Genes that are represented within the neural induction gene regulatory network (GRN) are underlined.
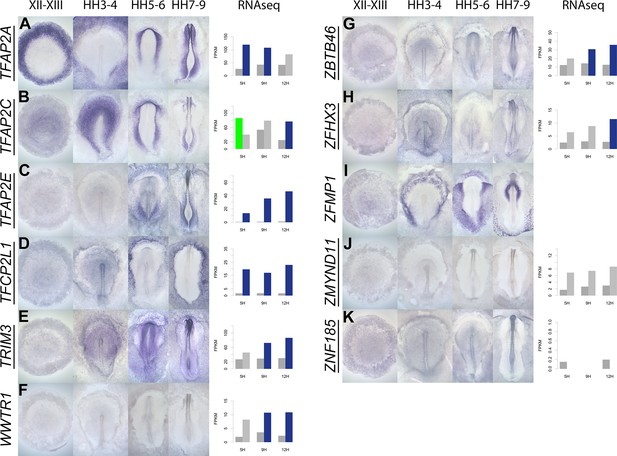
A spatiotemporal map in the normal embryo of transcripts that are differentially expressed during neural induction.
(A–K) In situ hybridization of genes encoding transcriptional regulators at four stages of embryonic development (EGKXII-XIII, HH3-4, HH5-6, and HH7-9) compared to their RNA-sequencing (RNAseq) expression after 5, 9, and 12 hr of a node graft. Induced values are plotted in dark grey and uninduced in light grey. Columns are shaded green when induced FPKM values are upregulated (1.5 fold change compared to uninduced) and >10. Columns are shaded blue when genes are downregulated (0.5 fold change compared to uninduced) and uninduced FPKM values are >10. Genes that are represented within the neural induction gene regulatory network (GRN) are underlined.
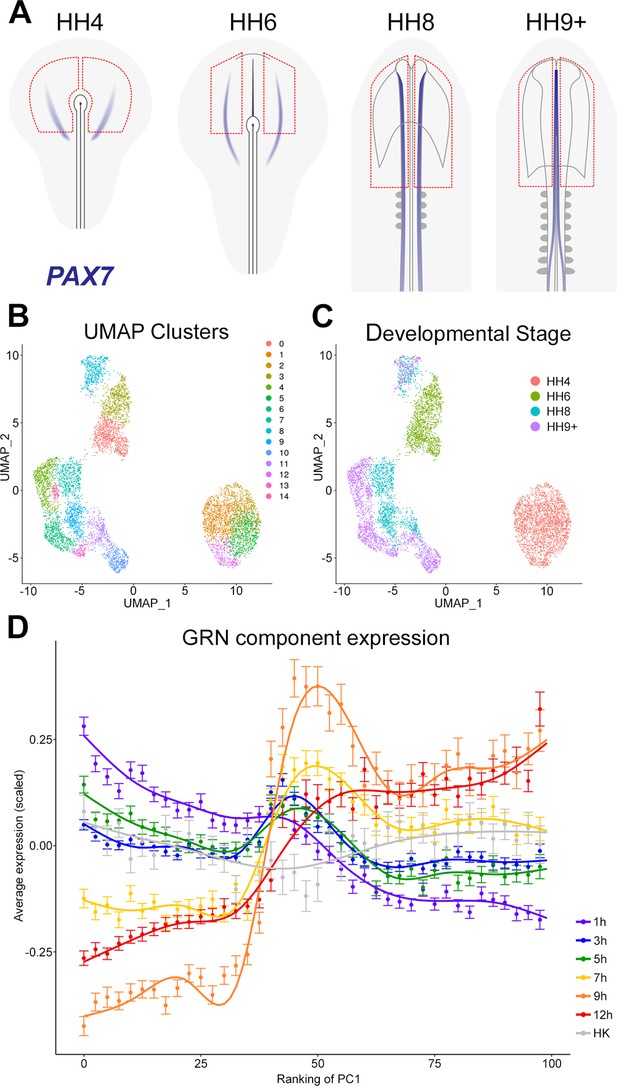
The gene regulatory network (GRN) describes a temporal hierarchy occurring in single cells during neural development.
(A) Broad regions of prospective neural plate, neural plate border and non-neural ectoderm were dissected at HH4, HH6, HH8, and HH9+ and processed for single-cell RNA-sequencing (scRNAseq). PAX7 expression marks the border between neural and non-neural tissues. (B) UMAP displaying unbiased clustering of cells into 15 clusters (0–14) representing distinct cell identities. (C) UMAP displaying the developmental stage from which the cells were collected; cells from HH4 and HH6 form independent clusters, whereas HH8 and HH9+ are more intermixed. (D) Temporal hierarchy of expression of GRN components in single cells during neural plate development. Prospective neural plate and neural tube cells (clusters 0, 1, 2, 5, 6, 7, 9, 13, and 14) were selected and ranked according to PC1 (see Figure 8—figure supplement 1C). The average gene expression (mean ± SE) is plotted for groups of components that are induced at each time point in the GRN, compared to housekeeping genes (HK). A general additive model was fitted to visualize the smoothed expression profile across the ranking of PC1 (using bins of 2.5).
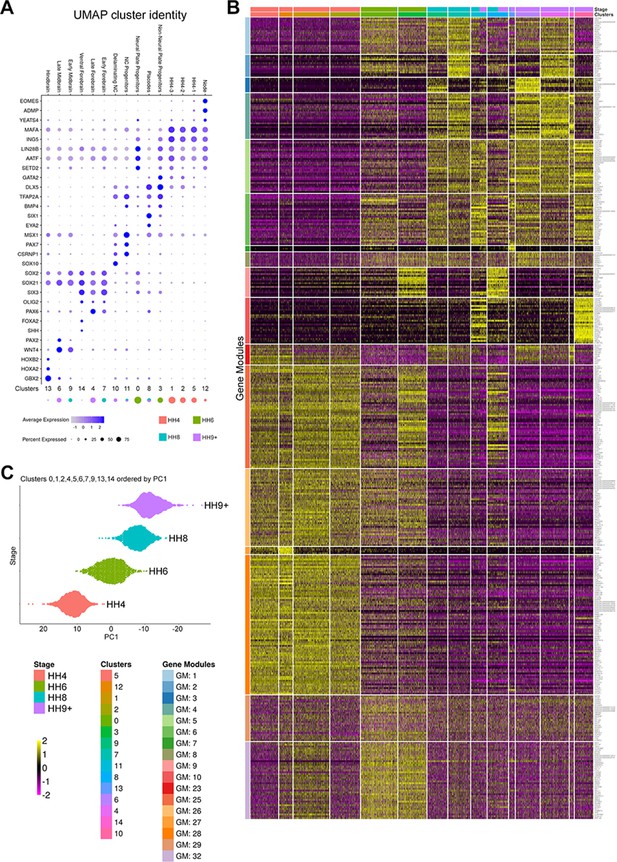
Network components are co-expressed in single cells during neural development.
(A) Identities were assigned to cells in clusters 0–14 based on the expression of known markers. (B) Gene module analysis identifies 17 modules of co-expressed genes associated with clusters 0–14. (C) Prospective neural plate and neural tube cells from clusters 0, 1, 2, 4, 5, 6, 7, 9, 13, and 14 were ranked according to their position along PC1, which inversely correlates with developmental time.
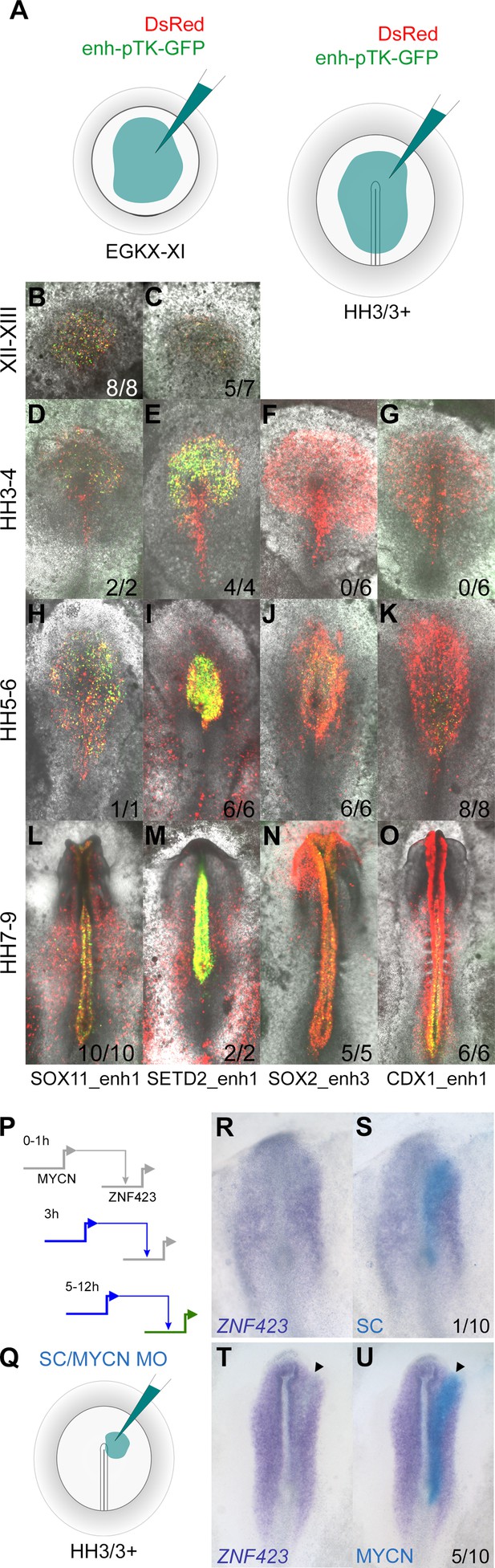
Enhancers identified by the neural induction assay have activity consistent with the expression of the gene during normal neural plate development.
(A) Putative enhancers were cloned into the E-pTK-EGFP vector and co-electroporated into a broad region of the pre-streak stage (EGKX-XI) or HH3+ epiblast together with DsRed. (B–C) Activity of SOX11_enh1 and SETD2_enh1 at EGKXII-XIII; (D–G) activity of SOX11_enh1, SETD2_enh1, SOX2_enh3, and CDX1_enh1 at HH4; (H–K) HH5-6; (L–O) HH7-9. (P–U) Effect of knockdown of predicted network targets by electroporation of a morpholino (MO): a MYCN MO knocks down the expression of predicted target ZNF423, compared to a control MO.
-
Figure 9—source data 1
Sequences of primers used for cloning enhancers.
- https://cdn.elifesciences.org/articles/73189/elife-73189-fig9-data1-v2.xlsx
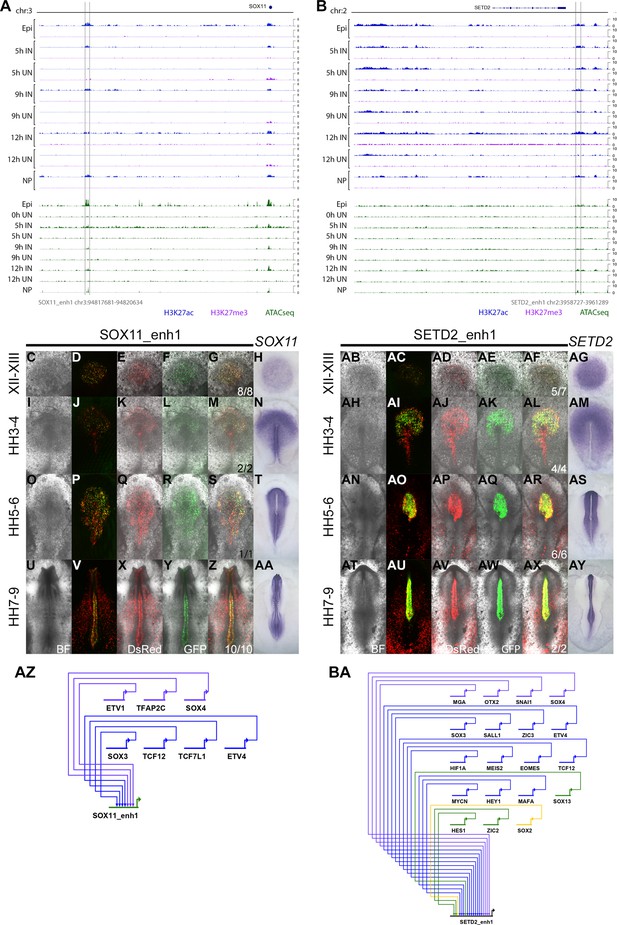
Detailed evidence of SOX11_enh1 and SETD2_enh1 activity.
(A–B) ChIPseq and ATACseq track view for SOX11_enh1 (A) and SETD2_enh1 (B). (C–AA) Brightfield and fluorescence images for DsRed and SOX11_enh1 EGFP activity, compared to SOX11 in situ hybridization at EGKXII-XIII (C–H), HH3-4 (I–N), HH5-6 (O–T), and HH7-9 (U–AA). (AB-AY) Brightfield and fluorescence images for DsRed and SETD2_enh1 EGFP activity, compared to SETD2 in situ hybridization at EGKXII-XIII (AB-AG), HH3-4 (AH-AM), HH5-6 (AN-AS), and HH7-9 (AT-AY). (AZ-BA) Predicted regulators of SOX11_enh1 (AZ) and SETD2_enh1 (BA) at 5 hr.
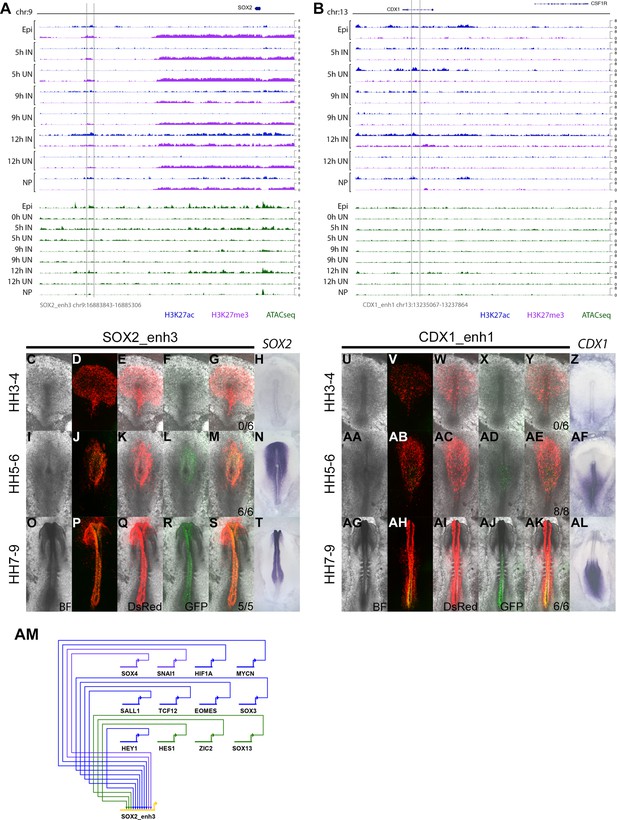
Detailed evidence of SOX2_enh3 and CDX1_enh1 activity.
(A–B) ChIPseq and ATACseq track view of SOX2_enh3 (A) and CDX1_enh1 (B). (C–T) Brightfield and fluorescence images for DsRed and SOX2_enh3 EGFP activity, compared to SOX2 in situ hybridization at HH3-4 (C–H), HH5-6 (I–N), and HH7-9 (O–T). (U–AL) Brightfield and fluorescence images for DsRed and CDX1_enh1 EGFP activity, compared to CDX1 in situ hybridization at HH3-4 (U–Z), HH5-6 (AA-AF), and HH7-9 (AG-AL). (AM) Predicted regulators of SOX2_enh3 at 9 hr.
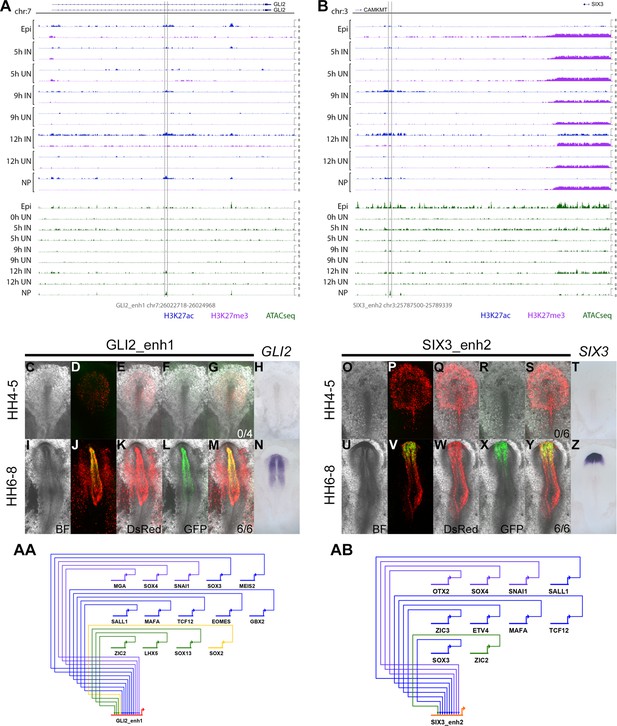
Detailed evidence of GLI2_enh1 and SIX3_enh2 activity.
(A–B) ChIPseq and ATACseq track view of GLI2_enh1 (A) and SIX3_enh2 (B). (C–N) Brightfield and fluorescence images and composites for DsRed and GLI2_enh1 EGFP activity, compared to GLI2 in situ hybridization at HH4-5 (C–H) and HH6-8 (I–N). (O–Z) Brightfield and fluorescence images and composites for DsRed and SIX3_enh2 EGFP activity, compared to SIX3 in situ hybridization at HH4-5 (O–T) and HH6-8 (U–Z). (AA-AB) Predicted regulators of GLI2_enh1 (AA) and SIX3_enh2 (AB) at 9 hr.
Additional files
-
MDAR checklist
- https://cdn.elifesciences.org/articles/73189/elife-73189-mdarchecklist1-v2.pdf
-
Supplementary file 1
BioTapestry File (.BTN) of the full network - to open/view, please download the BioTapestry software from http://www.biotapestry.org.
- https://cdn.elifesciences.org/articles/73189/elife-73189-supp1-v2.zip
-
Supplementary file 2
Screen shots from the BioTapestry representation, showing the GRN at each successive time point.
- https://cdn.elifesciences.org/articles/73189/elife-73189-supp2-v2.pdf





