Stress conditions promote Leishmania hybridization in vitro marked by expression of the ancestral gamete fusogen HAP2 as revealed by single-cell RNA-seq
Figures
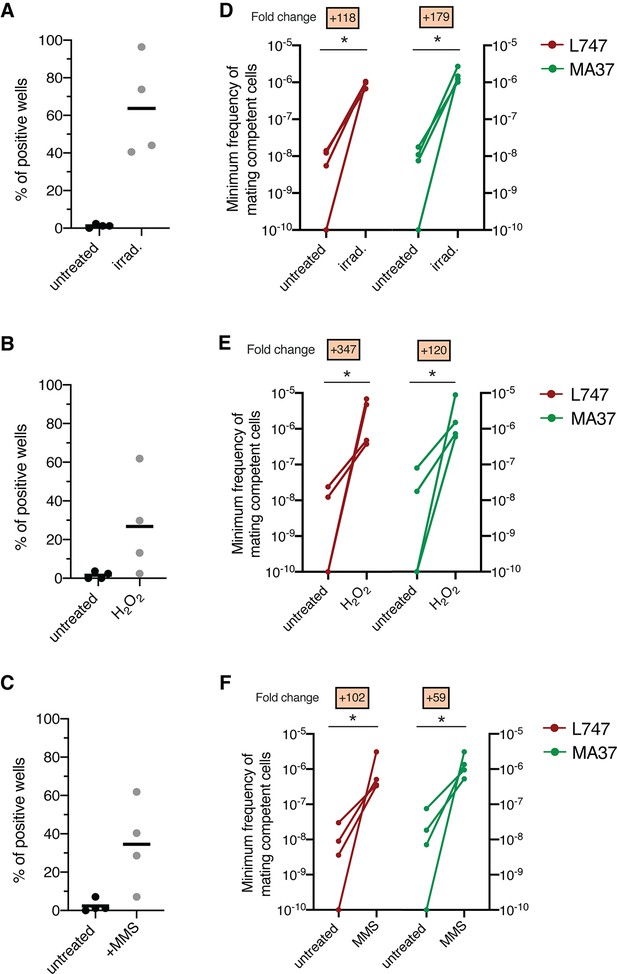
Stress treatments strongly enhance in vitro hybridization of L. tropica L747 and MA37.
(A–C) Proportion of culture wells exhibiting growth of LMA hybrids when exposed to γ-radiation (A), hydrogen peroxide (H2O2) (B), or methyl methanesulfonate (MMS) (C); data are presented as mean values (black line) and individual measurements. (D, E) Minimum frequency of hybridization – competent cells for both L747 and MA37 parental strains after exposure to γ-radiation (D), H2O2 (E) or MMS (F). Four independent experiments were performed for each treatment. The frequencies calculated for the control and treated conditions in each experiment are linked by a line. *p=0.0286 (Mann–Whitney test).
-
Figure 1—source data 1
Percentage of hybrid-positive wells in L. tropica in vitro crosses using parental lines submitted to different DNA stress.
- https://cdn.elifesciences.org/articles/73488/elife-73488-fig1-data1-v2.xls
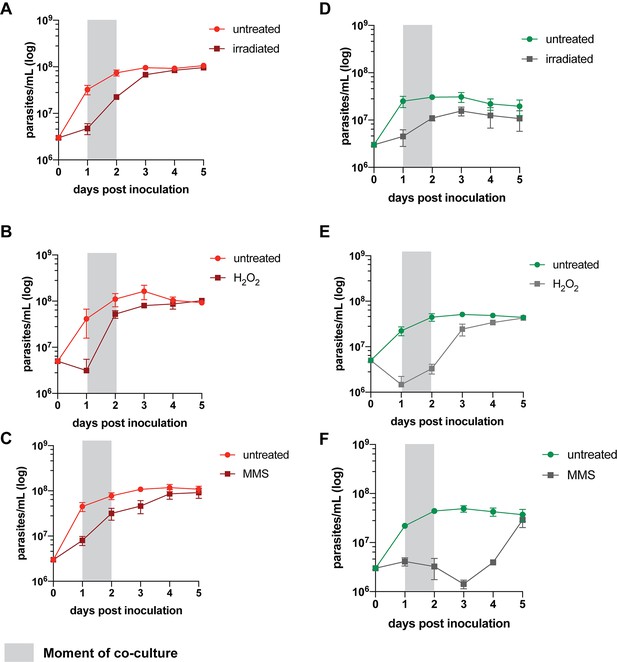
Effect of different stress treatments on L. tropica promastigote growth in vitro.
(A–C) Growth curves of L747 after exposure to γ-radiation (A), H2O2 (B), and methyl methanesulfonate (MMS) (C). (D–F) Growth curves of MA37 after exposure to γ-radiation (D), H2O2 (E), and MMS (F).
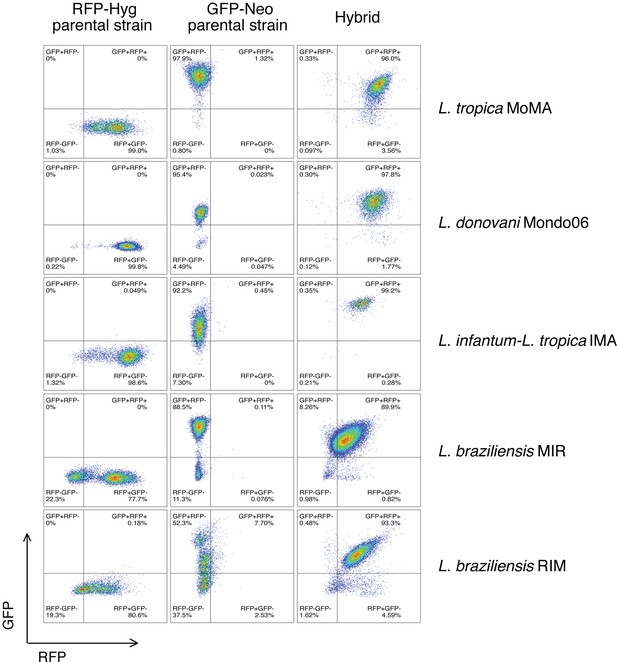
Flow cytometry data of a representative double-fluorescent irradiation-facilitated hybrid from the different crosses where each parent expresses either GFP or RFP.
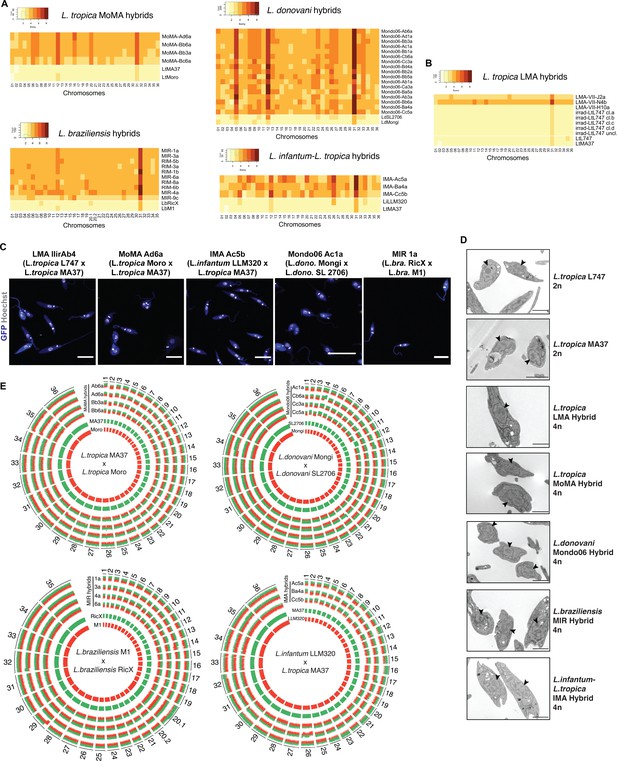
Whole-genome sequencing of irradiation-facilitated experimental hybrids reveals chromosome biparental inheritance and recombination breakpoints.
(A) Normalized sequence read depth of the different Leishmania chromosomes (35 chrs in L. braziliensis and 36 in the other species shown) was used to infer somy values in parental strains (bottom two rows in each heatmap) and hybrids. (B) Normalized sequence read depth of L. tropica LMA hybrids of different ploidies generated in untreated in vitro hybridization, and of four independent subclones and one uncloned line of the irradiated L747 parent. (C) Fluorescence confocal microscopy images of tetraploid LMA, MoMA, IMA, Mondo06, and MIR irradiation-facilitated hybrids. Nuclear and mitochondrial DNA (kDNA) are shown in white (Hoechst 33342 staining) and cell bodies in blue (GFP expression). Scale bar: 10 µm. (D) Transmission electron micrographs of representative diploid (2n) parental strains and different tetraploid (4n) hybrids depicting a single nucleus (arrowheads) in promastigote forms not undergoing cell division. Scale bar: 2 µm. (E) Circos plots representing the inheritance patterns of all the homozygous SNP differences between the two parental strains in four L. tropica MoMA intraspecific hybrids, three L. infantum-L. tropica IMA interspecific hybrids, four L. donovani Mondo06 intraspecific hybrids, and in four L. braziliensis MIR intraspecific hybrids. Each group separated by radial white lines represents a different chromosome, and chromosome ids are shown on the outer circle. Hybrid clones ids are indicated at the start of each circular track. Red and green histograms depict inferred parental contribution from homozygous SNPs specific to the RFP-expressing parental line and the GFP-expressing parental line, respectively. Whole-genome sequencing analyses were performed using the PAINT software (Shaik et al., 2021) and reference genomes available on Tritrypdb (tritrypdb.org).

DNA content analysis of irradiation-facilitated hybrids by propidium iodide (PI) staining followed by flow cytometry.
Parental strains expressing RFP (in red) or GFP (in green) are considered to have close to diploid genomes (2n) while hybrids generated post-irradiation are close to triploid (3n) or tetraploid (4 n). Estimated ploidy was assigned based on both PI staining and whole-genome sequencing analysis for hybrids generated from L. tropica MoMA (A), L. donovani Mondo06 (B), L. braziliensis MIR and RIM (C), and L. infantum-L. tropica IMA (D) crosses. Control L. tropica LMA hybrids of different ploidies generated in untreated in vitro hybridization are shown (E).

Description of the different approaches attempted to reduce the ploidy of LMA irradiation-facilitated hybrids (hybrids LMIIirAb4 and IIirBb1).
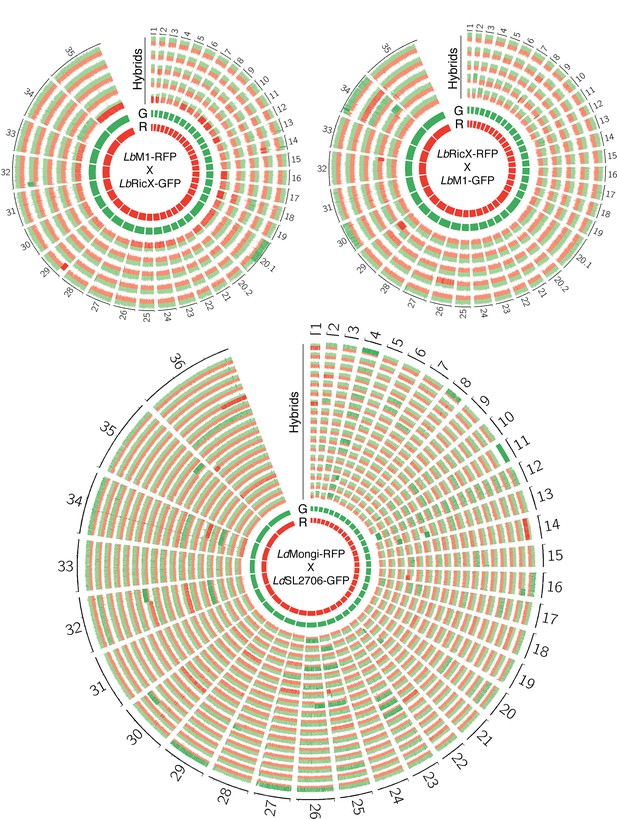
Circos plots showing inferred parental SNP contribution in all L. braziliensis and L. donovani sequenced hybrid genomes.
Red and green histograms refer to homozygous SNPs specific to the RFP-expressing parental line (R) and the GFP-expressing parental line (G), respectively. Parental allele frequencies higher than 0.7 are highlighted in dark red or dark green; frequencies lower than 0.7 are in light red or light green.
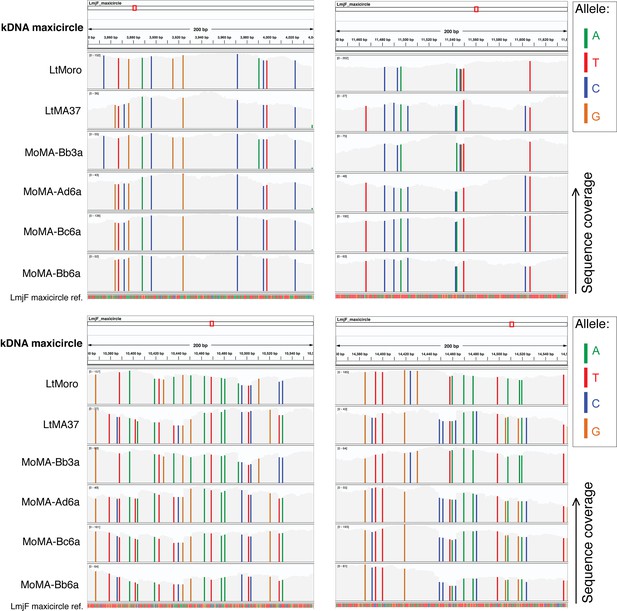
Parental SNP contribution in the kDNA maxicircle sequences from L. tropica MoMA hybrids.
Nucleotide-level sequence coverage is shown for each of the parental strains (LtMoro and LtMA37) and F1 hybrid clones (MoMA) in four different 200-bp-long sequences of the kDNA maxicircles. Each alternative allele is color-coded as shown (green: A; red: T; blue: C; orange: G). A complete match with the reference sequence is in light gray.
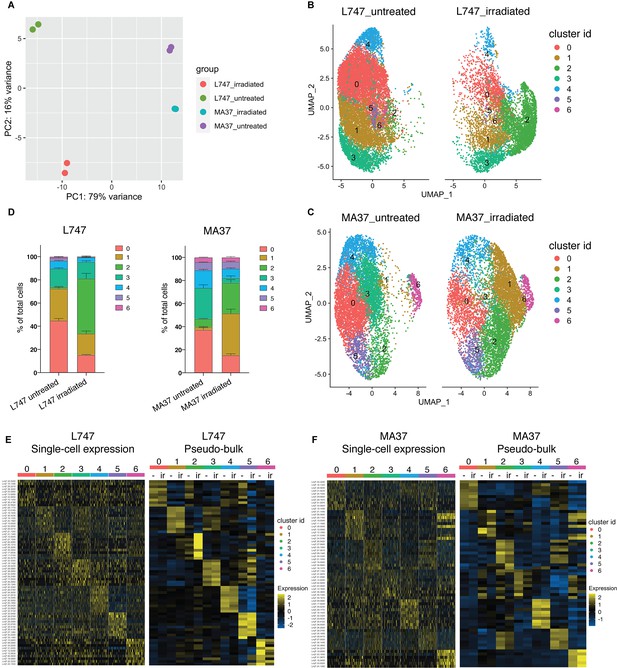
Single-cell RNA sequencing analysis of L. tropica parental strains identify discrete clusters of cells expanded after irradiation.
(A) Principal component analysis (PCA) plot of average gene expression comparing scRNA-seq biological replicates of L747 and MA37 promastigote cultures 1 day post-exposure to 6.5 Gy of γ-radiation or not treated (n = 2 for each group). (B, C) Unifold Manifold Approximation and Projection (UMAP) visualization of the seven heterogeneous clusters of cells identified in L747 (B) and MA37 (C) according to their transcriptomic profiles using the Seurat R package (Hao et al., 2021). Cluster identities in L747 and MA37 are completely independent and unrelated with each other. Data presented are a combination of two biological scRNA-seq replicates for each group. (D) Representation (%) of each cell cluster per sample in irradiated or untreated L747 and MA37 in the two replicates. (E, F) Left panel: heatmap representation of the expression (log2 of fold change comparing a single cell versus all other cells within the same sample) of the top 10 transcript markers in each cluster identified for L747 (E) and MA37 (F), downsampled to 100 cells in each cluster for visualization (Wilcoxon rank-sum test adj. p<0.05). Right panel: a pseudo-bulk analysis shows the average gene expression of the same top 10 markers, highlighting the differences and similarities between untreated (-) and irradiated (Ir) samples using data from all cells assigned to each cluster.
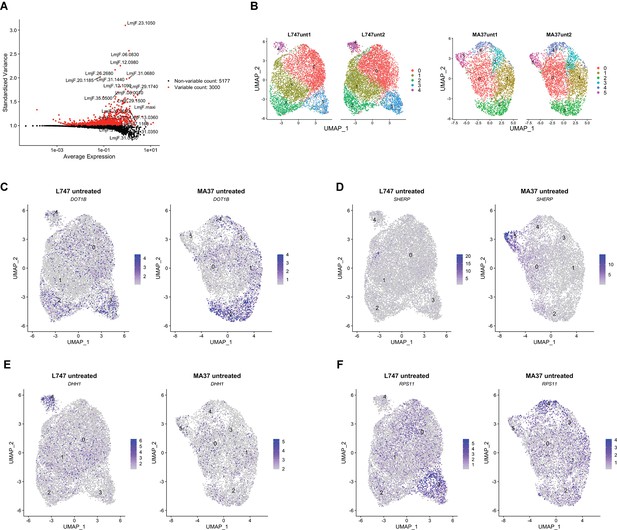
Transcriptomic heterogeneity in untreated L. tropica strains.
(A) Variable features selected for the Seurat R package analysis of the scRNA-seq data (3000 genes). (B) Unifold Manifold Approximation and Projection (UMAP) visualization of the untreated L. tropica clusters for L747 (left) and MA37 (right) strains. Two biological replicates analyzed are shown for each strain. Cluster denomination is arbitrary and unrelated between the strains. (C, D) Relative single-cell expression of different transcripts (log2FC) associated with procylic promastigotes (C), metacyclic promastigotes (D), stress granules (E), or protein synthesis (F).
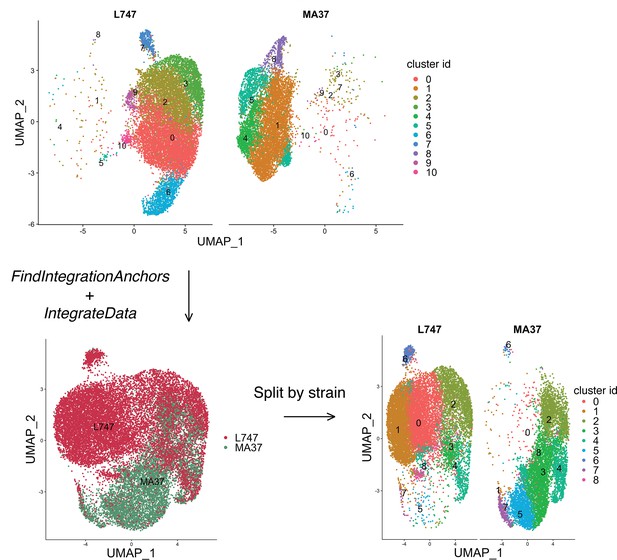
Differential single-cell gene expression in integrated scRNA-seq data from untreated L747 and MA37 cells.
The standard Seurat pipeline for scRNA-seq data analysis revealed no significant co-clustering of untreated cells, as shown in the Unifold Manifold Approximation and Projection (UMAP) projections of combined untreated cells in L747 and MA37 scRNA-seq data (top panel). After identification of matched cell populations (‘anchors’) between the strains and integrating the data, new clustering was performed revealing limited overlapping between the strains (bottom panels). A split view of the integrated UMAP projection is also shown, in which all of the different clusters are identified (bottom-right panel). A complete list of upregulated genes in each cluster post-integration is presented in Supplementary file 2.
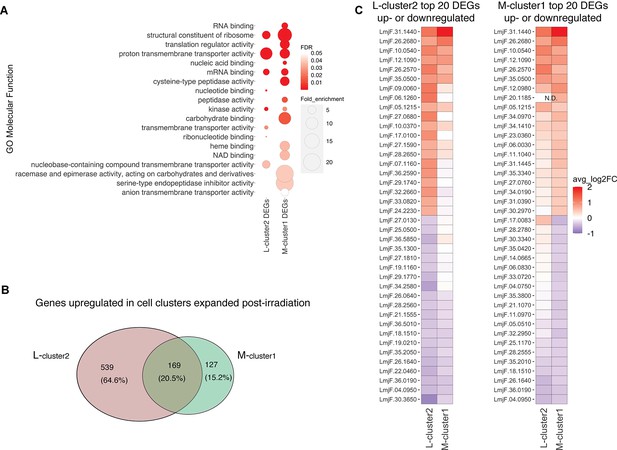
Irradiation-induced cell clusters in L747 and MA37 share a common transcriptomic signature.
(A) Gene Ontology (GO) molecular function enrichment analysis was performed for the lists of differentially expressed genes (DEGs; |log2FC| > 0.1; Wilcoxon rank-sum test adj. p<0.05) in L747 cell cluster 2 (L-cluster2) and MA37 cell cluster 1 (M-cluster1) using TritrypDB online tools (Benjamini–Hochberg FDR < 0.05). (B) Venn diagram representation of the total number of genes upregulated in L-cluster2 and/or M-cluster1 compared to the other clusters (log2FC > 0.1; Wilcoxon rank-sum test adj. p<0.05). (C) Heatmap representation of the expression of the top 20 genes up- or downregulated in L-cluster2 (left panel) or M-cluster1 (right panel). Data are presented as the average gene expression in cells from each of the clusters compared with the other clusters in the same sample. Genes are ranked according to their mean expression in both clusters. Expression of gene LmjF.20.1185 was not detected (N.D.) in the L-cluster2 cells.
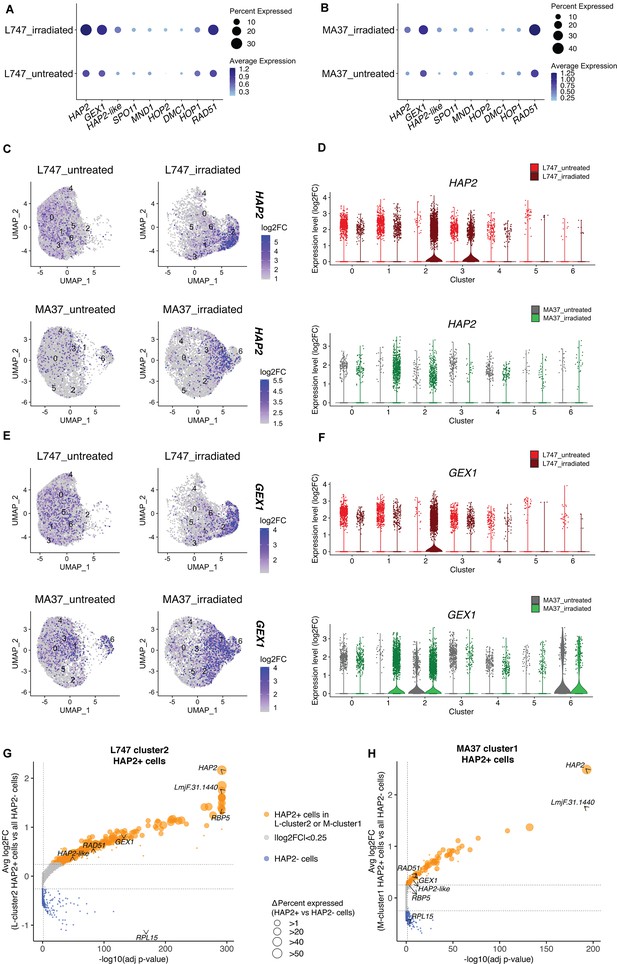
Expression of HAP2 and GEX1 is enriched in irradiation-induced L747 cluster 2 and MA37 cluster 1.
(A, B) Dot plots depicting the average expression of Leishmania homologues of genes involved in genetic exchange and meiosis in other organisms, shown for L747 (A) and MA37 (B) populations with and without exposure to irradiation (pseudo-bulk analysis). The dot size in the plots represents the percentage of cells expressing those genes in each sample. Meiotic genes tested are HAP2 (LmjF.35.0460), GEX1 (LmjF.04.0100), HAP2-like (LmjF.36.3860), SPO11 (LmjF.16.0630), MND1 (LmjF.24.1010), HOP2 (LmjF.27.2420), DMC1 (LmjF.35.4890), HOP1 (LmjF.36.1110), and RAD51 (LmjF.28.0550). (C–E) Unifold Manifold Approximation and Projection (UMAP) plots showing the expression of HAP2 (C) and GEX1 (E) in the different cell clusters identified in L747 (upper panels) and MA37 (lower panels) untreated or irradiated. (D–F) Violin plots representation of the single-cell expression data of HAP2 (D) and Gex1 (F) in L747 (upper panel) and MA37 (lower panel), comparing cells from untreated and irradiated cultures. (G, H) Volcano plot representation of differentially expressed genes (DEGs) in HAP2+ cells (i.e., cells with HAP2 expression log2FC > 1.0) identified in L-cluster2 (G) and M-cluster1 (H). Delta percent expressed (%HAP2+ minus %HAP2- cells expressing a particular gene) is presented as different data point sizes in the plots. Threshold values for adjusted p<0.05 (vertical dotted line; Wilcoxon rank-sum test) and |log2FC| > 0.25 (horizontal dotted lines) were used.
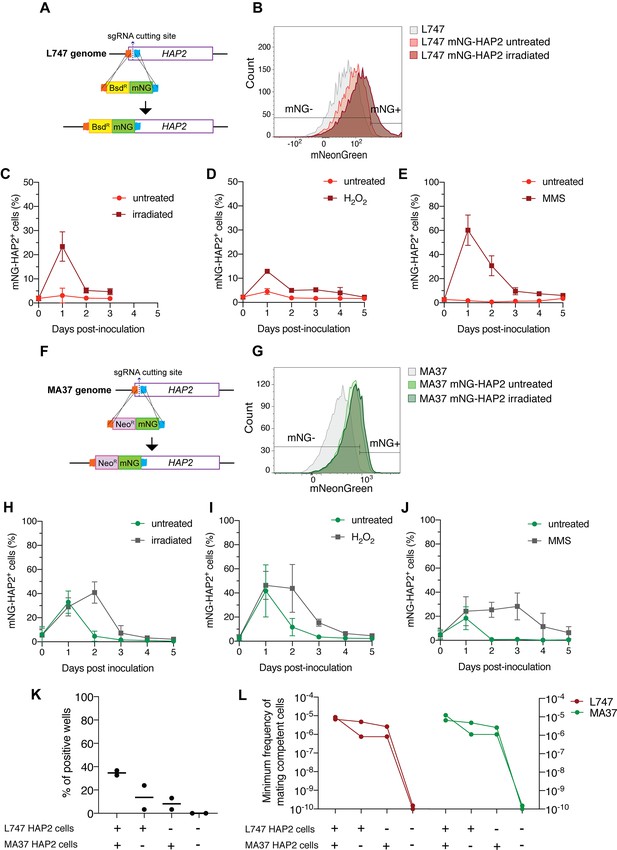
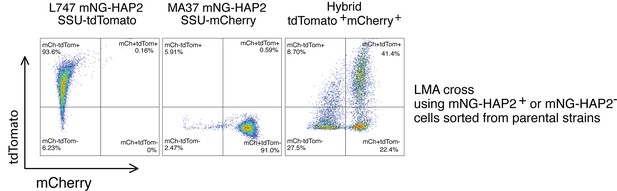
HAP2 protein expression in L. tropica promastigotes is associated with in vitro hybridization capacity.
Schematic representation of the mNeonGreen (mNG)-HAP2 fusion reporter construct generated in the L747 strain (A) and in the MA37 strain (F). Stationary phase promastigotes of either L747 mNG-HAP2 or MA37 mNG-HAP2 parental lines were used to inoculate fresh cultures that were immediately divided into two flasks, with one flask remaining untreated and the other exposed to 6.5 Gy of γ-radiation or supplemented with 250 μM of H2O2 or 0.005% of methyl methanesulfonate (MMS). Flow cytometry histogram plots showing the fluorescence intensity of mNG-HAP2 in L747 (B) and in MA37 (G) 1 day post-inoculation, with or without irradiation. Proportion of mNG-HAP2+ cells over time in L747 mNG-HAP2 cultures with or without exposure to γ-radiation (C), to H2O2 (D) or to MMS (E), n = 3 replicates. Proportion of mNG-HAP2+ cells in MA37 transfectants after exposure to γ-radiation (H), to H2O2 (I), or to MMS (J), n = 4 replicates. (K) Proportion of culture wells exhibiting growth of LMA hybrids after in vitro crosses using the different indicated pairwise combinations of FACS-sorted mNG-HAP2+ and mNG-HAP2- cells 1 day after irradiation; data are presented as mean values (black line) and individual measurements. (L) Minimum frequency of hybridization-competent cells for both L747 and MA37 parental strains in in vitro crosses using different combinations of FACS-sorted mNG-HAP2+ and mNG-HAP2- cells (n = 2 independent experiments). Frequencies collected from each single experiment are linked by a line. Red lines and green lines represent data from L747 and MA37 parental strains, respectively.
-
Figure 6—source data 1
Effect of different stress treatments on the percentage of mNG+ cells during growth of mNG-HAP2 L. tropica transfectants in vitro.
- https://cdn.elifesciences.org/articles/73488/elife-73488-fig6-data1-v2.xls
-
Figure 6—source data 2
Percentage of hybrid-positive wells after mixing sorted mNG-HAP2+ and/or mNG-HAP2- L. tropica promastigotes.
- https://cdn.elifesciences.org/articles/73488/elife-73488-fig6-data2-v2.xls
Tables
List of the different intra- and interspecific irradiation-facilitated hybrids (from in vitro hybridization after exposure of parental strains to γ-radiation) generated in this study with their respective ploidies according to propidium iodide staining, flow cytometry, and whole-genome sequencing.
| Type of cross | Parental strains | Hybrid denomination | Frequency of hybrid recovery: # positive wells/# total wells | Ploidy of irradiation-facilitated hybrids: # hybrids/# hybrids tested | ||||
|---|---|---|---|---|---|---|---|---|
| A | B | No treatment | Irradiation | 2n | 3n | 4n | ||
| Intraspecific | L. tropica L747 RFP-Hyg | L. tropica MA37 GFP-Neo | LMA | 4/336 | 214/336 | 0/44 | 4/44 | 40/44 |
| Intraspecific | L. tropica Moro RFP-Hyg | L. tropica MA37 GFP-Neo | MoMA | 1/84 | 20/84 | 0/4 | 1/4 | 3/4 |
| Interspecific | L. infantum LLM320 RFP-Hyg | L. tropica MA37 GFP-Neo | IMA | 0/84 | 3/84 | 0/3 | 0/3 | 3/3 |
| Intraspecific | L. donovani Mongi RFP-Hyg | L. donovani SL2706 GFP-Neo | Mondo06 | 0/84 | 35/84 | 0/16 | 0/16 | 16/16 |
| Intraspecific | L. braziliensis M1 RFP-Hyg | L. braziliensis RicX GFP-Neo | MIR | 0/84 | 9/84 | 0/7 | 1/7 | 6/7 |
| Intraspecific | L. braziliensis RicX RFP-Hyg | L. braziliensis M1 GFP-Neo | RIM | 0/84 | 9/84 | 0/6 | 0/6 | 6/6 |
| Intraspecific | L. major FV1 Sat | L. major LV39 RFP-Hyg | LVFV | 0/84 | 0/84 | * | * | * |
-
*Not detected.
-
RFP: red fluorescent protein; GFP: green fluorescent protein; mNG-yTub: mNeonGreen-gammaTubulin fusion; Hyg: hygromycin B resistance marker; Neo: neomycin resistance marker; Bsd: blasticidin S resistance marker.
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Gene (species)(Leishmania tropica) | Hapless2 (Hap2) | TritrypDB | LmjF.35.0460 | Also called GCS1 |
| Strain, strain background (L. tropica) | L747 RFP-Hyg | Inbar et al., 2019 | MHOM/IL/02/LRC-L747 | https://doi.org/10.1371/journal.pgen.1008042 |
| Strain, strain background(L. tropica) | MA37 GFP-Neo | Inbar et al., 2019 | MHOM/JO/94/MA37 | https://doi.org/10.1371/journal.pgen.1008042 |
| Strain, strain background(Leishmania major) | LV39 RFP-Hyg | Akopyants et al., 2009 | MRHO/SU/59/P-strain | https://doi.org/10.1371/journal.pgen.1008042 |
| Strain, strain background(L. major) | FV1-Sat | Akopyants et al., 2009 | MHOM/IL/80/Friedlin | https://doi.org/10.1371/journal.pgen.1008042 |
| Strain, strain background(Leishmania infantum) | LLM320 RFP-Hyg | Romano et al., 2014 | MHOM/ES/92/LLM-320; isoenzyme typed MON-1 | https://doi.org/10.1073/pnas.1415109111 |
| Strain, strain background(L. tropica) | Moro RFP-Hyg | This paper | MHOM/AF/19/Moro | Patient from Afghanistan; cell line maintained in D. Sacks lab |
| Strain, strain background(Leishmania donovani) | Mongi RFP-Hyg | This paper | MHOM/IN/83/Mongi-142 | Patient from India; cell line maintained in D. Sacks lab |
| Strain, strain background(L. donovani) | SL2706 GFP-Neo | This paper | MHOM/LK/19/2706 | Patient from Sri Lanka; cell line maintained in D. Sacks lab |
| Strain, strain background(Leishmania braziliensis) | M1 GFP-Neo | This paper | MHOM/BR/00/BA779 | Patient from Brazil; cell line maintained in D. Sacks lab |
| Strain, strain background(L. braziliensis) | M1 RFP-Hyg | This paper | MHOM/BR/00/BA779 | Patient from Brazil; cell line maintained in D. Sacks lab |
| Strain, strain background(L. braziliensis) | RicX GFP-Neo | This paper | MHOM/PE/19/RicX | Patient from Peru; cell line maintained in D. Sacks lab |
| Strain, strain background(L. braziliensis) | RicX RFP-Hyg | This paper | MHOM/PE/19/RicX | Patient from Peru; cell line maintained in D. Sacks lab |
| Genetic reagent(L. tropica) | L747-mNG-HAP2 | This paper | MHOM/IL/02/LRC-L747 | Cell line maintained in D. Sacks lab |
| Genetic reagent(L. tropica) | MA37-mNG-HAP2 | This paper | MHOM/JO/94/MA37 | Cell line maintained in D. Sacks lab |
| Genetic reagent(L. tropica) | L747-mNG-HAP2 SSU-tdTom | This paper | MHOM/IL/02/LRC-L747 | Cell line maintained in D. Sacks lab |
| Genetic reagent(L. tropica) | MA37-mNG-HAP2 SSU-mCherry | This paper | MHOM/JO/94/MA37 | Cell line maintained in D. Sacks lab |
| Recombinant DNA reagent (plasmid) | pA2-GFP-Neo | Chagas et al., 2014 | https://doi.org/10.1371/journal.ppat.1003923 | |
| Recombinant DNA reagent (plasmid) | pA2-RFP-Hyg | Chagas et al., 2014 | https://doi.org/10.1371/journal.ppat.1003923 | |
| Recombinant DNA reagent (plasmid) | pSSU-tdTomato-Neo | Dr Deborah Smith, University of York, UK | ||
| Recombinant DNA reagent (plasmid) | pLEXSY-cherry-Sat2 | Jena Bioscience | Cat # EGE-236 | |
| Chemical compound, drug | CM199 | Inbar et al., 2017 | https://doi.org/10.1128/mBio.00029-17 | |
| Chemical compound, drug | G418 (Geneticin) | Thermo Fisher | Cat # J63871.AB | ‘Neo’ antibiotic |
| Chemical compound, drug | Hygromycin B | Sigma-Aldrich | Cat # H3274 | ‘Hyg’ antibiotic |
| Chemical compound, drug | Nourseothricin | Sigma-Aldrich | Cat # 74667 | ‘Sat’ antibiotic |
| Chemical compound, drug | Blasticidin | Fisher Scientific | Cat # 10264913 | ‘Blast’ antibiotic |
| Chemical compound, drug | Hydrogen peroxide | Sigma-Aldrich | Cat # 216763 | H2O2 |
| Chemical compound, drug | Methyl methane sulfonate | Sigma-Aldrich | Cat # 129925 | MMS |
| Chemical compound, drug | Propidium iodide | Sigma-Aldrich | Cat # P4170 | |
| Chemical compound, drug | Hoechst 33342 | Thermo Fisher | Cat # 62249 | |
| Commercial assay or kit | AMAXA Nucleofactor 4D | Lonza | Cat # V4XP-3024 | |
| Commercial assay or kit | DNeasy Blood and Tissue Kit | QIAGEN | Cat # 69504 | |
| Commercial assay or kit | TruSeq Nano DNA Library Prep kit | Illumina | Cat # 20015965 | |
| Commercial assay or kit | Chromium Next GEM Single Cell 3′ kit v3.1 | 10X Genomics | Cat # NC1690752 | |
| Commercial assay or kit | dsDNA HS Assay Kit | Invitrogen | Cat # Q32854 | |
| Software, algorithm | FACSDIVA | BD Biosciences | RRID:SCR_001456 | |
| Software, algorithm | FlowJo v.10.7 | Becton, Dickinson and Company | RRID:SCR_008520 | |
| Software, algorithm | PAINT | Shaik et al., 2021 | https://doi.org/10.3390/genes12020167 | |
| Software, algorithm | Cell Ranger v.5.0 | 10X Genomics | RRID:SCR_017344 | |
| Software, algorithm | Seurat R package v.4.0.3 | Hao et al., 2021 | RRID:SCR_016341 | |
| Software, algorithm | RStudio v.1.4.1717 | RStudio, PBC | RRID:SCR_000432 | |
| Software, algorithm | GraphPad Prism v.8.0 | GraphPad | RRID:SCR_002798 |
Additional files
-
Supplementary file 1
Calculations of the minimum frequencies of hybridization-competent cells for each L747 × MA37 cross.
- https://cdn.elifesciences.org/articles/73488/elife-73488-supp1-v2.xls
-
Supplementary file 2
Differentially expressed genes for each of the nine cell clusters identified in integrated untreated L747 and MA37 scRNA-seq data (after using FindIntegrationAnchors and IntegrateData commands) according to FindAllMarkers function in Seurat R package, with the following cutoff values: percentage of cells within a cluster expressing a given gene >10%, log2FC > 0.1, and Wilcoxon rank-sum test adjusted p-value < 0.05. Pct.1, percentage of cells in the cluster expressing a given gene; Pct.2, percentage of cells in all the other clusters expressing a given gene.
- https://cdn.elifesciences.org/articles/73488/elife-73488-supp2-v2.xls
-
Supplementary file 3
Differentially expressed genes for each of the seven cell clusters identified in L747 and MA37 (integrated irradiated and untreated samples) according to FindAllMarkers function in Seurat R package, with the following cutoff values: percentage of cells within a cluster expressing a given gene >10%, |log2FC| > 0.1, and Wilcoxon rank-sum test adjusted p-value < 0.05. Pct.1, percentage of cells in the cluster expressing a given gene; Pct.2, percentage of cells in all the other clusters expressing a given gene. Sheets containing the lists of the top 10 markers in each cluster or the genes upregulated in HAP2+ cells in clusters L-cluster2 and M-cluster1 vs. all the Hap2- cells in each strain are presented as additional tabs.
- https://cdn.elifesciences.org/articles/73488/elife-73488-supp3-v2.xls
-
Supplementary file 4
List of genes upregulated in both L747 cluster 2 and MA37 cluster 1 with the following threshold cutoffs: percentage of cells within a cluster expressing a given gene >10%, log2FC > 0.1, and Wilcoxon rank-sum test adjusted p-value < 0.05.
- https://cdn.elifesciences.org/articles/73488/elife-73488-supp4-v2.xls
-
Supplementary file 5
List of the primers used in this work.
- https://cdn.elifesciences.org/articles/73488/elife-73488-supp5-v2.xls
-
Supplementary file 6
Summary statistics of whole-genome sequencing read and alignment quality.
- https://cdn.elifesciences.org/articles/73488/elife-73488-supp6-v2.xls
-
Supplementary file 7
Summary statistics of single-cell RNA-sequencing reads and alignment quality.
- https://cdn.elifesciences.org/articles/73488/elife-73488-supp7-v2.xls
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/73488/elife-73488-transrepform1-v2.docx
-
Source code 1
Code for preparing files for irradiated and untreated Ltrop scRNA-seq data analysis.
- https://cdn.elifesciences.org/articles/73488/elife-73488-supp8-v2.txt