The anterior paired lateral neuron normalizes odour-evoked activity in the Drosophila mushroom body calyx
Figures
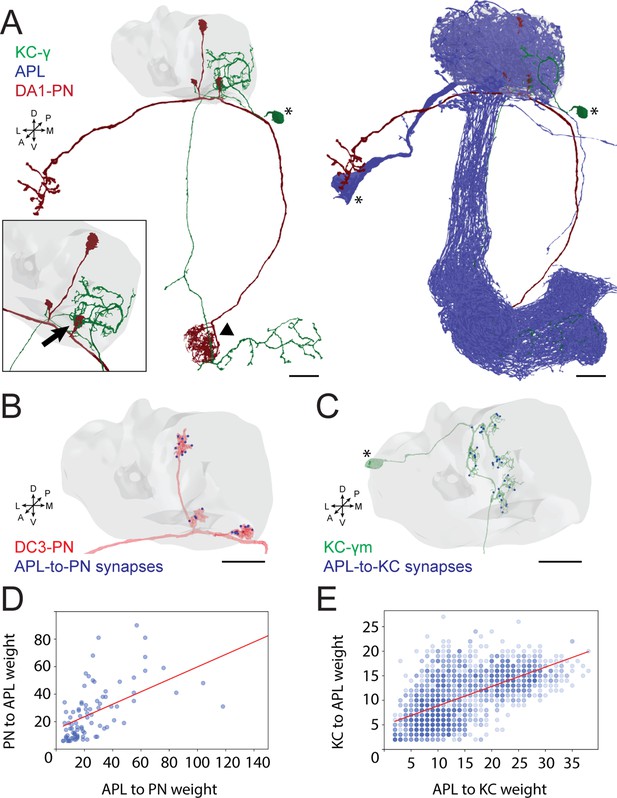
Anterior paired lateral (APL) participates in the microglomerulus (MG) microcircuit with reciprocal synapses.
(A) Left: example of a projection neuron (PN) (red) sending collateral boutons into the mushroom body (MB) calyx (grey volume), where it connects onto Kenyon cells (KCs) claws via synaptic MGs. For simplicity, only one KC is visualized here (green). Note that the partial overlap between PN and KC indicated by the arrowhead is an artefact due to this particular view: the processes of these two neurons are located at different depths in this region. Bottom-left box: magnification of a PN bouton interacting with a KC claw (black arrow). Right: APL (blue) innervates the entire MB including lobes, peduncle, and the calyx. Asterisks indicate cell bodies. Scale bar = 10 μm. Axes indicate the orientation of the reconstruction; D (dorsal), V (ventral), L (lateral), M (medial), A (anterior), P (posterior). (B) Visualization of APL synapses (blue dots) onto a PN 3D mesh within the MB calyx. Most connections are localized on PN boutons. Scale bar = 10 μm. (C) Localization of APL synapses (blue) on a KC 3D mesh within the MB calyx. While most are localized on dendritic claws, some connections along dendritic branches could be seen as well (see also S1C). The cell body is marked by an asterisk. Scale bar = 10 μm. (D) Correlation between the number of PN-to-APL reciprocal synapses (r2 = 0.63) and KC-to-APL ones (E) (r2 = 0.60). The correlation was calculated among the entire synaptic weight (i.e. the total number of synapses reported in the dataset) that individual PNs or KCs had with APL. The gradient of blue in both scatter plots indicates how many neurons share the same connectivity values (lighter blue for fewer, darker blue for more). All 3D plots were created via the Neuprint-python package (see Materials and methods).
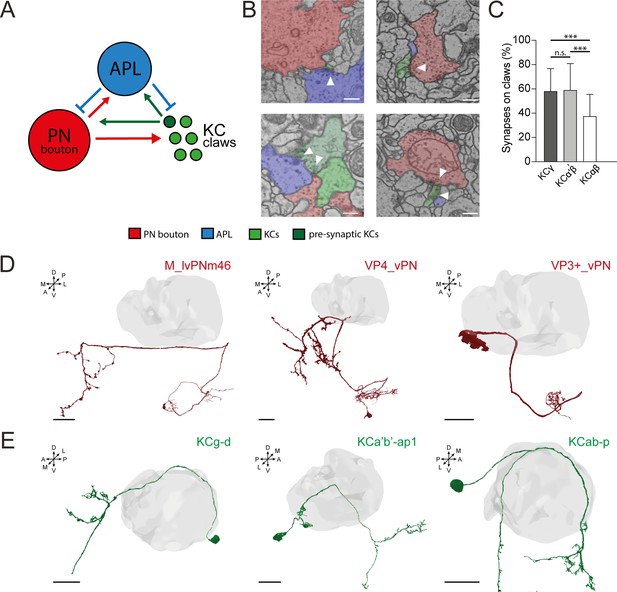
Anterior paired lateral (APL) in the microglomerular circuit.
(A) Schematized view of the connectivity patterns found in the DA1-projection neuron (PN) microglomerulus (MG) reconstructed in Baltruschat et al., 2021. Green circles represent Kenyon cell (KC) claws. Dark green circles represent presynaptic KCs. The red circle represents the reconstructed bouton of a DA1-PN. The blue circle represents APL. See Baltruschat et al., 2021 for further explanation, including the full connectome of this DA1-PN MG. (B) Examples of connectivity patterns involving APL in the DA1-PN MG reconstructed in Baltruschat et al., 2021. Top-left: Electron microscopy (EM) image of APL (blue) presynaptic to the PN bouton (red) and the claw of a KC (green). Top-right: PN bouton presynaptic to APL and three KC claws. Bottom-left: KC (dark green) presynaptic to PN bouton, APL, and another claw. Bottom-right: APL and PN bouton presynaptic to the same KC claw. Scale bar = 250 nm. (C) Spatial distribution of APL synapses among different KC types. The fraction of APL to KCαβ synapses localized on KC claws was lower than in other KC types. n = 210 (70 per KC type, randomly selected), p < 0.0001 (***), unpaired ANOVA with multiple comparisons. Whiskers indicate SD. (D) Examples of PNs not interacting with APL at the MB calyx (grey volume). Scale bar = 10 μm. Axes indicate the orientation of the reconstruction; D (dorsal), V (ventral), L (lateral), M (medial), A (anterior), P (posterior). (E) Examples of KCs not interacting with APL at the MB calyx (grey volume). 3D neuronal meshes were created via the Neuprint-python package (see Materials and methods). Scale bar = 10 μm.
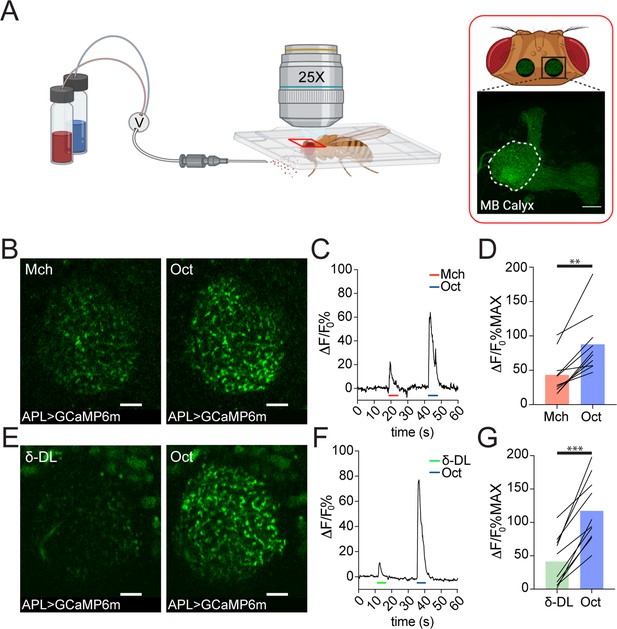
Anterior paired lateral (APL) responds to odours with variable calcium transients.
(A) Schematic view of the two-photon in vivo imaging setup. Scale bar = 20 μm. (B) Example of APL response to 4-methylcyclohexanol (Mch) or 3-octanol (Oct) in the calyx of APLi-GAL4> UAS-GCaMP6m flies. Scale bar = 10 μm. (C) Fluorescence intensity over time for the fly showed in (B). (D) APL showed higher intracellular calcium transients in response to Oct compared to Mch. n = 10, p = 0.002 (**), Wilcoxon matched-pairs test. (E) Example of APL GCaMP6m response to δ-decalactone (δ-DL) or Oct. Scale bar = 10 μm. (F) Fluorescence intensity over time for the fly showed in (E). (G) APL peak response comparison for the δ-DL vs. Oct odours sequence. n = 10, p < 0.0001 (***), paired t-test. Odours were diluted 1:100, bars indicate means.
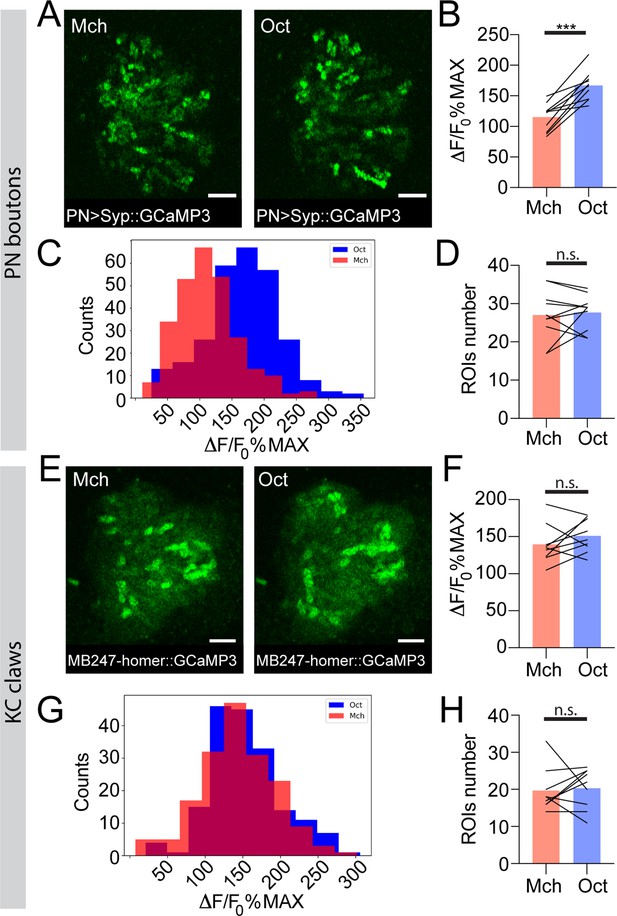
The strength of response to odour stimulation varies in an odour-dependent way in the projection neuron (PN) boutons, but is homogenous at the postsynaptic Kenyon cell (KC) claws.
(A) Example of PN boutons fluorescence increase in response to stimulation with 4-methylcyclohexanol (Mch) or 3-octanol (Oct) in NP225-GAL4> UAS-Syp::GCaMP3 flies. Scale bar = 10 μm. (B) The average activity peak among responding PN boutons was higher when flies were exposed to Oct compared to Mch. n = 10, p = 0.0002 (***), paired t-test. (C) Frequency distribution of PN boutons activity peaks in the Mch vs. Oct protocol. The Oct population was significantly shifted towards higher ΔF/F0%MAX values. n = 10, p < 0.0001 (***), Kolmogorov-Smirnov test. (D) The number of ROIs showing odour-evoked activity did not change between the two odour exposures. n = 10, p = 0.689, paired t-test. (E) Example of KC claws fluorescence levels in response to Mch and Oct in MB247-homer::GCaMP3 flies. Scale bar = 10 μm. (F) The average activity peak among responding KC claws was comparable between Mch and Oct exposures. n = 9, p = 0.1648, paired t-test. (G) Frequency distribution of KC claws activity peaks in the Mch vs. Oct protocol. The two populations had a similar shape and spread among similar ΔF/F0%MAX values. n = 9, p = 0.0982, Kolmogorov-Smirnov test. (H) The number of ROIs showing odour-evoked activity did not change between the two odour exposures. n = 9, p = 0.727, Wilcoxon matched-pairs test. Odours were diluted 1:100, bars indicate means.
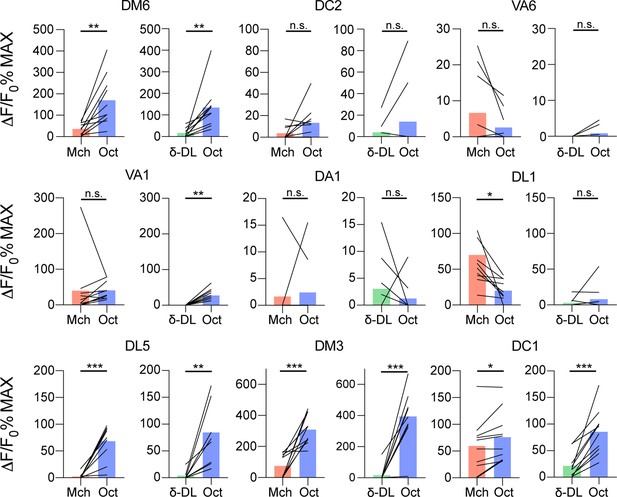
Single projection neuron (PN) glomeruli responses at the antennal lobe (AL).
GH146-GAL4> UAS-GCaMP6m flies were stimulated with either 4-methylcyclohexanol (Mch) vs. 3-octanol (Oct) or δ-DL vs. Oct. The activity peak per each glomerulus was extracted and compared. n = 10, paired t-test, p-value > 0.05 (n.s.), ≤ 0,05 (*), ≤ 0,01 (**), ≤ 0,001 (***). Odours were diluted 1:100, bars indicate means.
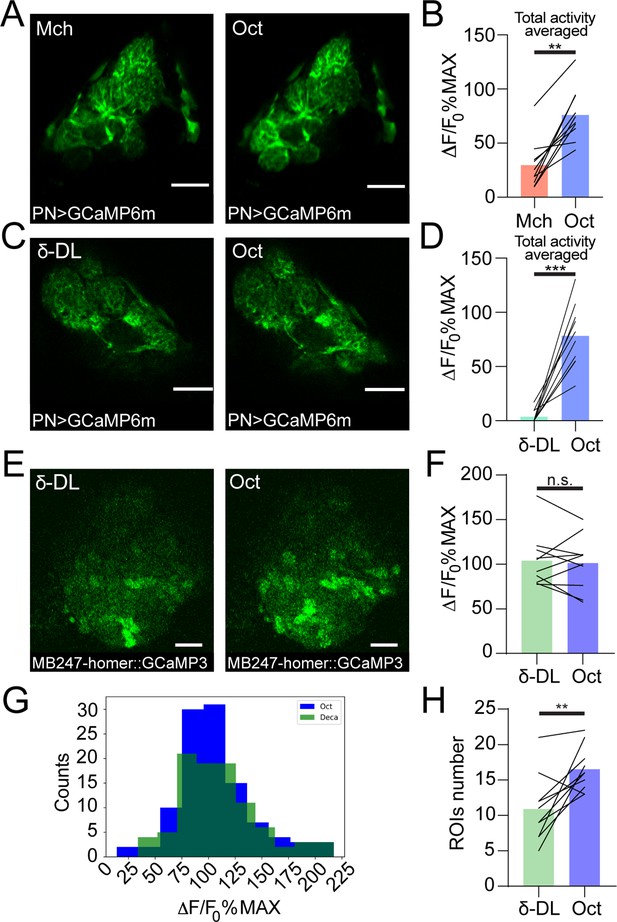
Additional data on projection neurons (PNs) and Kenyon cells (KCs) odour-evoked activity.
Examples of PN dendritic calcium transients in response to either 4-methylcyclohexanol (Mch) and 3-octanol (Oct) (A) or δ-decalactone (δ-DL) and Oct (C) at the antennal lobe in GH146-GAL4> UAS-GCaMP6m flies. Scale bar = 20 μm. (B) The average activity peak among responding glomeruli was higher when flies were exposed to Oct compared to Mch. n = 10, p = 0.002 (**), Wilcoxon matched-pairs test. (D) The average activity peak among responding glomeruli was higher when flies were exposed to Oct compared to δ-DL. n = 10, p < 0.0001 (***), paired t-test. (E) Example of KC claws fluorescence levels in response to δ-DL and Oct in MB247-homer::GCaMP3 flies. Scale bar = 10 μm. (F) The average activity peak in KC claws was comparable between δ-DL and Oct exposures. n = 10, p = 0.767, Wilcoxon matched-pairs test. (G) Frequency distribution of KC claws activity peaks in the δ-DL vs. Oct protocol. The two populations had a similar shape and spread among similar ΔF/F0%MAX values. n = 10, p = 0.9554, Kolmogorov-Smirnov test. (H) The number of ROIs responding to Oct was higher compared to the δ-DL ones. n = 10, p = 0.0059 (**), paired t-test. Odours were diluted 1:100 unless stated otherwise, bars indicate means.
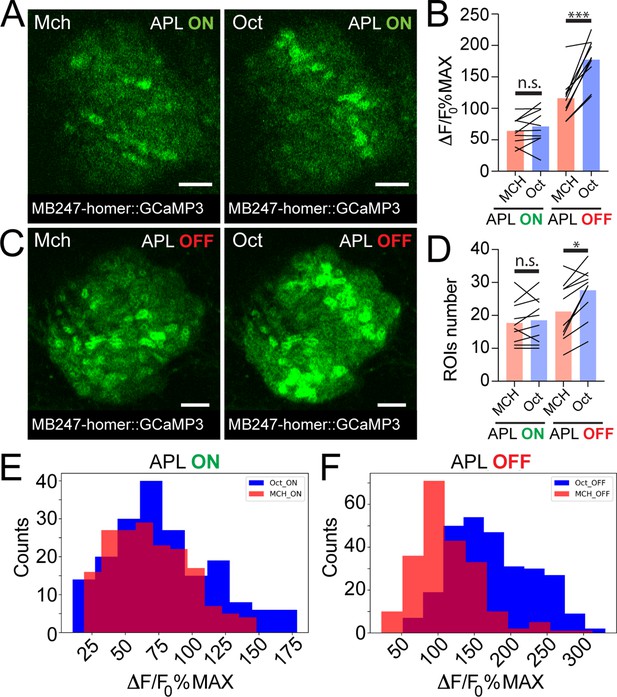
Anterior paired lateral (APL) silencing leads to more variable odour representations at the mushroom body (MB) calyx.
Examples of Kenyon cell (KC) claws fluorescence levels in response to 4-methylcyclohexanol (Mch) and 3-octanol (Oct) in control animals (A, APL ON) or in flies where the output from APL was blocked (C, APL OFF). The genotype used is APLi-GAL4> UAS TeTx, UAS-mCherry; MB247-homer::GCaMP3. Scale bar = 10 μm. (B) The average activity peak in KC claws was similar when APL was active (APL ON, p = 0.949), but was highly variable in the absence of APL output (APL OFF, p = 0.0003 (***)). n = 10, two-way ANOVA with Tukey’s multiple comparisons. (D) The number of odour-responding ROIs was comparable in the presence of active APL (APL ON, p = 0.995) and it was slightly increased in the absence of APL output (APL OFF, p = 0.047 (*)). n = 10, p = 0.047, two-way ANOVA with Tukey’s multiple comparisons. (E) Frequency distribution of activity peaks among microglomeruli (MGs) responding to a particular odour in the presence of APL inhibition. The two populations are highly overlapping, as in Figure 3G. n = 10, p = 0.0533, Kolmogorov-Smirnov test. (F) In the absence of APL activity, the distribution of MGs responding to Oct shifted towards higher values, resembling presynaptic PN boutons data shown in Figure 3C. n = 10, p < 0.0001 (***), Kolmogorov-Smirnov test. Odours were diluted 1:100, bars indicate means.
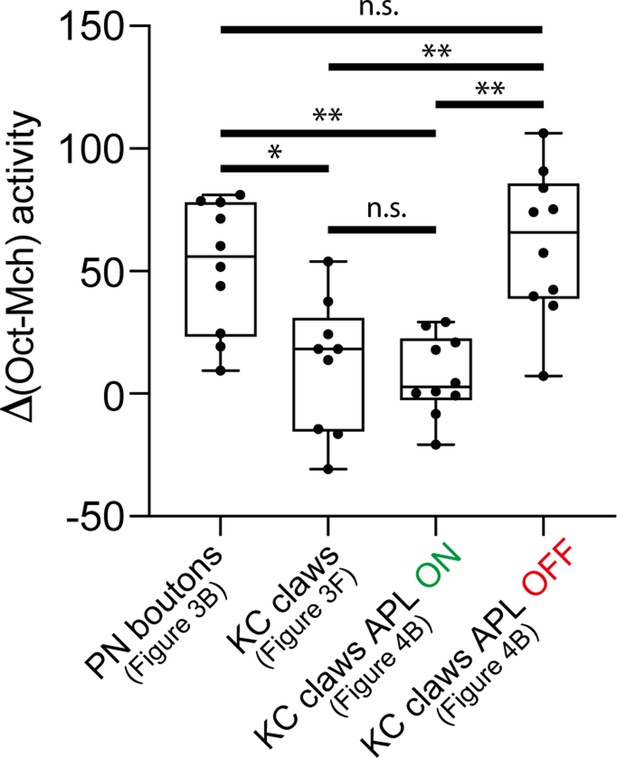
Anterior paired lateral (APL) restrains odour-evoked activity in Kenyon cell (KC) claws.
The gap between the 3-octanol (Oct) signal peak and the 4-methylcyclohexanol (Mch) one was higher in projection neuron (PN) boutons compared to KC claws. In the absence of APL output (APL OFF), the gap between the Oct signal peak and the Mch one increased in KC claws compared to control animals (APL ON) and resembled the value measured in PN boutons. n = 10, p = 0.0271 (*) for PN boutons vs. KC claws, p = 0.0024 (**) for PN boutons vs. KC claws APL ON, p = 0.0011 (**) for KC claws APL ON vs. KC claws APL OFF, p = 0.9698 (n.s.) for PN boutons vs. KC claws APL OFF; Brown-Forsythe and Welch ANOVA test with multiple comparisons.
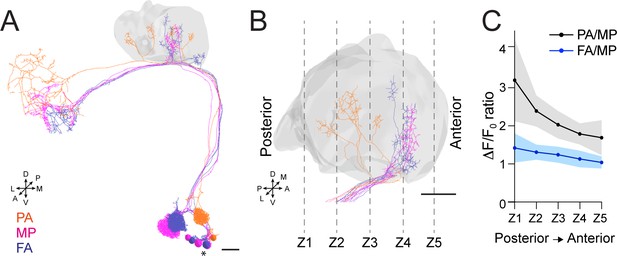
Anterior paired lateral (APL) inhibition is local within the mushroom body (MB) calyx.
(A) 3D skeletons of the projection neurons (PNs) activated by the odours used in this experiment: pentyl acetate (PA) (orange), methyl palmitate (MP) (magenta), farnesol (FA) (blue). Asterisk indicates PN cell bodies. Scale bar = 10 μm. Axes indicate the orientation of the reconstruction; D (dorsal), V (ventral), L (lateral), M (medial), A (anterior), P (posterior). (B) Side view of the calyx showing the distribution of the PN terminals for the odours used in this experiment. Note the higher spatial segregation between PA and MP projection neuron (PN) terminals compared to FA and MP ones. The ticked lines Z1-Z5 show an example of sectioning applied when acquiring image stacks over time. Scale bar = 10 μm. (C) The APL neuron calcium transient ratio in response to PA vs. MP (black line) was highly variable across different sections of the calyx compared to the FA vs. MP one (blue line), and the slope of the two curves was significantly different. n = 7, p = 0.0004 (***), curves slope comparison via linear regression analysis. Coloured areas represent SEM.
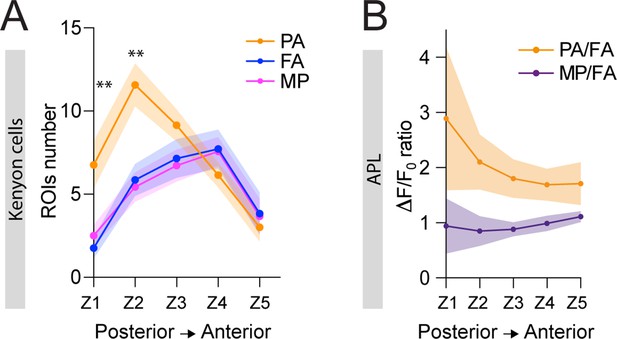
Localized inhibition within the mushroom body (MB) calyx.
(A) The number of ROIs showing odour-evoked activity in postsynaptically tagged MB247-homer::GCaMP3 flies reflected the segregated distribution of the projection neurons (PNs) delivering that particular odour information. In particular, pentyl acetate (PA) responding ROIs were significantly more compared to farnesol (FA) and methyl palmitate (MP) responsive ones in the two more posterior sections of the calyx (Z1 and Z2). n = 7, Z1: p = 0.001 (**) and 0.004 (**) for PA vs. FA and PA vs. MP, respectively; Z2: p = 0.004 (**) and p = 0.002 (**) for PA vs. FA and PA vs. MP, respectively. No significative differences were observed between FA and MP representations. Multiple t-tests. Coloured areas represent SEM. (B) Reciprocal analysis of the curves shown in Figure 5C. The anterior paired lateral (APL) neuron calcium transient ratio in response to PA vs. FA (orange line) was highly variable across different sections of the calyx compared to the MP vs. FA one (purple line), and the slope of the two curves was significantly different. n = 7, p = 0.0004 (***), curves slope comparison via linear regression analysis. Coloured areas represent SEM.

Cell Tracking by calcium macro.
Example of detection of active ROIs during odour exposure. Left, original raw file. Centre, mask of active ROIs obtained via the “Cell Tracking by calcium” ImageJ macro. Right, the obtained mask is then used to extract the pixel intensity values over time of the regions labelled as active ROI. Afterwards, ΔF/F0% and ΔF/F0% MAX values per each ROI are calculated and analysed.
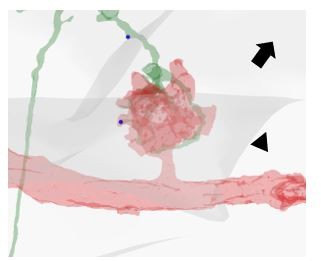
Distinction between synapses on KC dendritic endings (claws) vs not on claws.
In this example, one PN bouton providing input to a KC of interest is visualized. The KC dendritic specialization involved in the microglomerular structure (i. e. the claw) is defined by the fact that it is in direct contact with the bouton. The APL presynaptic site marked by an arrowhead is located on a claw (note the contact between the green claw and the red bouton). In contrast, the localization of the APL presynaptic site marked by an arrow is not on a claw but along the dendritic branch.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Genetic reagent (D. melanogaster) | GH146-Gal4 | Stocker et al., 1997 | BDSC:91812 | |
| Genetic reagent (D. melanogaster) | NP225-Gal4 | Hayashi et al., 2002 | DGRC:112095 | |
| Genetic reagent (D. melanogaster) | NP2631-Gal4 | Hayashi et al., 2002 | DGRC: 104266 | |
| Genetic reagent (D. melanogaster) | GH146-Flp | Hong et al., 2009 | FLYB: FBtp0053491 | |
| Genetic reagent (D. melanogaster) | tubP-Gal80ts | McGuire et al., 2003 | BDSC: 7017 | |
| Genetic reagent (D. melanogaster) | tubP-FRT-GAL80-FRT | Gao et al., 2008; Gordon and Scott, 2009 | BDSC: 38880; 38881 | |
| Genetic reagent (D. melanogaster) | UAS-GCaMP6m | Chen et al., 2013 | BDSC: 42750 | |
| Genetic reagent (D. melanogaster) | MB247-homer::GCaMP3 | Pech et al., 2015 | Gift from A Fiala | |
| Genetic reagent (D. melanogaster) | UAS-Syp::GCaMP3 | Pech et al., 2015 | FLYB: FBtp0130846 | |
| Genetic reagent (D. melanogaster) | UAS-TeTx | Sweeney et al., 1995 | BDSC: 28838 | |
| Genetic reagent (D. melanogaster) | UAS-mCherry::CAAX | Kakihara et al., 2008 | FLYB: FBtp0041366 |





