Early detection of cerebrovascular pathology and protective antiviral immunity by MRI
Figures
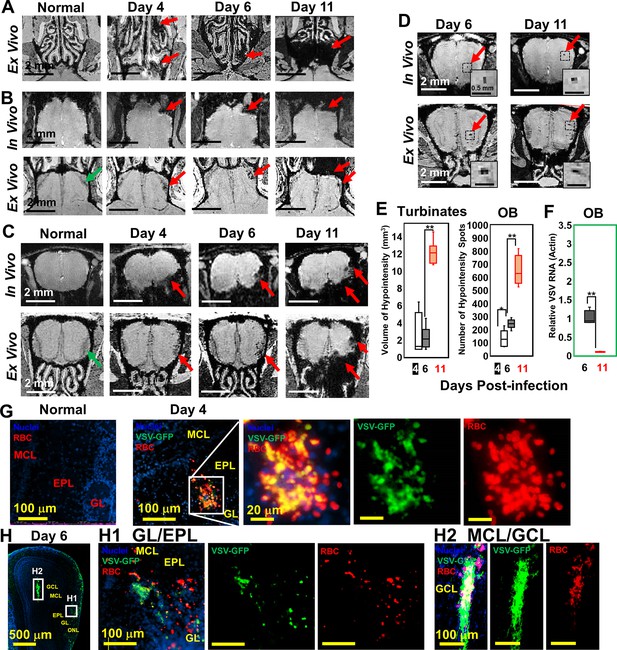
Magnetic resonance imaging (MRI) detected microbleeds in turbinates and olfactory bulb (OB) since day 4 post-infection.
(A) Ex-vivo MRI of turbinates (axial view) from a normal mouse and VSV-infected mice on day 4, 6, and 11 post-infection. (B) Axial view and (C) coronal view of in-vivo (upper panel) and ex-vivo (lower panel) MRI of OB. The coronal view was sectioned from the bleeding site on the axial view, as indicated by red arrows. (D) At the center of the OB, microbleeds were detected since around day 6 by in-vivo MRI. The inserted figure was the enlarged views of the framed bleeding sites in the full views. The scale bar in the full view: 2 mm. The scale bar in the inserted view: 0.5 mm (D). (E) Quantification of volume of hypointensity in the turbinates and numbers of hypointensity spots in the OB on day 4, 6, and 11 (n=6). * p=0.046; ** p<0.0001. (F) Viral titers, represented as relative to actin RNA, at the OB on day 6 and 11 (n=6). ** p<0.0001. (G, H) Immunohistochemical (IHC) study to show microbleeds and vesicular stomatitis virus (VSV) in the OB of VSV-infected mice on day 4 (G) and 6 (H). EPL, external plexiform layer; GCL, granule cell layer; GL, glomerular layer; MCL, mitral cell layer; ONL, olfactory nerve layer. Blue, DAPI for nuclei; Green, VSV-GFP; Red, Alexa Fluor 647-conjugated anti-Ter119 for RBCs.
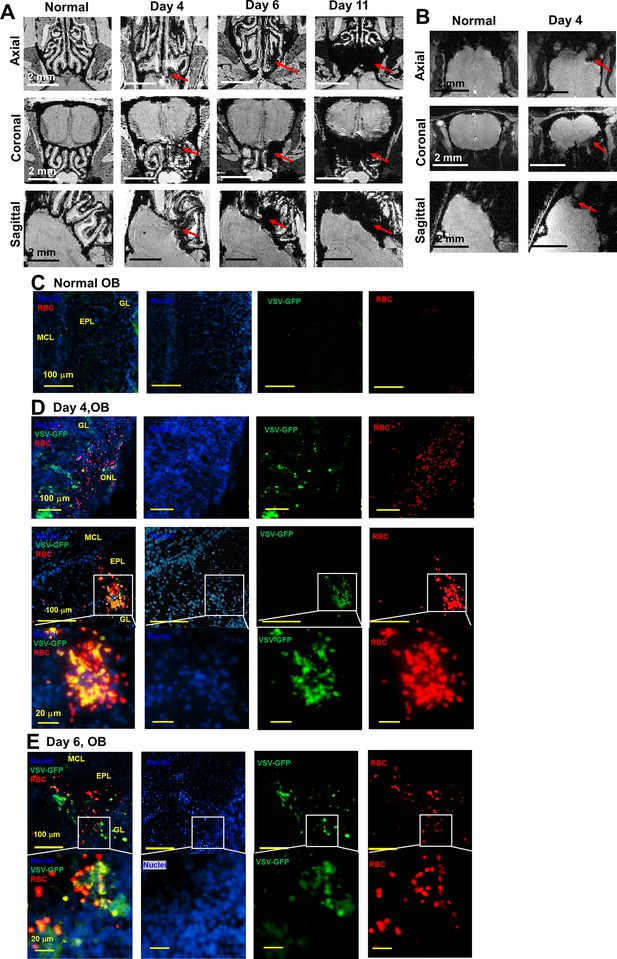
Magnetic resonance imaging (MRI) detected microbleeds in the turbinate and olfactory bulb (OB) during vesicular stomatitis virus (VSV) infection.
(A) 3D view of ex-vivo MRM of turbinates from normal mouse and VSV-infected mice on day 4, 6, and 11. (B) 3D view of in-vivo MRI of OB from normal mouse and VSV-infected mice on day 4. (C–E) IHC verification of the MRI results. Separated color channels of fluorescence images of the OB from a normal mouse (C) and VSV-infected mice on day 4 (D) and 6 (E). GL, glomerular layer; EPL, external plexiform layer; MCL, mitral cell layer. Blue, DAPI for nuclei; Green, VSV-GFP; Red, Alexa Fluor 647-conjugated anti-Ter119 for RBCs.
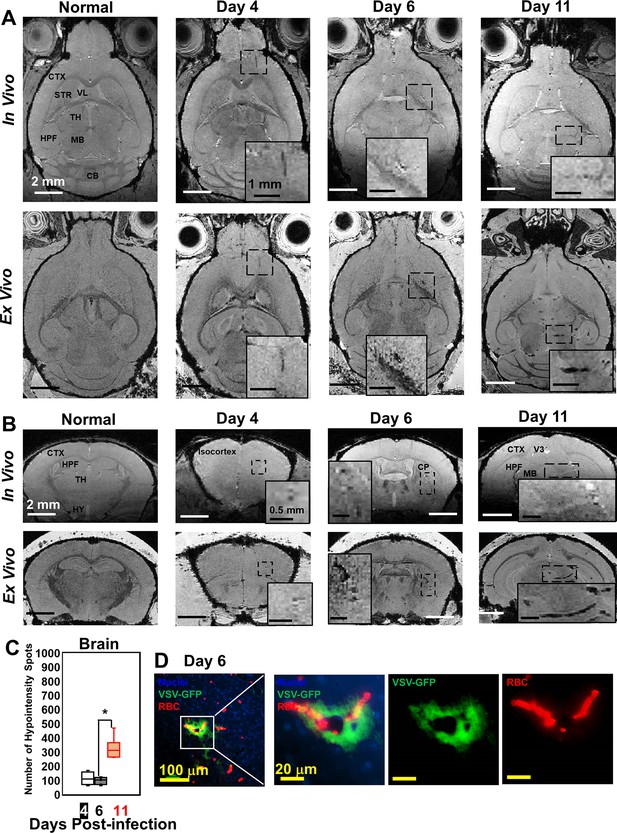
Magnetic resonance imaging (MRI) monitored vessel breakdown in the rest of brain.
(A) Axial view and (B) coronal view of in-vivo (upper panel) and ex-vivo (lower panel) MRI of brain, showing the breakdown of vessels from frontal brain to midbrain. The scale bar in the full view: 2 mm. The scale bar in the inserted view: 1 mm (A) and 0.5 mm (B). CB, cerebellum; CP, caudoputamen; CTX, cerebral cortex; HPF, hippocampal formation; HY, hypothalamus; MB, midbrain; STR, striatum; TH, thalamus; V3, third ventricle; VL, lateral ventricle. (C) Quantification of numbers of hypointensity spots in the brain (not including OB) on day 4, 6, and 11 (n=6). * p<0.0001. (D) Immunohistochemical (IHC) staining to show microbleeds and VSV in the forebrain on day 6. Blue, DAPI for nuclei; Green, VSV-GFP; Red, Alexa Fluor 647-conjugated anti-Ter119 for RBCs.
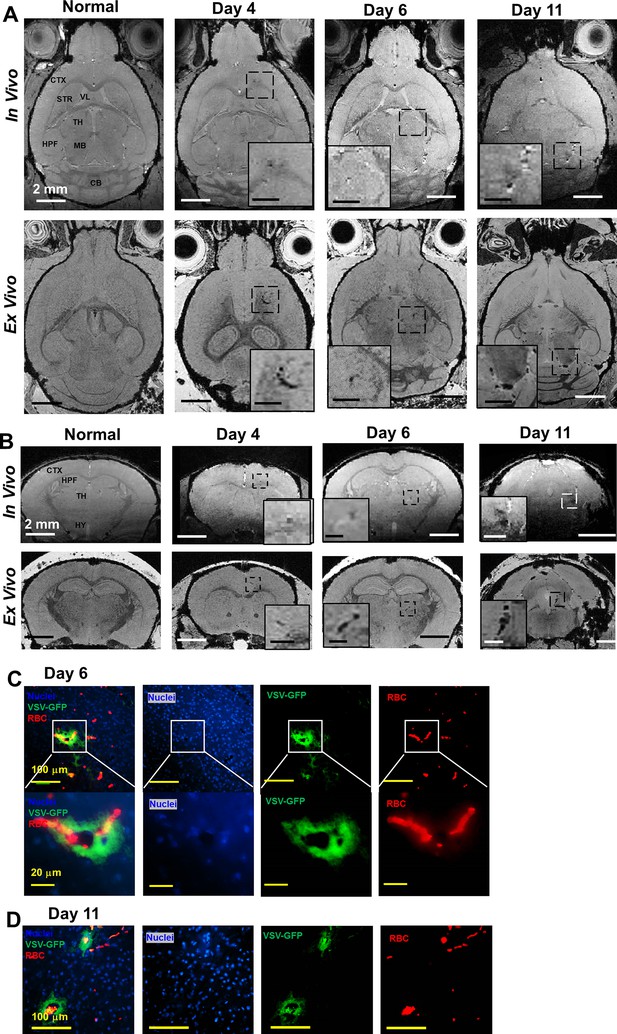
Magnetic resonance imaging (MRI) monitored vessel breakdown in the rest of brain.
More (A) axial view and (B) coronal view of in-vivo (upper panel) and ex-vivo (lower panel) MRI of brain, showing the breakdown of vessels from frontal brain to midbrain. (C, D) IHC verification of the MRI results. Separated color channels of fluorescence images to show microbleeds and vesicular stomatitis virus (VSV) in the forebrain on day 6 (C) and midbrain on day 11 (D). Blue, DAPI for nuclei; Green, VSV-GFP; Red, Alexa Fluor 647-conjugated anti-Ter119 for RBCs.
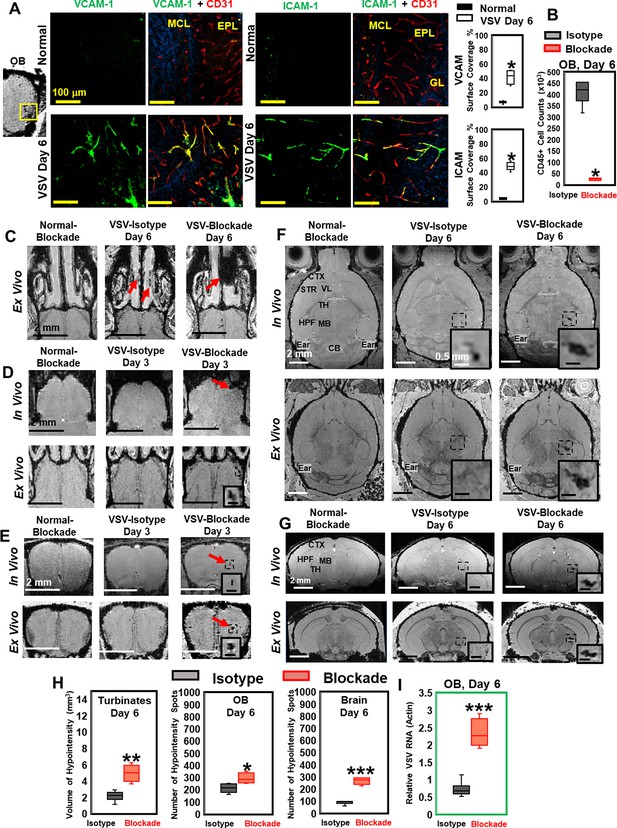
Vesicular stomatitis virus (VSV) infection caused vascular endothelium activation and blocking immune cell infiltration by anti-LFA-1/VLA-4 antibodies promoted bleeding in the turbinates, olfactory bulb (OB), and brain.
(A) Immunohistochemical (IHC) staining showed increased expression of endothelial VCAM-1 and ICAM-1 in the OB on day 6 of infection. n=3 per group. * p<0.0001. (B) Anti-LFA-1/VLA-4 blockade antibodies decreased CD45+ cells over 95% in the OB on day 6. n=4 per group. * p<0.0001. (C) Treatment with anti-LFA-1/VLA-4 blockade antibodies increased bleeding in the turbinates. Ex-vivo MRI of the turbinates (axial view) from normal mouse treated with the blockade antibodies, VSV infected mouse treated with isotype control (rat IgG2a) antibody on day 6, and VSV infected mouse treated with the blockade antibodies on day 6. In the glomerular layer (GL) (D) and the granule cell layer (GCL) of OB (E), MRI detected bleeding earlier upon treatment with the blockade antibodies. (F) Axial and (G) coronal view of MRI of the brain showed a large area of hemorrhage at the thalamus of anti-LFA-1/VLA-4 treated mouse on day 6. The coronal view was sectioned from the hemorrhage site on the axial view, as shown in the frame. In (F) and (G), scale bar in the full view: 2 mm; in the inserted view: 0.5 mm. (H) Quantification of the volume of bleeding in the turbinates and the numbers of hypointensity spots in the OB and brain on day 6, in the isotype control group and anti-LFA-1/VLA-4 antibodies treated group (n=4). * p=0.039; ** p=0.005; *** p<0.0001. (I) Blocking immune cell infiltration increased viral RNA in the OB on day 6 (n=4). *** p<0.0001.
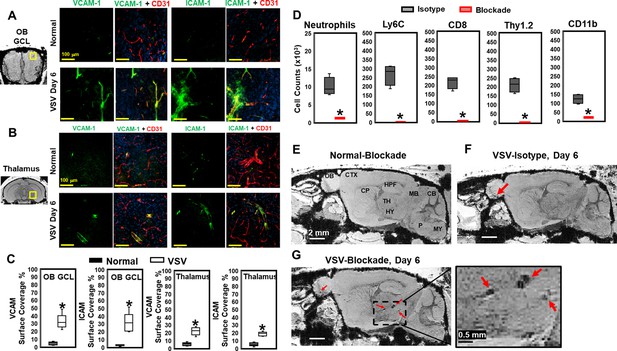
Vesicular stomatitis virus (VSV) infection caused vascular endothelium activation and blocking immune cell infiltration by anti-LFA-1/VLA-4 antibodies promoted bleeding in the brain.
(A–C) Immunohistochemical (IHC) staining showed increased expression of endothelial VCAM-1 and ICAM-1 in the OB (A) and thalamus (B) on day 6 of infection, as quantified in (C) n=3 per group. * p<0.0001. (D) Anti-LFA-1/VLA-4 blockade antibodies decreased inflammatory cells (neutrophiles, Ly6C+, CD8+, and CD11b+) in the olfactory bulb (OB) on day 6 significantly. Neutrophils is defined as Ly6Cint, Ly6Ghi, CD11b+. n=4 per group. * p<0.0001. (E–G) Sagittal view of Magnetic resonance imaging (MRI) of the brain showed a large area of hemorrhage at the thalamus of anti-LFA-1/VLA-4 treated mouse on day 6.
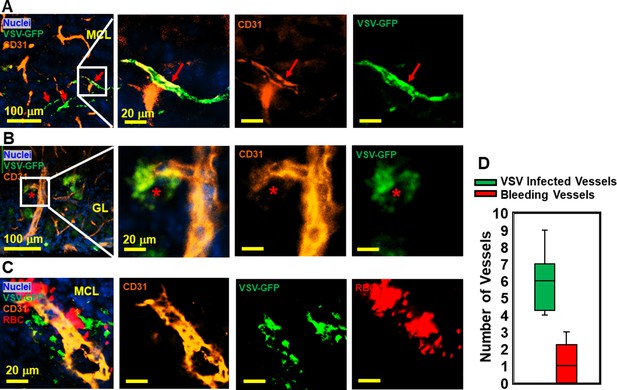
Immunohistochemical (IHC) study revealed that vesicular stomatitis virus (VSV) can infect vascular endothelial cells.
(A, B) Representative views of VSV infected vessels in the mitral cell layer (MCL) and glomerular layer (GL), as indicated by the red arrows and red star. Blue, DAPI for nuclei; Green, VSV-GFP; Orange, Alexa Fluor 647 conjugated anti-CD31. (C) Bleedings were observed from VSV infected vessels in the MCL. (D) Quantification of vessels colocalized with virus only or colocalized with virus and RBCs in the GL on day 6, under 40 x view (0.2 mm x 0.2 mm). n=3 mice. 6 views were counted manually per mouse. Orange, Alexa Fluor 594 conjugated anti-CD31; Red, Alexa Fluor 700 conjugated anti-Ter119 for RBCs.
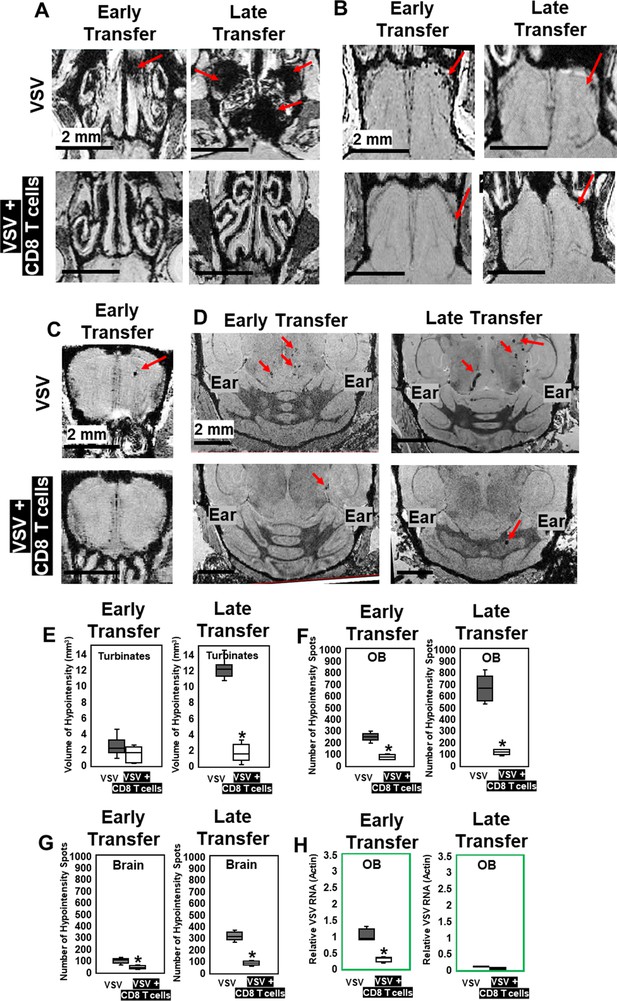
MRI study showed that adoptive CD8 T cells transfer reduced cerebrovasculature breakdown.
(A–D) MRI showed that both early and late transfer of OT-I CD8 T cells reduced bleeds in the turbinates (A), GL of the OB (B), center of the OB (C) and brain (D). Red arrows, microbleeds. Quantification of volume of bleeding in the turbinates (E) and numbers of hypointensity spots in the OB (F) and brain (G) without or with T cells early and late transfer (n=5–7 mice per group). * p<0.0001. (H) CD8 T cells reduced viral RNA on day 6 (n=4). * p<0.0001.
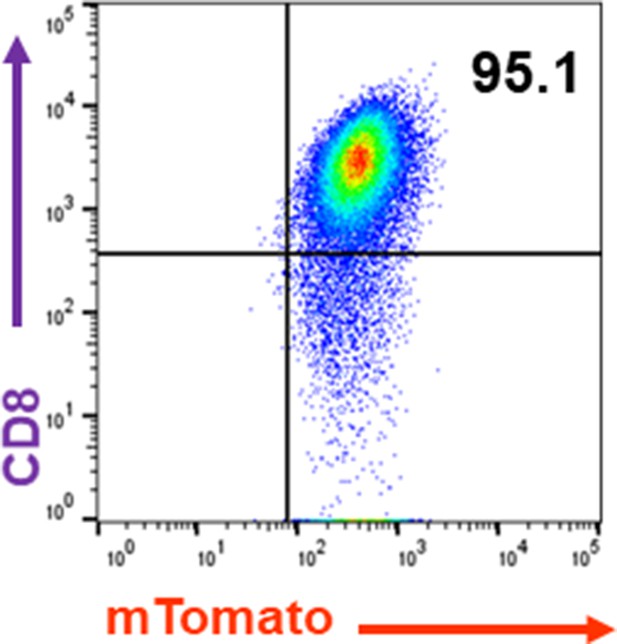
Purity of CD8 T cells used in this study.
CD8 T cells were isolated using the Dynabeads Untouched Mouse CD8 Cells Kit.
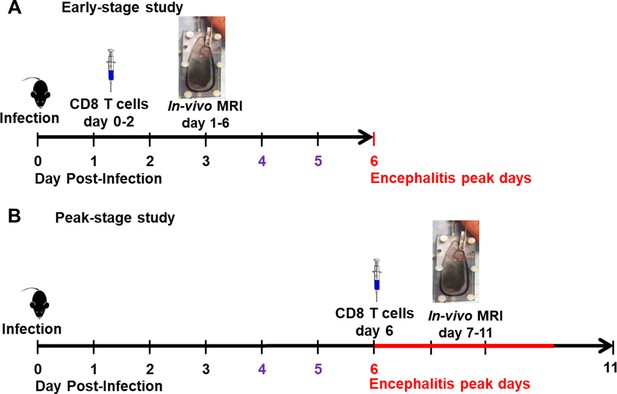
Experiment paradigms.
(A) Paradigm for CD8 T cell transfer study during early stage infection. CD8 T cells were transferred on day 0–2. In-vivo MRI study was performed from day 1–6. The encephalitis peak time was day 6, which was labeled in red. Remarkable amount of CD45+ cells infiltration into the olfactory bulb (OB) was reported since day 4, which was labeled in purple. (B) Paradigm for CD8 T cell transfer study during peak-stage infection. CD8 T cells were transferred on day 6. In-vivo MRI study was performed from day 7–11.
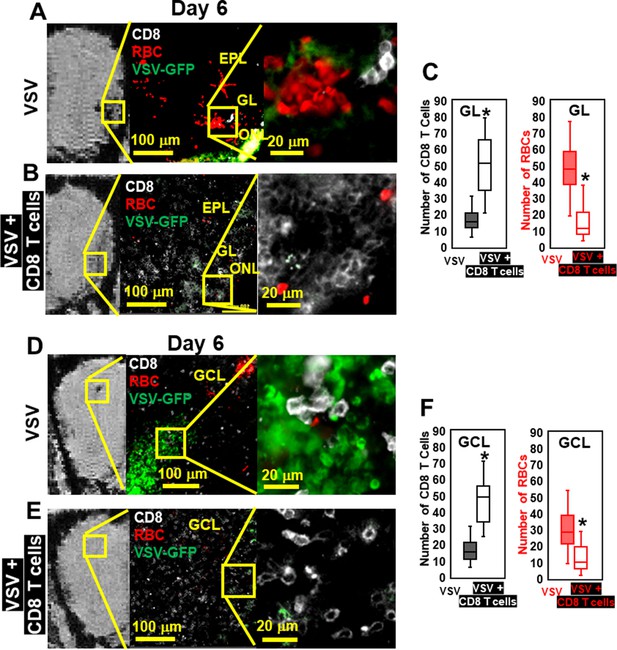
Immunohistochemical (IHC) study verified that antiviral CD8 T cells reduced VSV-induced brain bleeding.
(A) On day 6, VSV-induced bleeding at the olfactory nerve layer (ONL) and glomerular layer (GL) and there was a small number of CD8 T cells. (B) CD8 T cells transfer reduced bleeding and virus and there was a larger number of CD8 T cells at the ONL and GL. (D, E) In the granule cell layer (GCL), T cell transfer also reduced bleeding and virus comparing with non-T cells transferred. White, Brilliant Violet 421 conjugated anti-CD8 for T cells; Green, VSV-GFP; Red, Alexa Fluor 647 conjugated anti-Ter119 for RBCs. (C, F) Quantification of CD8 T cells and bleeds in the GL and GCL, under 40 x view (0.2 mm x 0.2 mm). n=4–6 mice per group. Six views were counted manually per mouse. * p<0.0001.
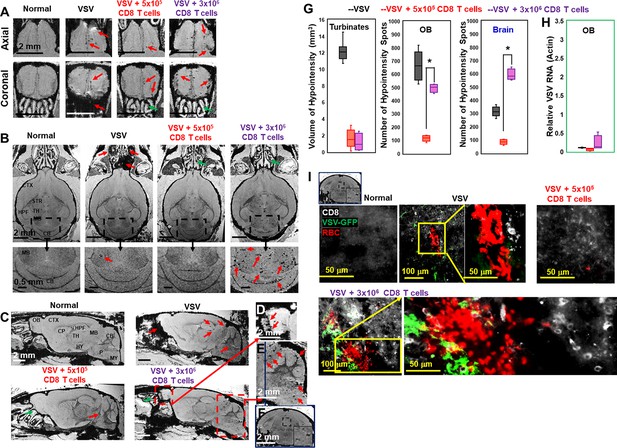
Adoptive transfer of overdose of CD8 T cells (3 × 106 T cells) at the peak stage caused bleeding in the olfactory bulb (OB) and brain.
T cells are transferred on day 6 and images were shown for day 11. Axial views (A), coronal views (B), and sagittal views (C) of normal mice, vesicular stomatitis virus (VSV) infected mice with no T cells transfer, with the transfer of 5 × 105 CD8 T cells, with the transfer of 3 × 106 CD8 T cells. (D, E, F) were enlarged views. Green arrows, reduced bleeding in the turbinates. Red arrows, bleeding in the turbinates, OB, or brain. (G) Quantification of bleeding volume in turbinates, and number of hypointensity spots in OB and brain without (black bar, n=7) or with T cell transfer (red bar, 5 × 105 T cells, n=5; purple bar, 3 × 106 T cells, n=5) on day 11. * p<0.0001 comparing with 5 × 105 T cells. (H) Viral titer. (I) IHC study showed large amount of VSV, CD8 T cells, and RBCs in the midbrain of overdose treated mice. Green, VSV-GFP; White, OT-1 CD8 T cells; Red, Ter119 for RBCs.
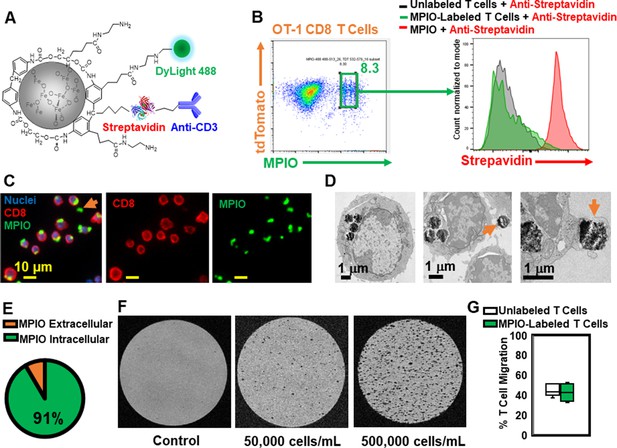
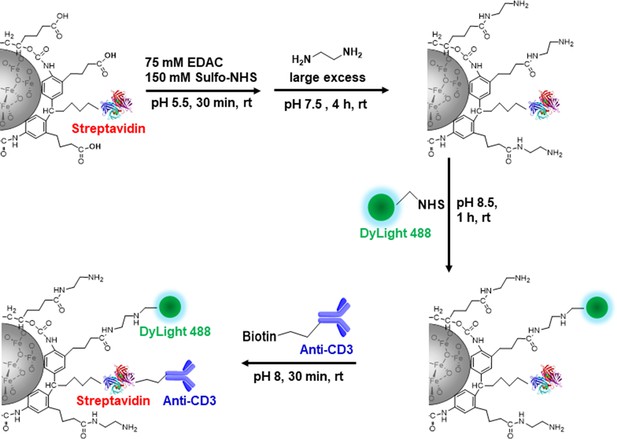
Label CD8 T cells with MPIO for Magnetic resonance imaging (MRI) investigation.
(A) A representative structure of the conjugated MPIO particle used in this study to label T cells. (B) FACS MPIO-labeled CD8 T cells, defined as DAPI-, mTomato+, DyLight 488+, DyLight 650-. (C) Fluorescence images of MPIO-labeled T cells. Green, MPIO-DyLight 488; Red, Alexa Fluor 647 conjugated anti-CD8. (D) EM images of CD8 T cells labeled with MPIO intracellularly and extracellularly. (E) Quantification of intracellular and extracellular MPIO through EM images. (F) MR sensitivity of labeled T cells. (G) Percentage of migrating T cells in the migration assay (n=6).
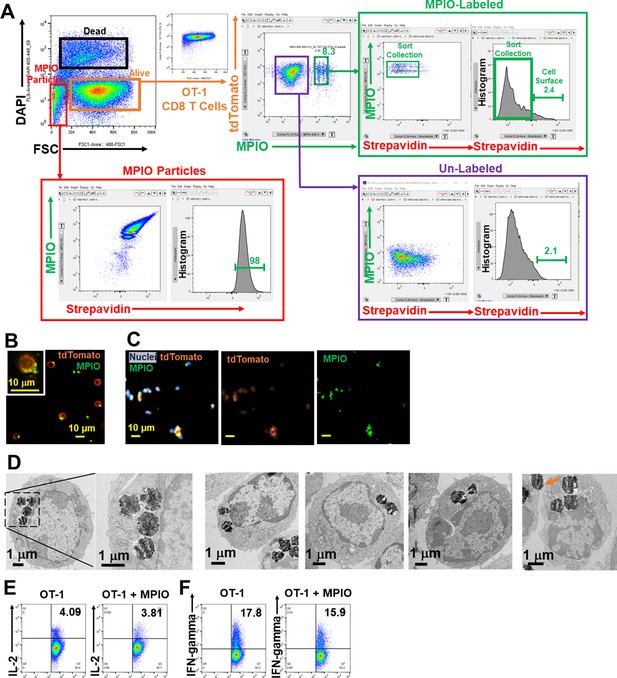
Label CD8 T cells with MPIO.
(A) FACS method for isolating MPIO-labeled T cells. (B, C) Fluorescence images of MPIO-labeled T cells in the cell culture (B) and after fixation with 2% PFA for 15 min (C). (D) Representative EM images of MPIO-labeled T cells. (E, F) Labeling T cells with MPIO did not affect T cell function on IL-2 (E) and IFN-gamma release (F).
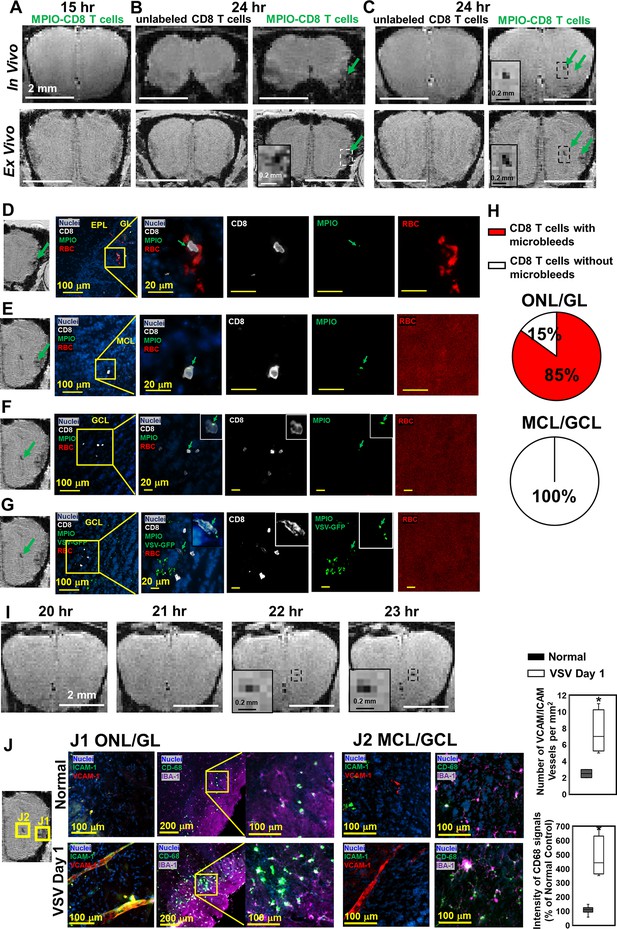
Magnetic resonance imaging (MRI) tracking of virus-specific CD8 T cells and detection of brain viral infection on day 1.
(A) No hypointensity was detected at 15 hr post-infection in the olfactory bulb (OB). (B, C) The earliest hypointensity spots were detected near the GL (B) and the center, mitral cell layer (MCL) to granule cell layer (GCL) (C), at 24 hr post-infection. Similar hypointensities were not detected in the controls infused with unlabeled CD8 T cells. RBCs were detected at the GL (D), but not near the MCL (E) and GCL (F, G), as shown by Immunohistochemical (IHC). In (D–F), Vesicular stomatitis virus (VSV) with no GFP was used to verify the presence of MPIO-DyLight 488 in the T cells. White, Alexa Fluor 594 conjugated anti-CD8 for T cells. Green, VSV-GFP or MPIO-DyLight 488; Red, Alexa Fluor 700 conjugated anti-Ter119 for RBCs. (H) Frequencies of MPIO-labeled T cells present with microbleeds versus without microbleeds at the ONL/GL and MCL/GCL [under 40 x view (0.2 mm x 0.2 mm); n=4 mice; 35 MPIO-labeled T cells total: 26 cells at the ONL/GL and 9 cells at the MCL/GCL]. (I) The appearance of MPIO-labeled T cells at the center at 22 hr post-infection. (J) Activation of vessel endothelium cells and microglia were found at the ONL/GL (J1) and MCL/GCL (J2) on day 1. n=3 per group. 3–6 views were quantified per mouse. * p<0.0001.
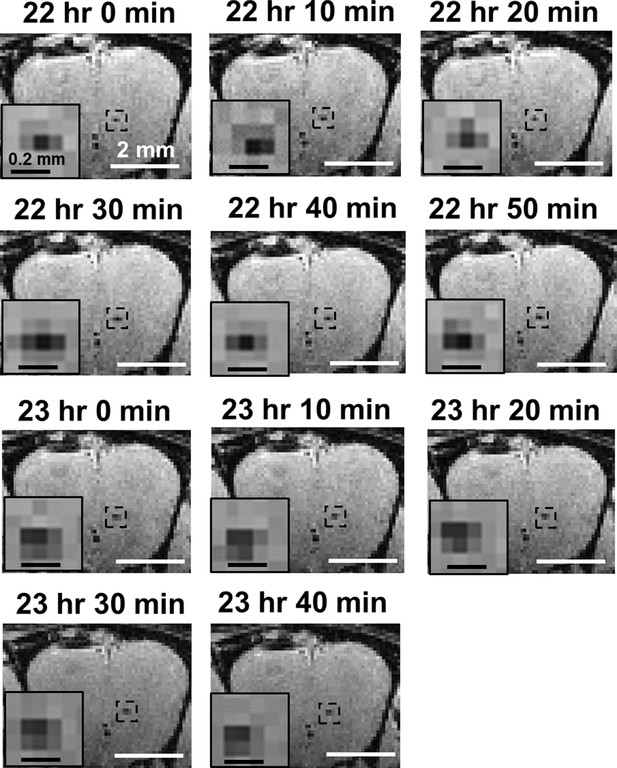
Time-lapse magnetic resonance imaging (MRI) showing the possible movement of MPIO-labeled T cells at the center of olfactory bulb (OB) from 22–24 hr-post-infection.
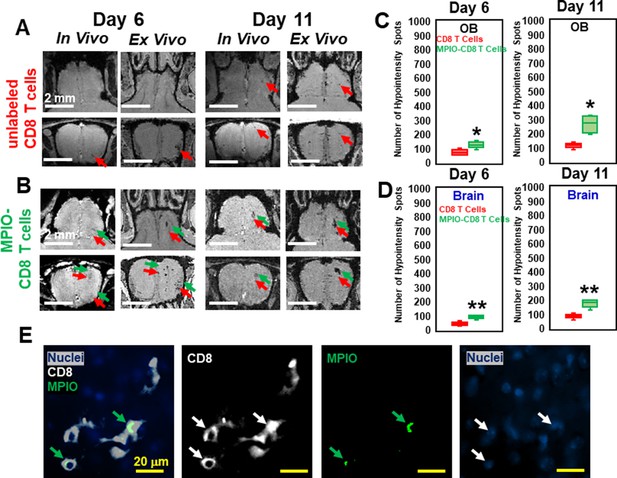
MPIO-labeled T cells increased magnetic resonance imaging (MRI) sensitivity to microbleeds.
(A) MRI, on day 6 and 11, of olfactory bulb (OB) from the mice transferred with unlabeled CD8 T cells. (B) MRI from the mice transferred with MPIO-labeled CD8 T cells. Red arrows, hypointensities caused by microbleeds. Green arrows, hypointensities caused by infiltration of MPIO-labeled CD8 T cells. Quantification of the number of hypointensity spots in OB (C) and brain (D) on day 6 and 11, upon transfer of unlabeled CD8 T cells (n=5 mice per group) or MPIO-labeled CD8 T cells (n=7 mice per group). Red bar, quantification from transfer of unlabeled T cells. Green bar, quantification from transfer of MPIO-labeled T cells. * p<0.001; ** p<0.0001. (E) Immunohistochemical (IHC) study showed the infiltration of MPIO-labeled CD8 T cells. Green arrows, MPIO. Blue, nuclei; White, CD8 T cell; Green, MPIO.
Videos
Coronal views of the whole brain at 21 hr post-infection, before the appearance of the hypointensity.
Coronal views of the whole brain at 22 hr post-infection, after the appearance of the hypointensity.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Cell line (Mus musculus) | C57BL/6-Tg(TcraTcrb)1,100Mjb/J (OT-I) mice | The Jackson Laboratory | Cat#: 003831 | |
| Cell line (Mus musculus) | B6.129(Cg)-Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J (mTomato) | The Jackson Laboratory | Cat#: 007676 | |
| Antibody | Brilliant Violet 421 conjugated anti-CD8 (Clone 53–6.7) (Rat monocolonal) | BioLegend | Cat# 100,753 | (1:100 dilution) (2 μg/mL) for IHC |
| Antibody | Alexa Fluor 594 conjugated anti-CD8 (Clone 53–6.7) (Rat monocolonal) | BioLegend | Cat# 100,758 | (1:100 dilution) (2 μg/mL) for IHC |
| Antibody | Alexa Fluor 700 conjugated anti-CD8 (Clone 53–6.7) (Rat monocolonal) | BioLegend | Cat# 100,730 | (1:80 dilution) ≤0.25 µg per 106 cells in 100 µl volume for flow cytometry |
| Antibody | Brilliant Violet 421 conjugated anti-CD45 (Clone 30-F11) (Rat monocolonal) | BioLegend | Cat# 103,134 | (1:80 dilution) ≤0.25 µg per 106 cells in 100 µl volume for flow cytometry |
| Antibody | APC conjugated anti-Thy1.2 (Clone 30-H12) (Rat monocolonal) | BioLegend | Cat# 105,312 | (1:80 dilution) ≤0.25 µg per 106 cells in 100 µl volume for flow cytometry |
| Antibody | Brilliant Violet 605 conjugated anti-CD11b (Clone M1/70) (Rat monocolonal) | BioLegend | Cat# 101,257 | (1:80 dilution) ≤0.25 µg per 106 cells in 100 µl volume for flow cytometry |
| Antibody | Brilliant Violet 785 conjugated anti-Ly6C (Clone HK1.4) (Rat monocolonal) | BioLegend | Cat# 128,041 | (1:80 dilution) ≤0.25 µg per 106 cells in 100 µl volume for flow cytometry |
| Antibody | PE conjugated anti-Ly6G (Clone 1A8) (Rat monocolonal) | BioLegend | Cat# 127,608 | (1:80 dilution) ≤0.25 µg per 106 cells in 100 µl volume for flow cytometry |
| Antibody | Alexa Fluor 594 conjugated anti-CD31 (Clone 390) (Rat monocolonal) | BioLegend | Cat# 102,432 | (1:250 dilution) (2 μg/mL) for IHC |
| Antibody | Alexa Fluor 647 conjugated anti-CD31 (Clone 390) (Rat monocolonal) | BioLegend | Cat# 102416 | (1:250 dilution) (2 μg/mL) for IHC |
| Antibody | Alexa Fluor 647 conjugated anti-TER-119 (Rat monocolonal) | BioLegend | Cat# 116,218 | (1:200 dilution) (2.5 μg/mL) for IHC |
| Antibody | Alexa Fluor 647 conjugated anti-TER-119 (Rat monocolonal) | BioLegend | Cat# 116,220 | (1:200 dilution) (2.5 μg/mL) for IHC |
| Antibody | anti-IBA-1 (Rabbit polycolonal) | FUJIFILM Wako | Distributor Barcode No 019–19741 4987481428584 | (1:500 dilution) (0.4 μg/mL) for IHC |
| Antibody | anti-CD68 antibody (Clone FA-11) (Rat monocolonal) | Bio-Rad | Cat# MCA1957GA | (1:200 dilution) (5 μg/mL) for IHC |
| Antibody | Alexa Fluor 647 conjugated anti- CD106/VCAM-1 (Clone 429 or MVCAM.A) (Rat monocolonal) | BioLegend | Cat# 105,712 | (1:250 dilution) (2 μg/mL) for IHC |
| Antibody | Alexa Fluor 488 anti-CD54/ICAM-1 (Clone YN1/1.7.4) (Rat monocolonal) | BioLegend | Cat# 116,112 | (1:250 dilution) (2 μg/mL) for IHC |
| Antibody | Anti-LFA-1 (Clone M17/4) (Rat monocolonal) | BioCell | Cat# BE0006 | (500 μg each IP injection) |
| Antibody | anti-VLA-4 (Clone PS/2) (Rat monocolonal) | BioCell | Cat# BE0071 | (500 μg each IP injection) |
| Antibody | rat IgG2a (Rat monocolonal) | BioCell | Cat# BE0089 | (1000 μg each IP injection) |
| Antibody | APC conjugated anti-IFN-γ (Clone XMG1.2) (Rat monocolonal) | BioLegend | Cat# 505,809 | (1:20 dilution) ≤1 µg per 106 cells in 100 µl volume for flow cytometry |
| Antibody | PE/Cy7 conjugated anti-IL-2 (Clone JES6-5H4) (Rat monocolonal) | BioLegend | Cat# 503,831 | (1:160 dilution) ≤0.125 µg per 106 cells in 100 µl volume for flow cytometry |
| Antibody | anti-Streptavidin (Rabbit polycolonal) | Thermo Fisher Scientific | Cat# S6390 | (20 μl for 107T cells for FACS) |
| Antibody | biotin anti-CD3 (Clone 145–2 C11) (Rat monocolonal) | BioLegend | Cat# 100,304 | 25 μl (12.5 μg) for 5 × 109 MPIO particles during conjugation |
| Commercial assay or kit | Dynabeads Untouched mouse cd8 cells kit | Invitrogen | Cat# 11,417D | |
| Commercial assay or kit | QCM chemotaxis cell migration assay, 24-well (3 μm) | Millipore Sigma | Cat# ECM505 | |
| Commercial assay or kit | Cyto-Fast Fix/Perm buffer set | BioLegend | Cat# 426,803 | |
| Commercial assay or kit | miRNeasy mini kit | Qiagen | Cat# 217,084 | |
| Commercial assay or kit | iScript cDNA synthesis kit | Bio-Rad | Cat# 1708890 | |
| Commercial assay or kit | SYBR Green PCR master mix | Applied Biosystems | Cat# 1708890 | |
| Chemical compound, drug | DyLight 488-NHS | Thermo Fisher Scientific | Cat# 46,403 | |
| Chemical compound, drug | DyLight 650-NHS | Thermo Fisher Scientific | Cat# 62,266 | |
| Chemical compound, drug | recombinant murine JE/MCP-1 (CCL2) | Peprotech | Cat# 250–10 | 10 ng/mL for migration assay |
| Chemical compound, drug | OVA257-264 peptide | Sigma Aldrich | Cat# S7951 | 2 μg/ml for OT-I cell culture |
| Chemical compound, drug | 2-Mercaptoethanol | Sigma Aldrich | Cat# M3148 | |
| Chemical compound, drug | 2-(N-morpholino)ethanesulfonic acid | Sigma Aldrich | Cat# M3885 | |
| Chemical compound, drug | 1-Ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride | Sigma Aldrich | Cat# E6383 | |
| Chemical compound, drug | sulfo-N-hydroxysuccinimide | Sigma Aldrich | Cat# 130,672 | |
| Chemical compound, drug | Ethylenediamine | Sigma Aldrich | Cat# E26266-100ML | |
| Chemical compound, drug | brefeldin A | BioLegend | Cat# 420,601 | |
| Software, algorithm | MIPAV | http://mipav.cit.nih.gov | ||
| Other | DAPI stain | Invitrogen | Cat# D1306 | (1 µg/mL) |
| Other | flow cytometry staining buffer | Invitrogen | Cat# 00-4222-26 | |
| Other | Dynabeads MyOne Streptavidin C1 | Thermo Fisher Scientific | Cat# 65,001 |