Muscle systems and motility of early animals highlighted by cnidarians from the basal Cambrian
Figures
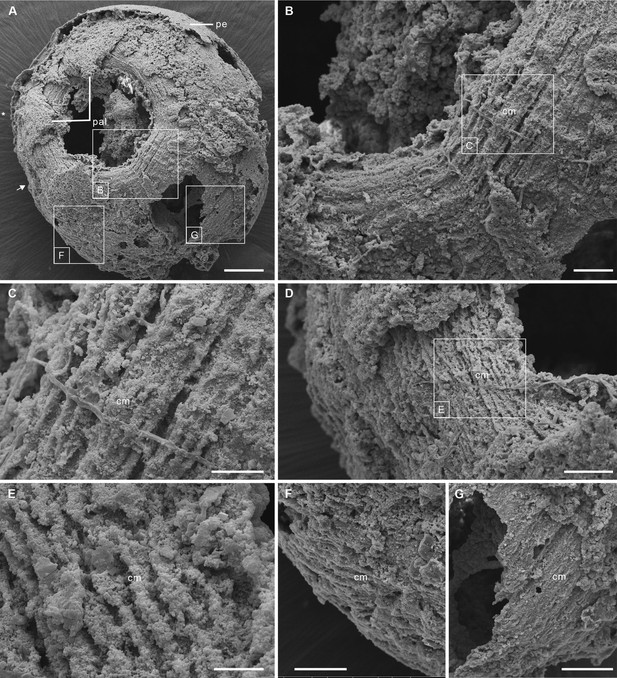
Post-embryonic stage of Olivooides sp. from the early Cambrian Kuanchuanpu Formation (ca. 535 Ma; South China), showing exposed muscle fibers. ELISN150-278. Scanning electron micrographs.
(A) General view of oral side. (B) Details of fiber bundles around aperture (location indicated in A). (C) Close-up showing individual fibers within each bundle. (D) Dense network of fibers (location indicated in A). (E) Close-up of individual fibers coated with fine grains of calcium phosphate. (F), (G) Circular fibers approximately half way between the oral and aboral poles. Abbreviations: cm, circular muscle; pal, perradial apertural lobe; pe, periderm; *, perradii; →, interradii. Scale bars: 100 μm in (A); 20 μm in (B); 10 μm in (C), (E); 20 μm in (D), (F) and (G).
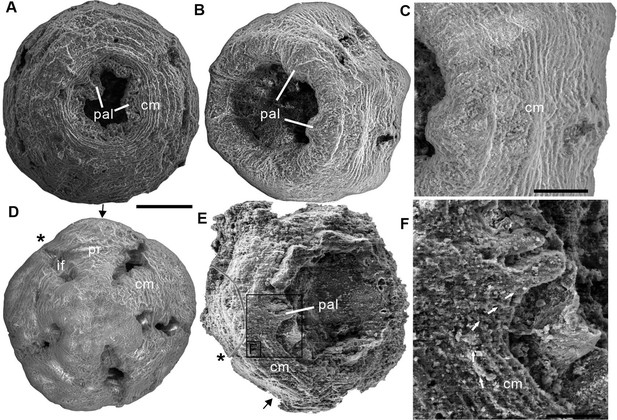
Three scanning electron micrographs of the Olivooides specimens.
ELISN012-16, ELISN045-143, and ELISN111-54 from the Kuanchuanpu Formation, south China. (A) Oral view showing the external morphology of ELISN045-143 without periderm. (A), (D) ELISN012-16. General view of oral side (A), details of muscles fibers in perradial apertural lobes and general view of aboral side (D). (B) Oral view showing the external morphology of ELISN045-143 without periderm. (C) Close-up view of a showing the circular muscles. (E) Oral view showing the external morphology of ELISN111-54. (F) The enlarged view of c showing that the circular muscles extending into the triangular perradial apertural lobes marked by the giant white arrows. Abbreviations: cm, circular muscle; pal, perradial apertural lobe; *, perradii; →, interradii. Scale bars represent: 200 μm in (A), (B), (D) and (E); 50 μm in (C); 25 μm in (F).
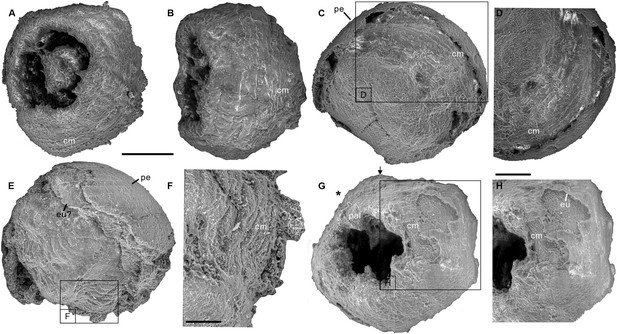
Four scanning electron micrographs of the Olivooides specimens.
ELISN087-64 (A, B), ELISN088-48 (C, D), ELISN087-33 (E, F) and ELISN98-19 (G, H) from the Kuanchuanpu Formation, south China. (A) General view of oral side. (B) Lateral view. (C) General view of aboral side. (D) The enlarged view of (C) showing the circular muscles. (E) Lateral view. (F) The enlarged view of (E) showing that the circular muscles. (G) General view of oral side. (H) The enlarged view of (G) showing the circular muscles. Abbreviations: cm, circular muscle; eu, exumbrella; pal, perradial apertural lobe; *, perradii; →, interradii. Scale bars represent: 200 μm in (A–C), (E) and (G); 50 μm in (F), (H); 25 μm in (D).
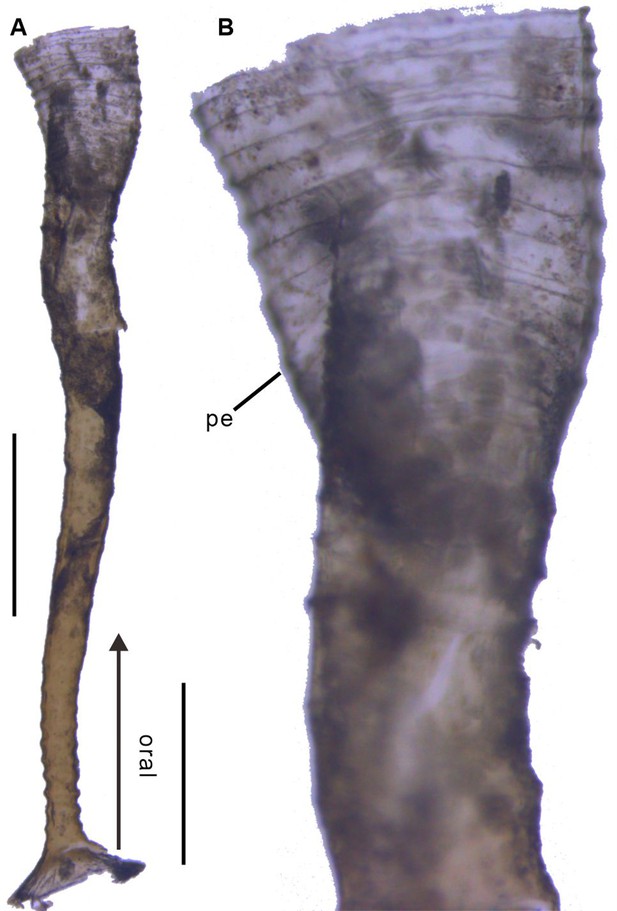
Polyp of Coronatae sp. from South China Sea showing the periderm.
(A), (B) Lateral view and details showing corrugations. Abbreviations are as follows: pe, periderm. Scale bars: 400 μm in (A); 100 μm in (B).
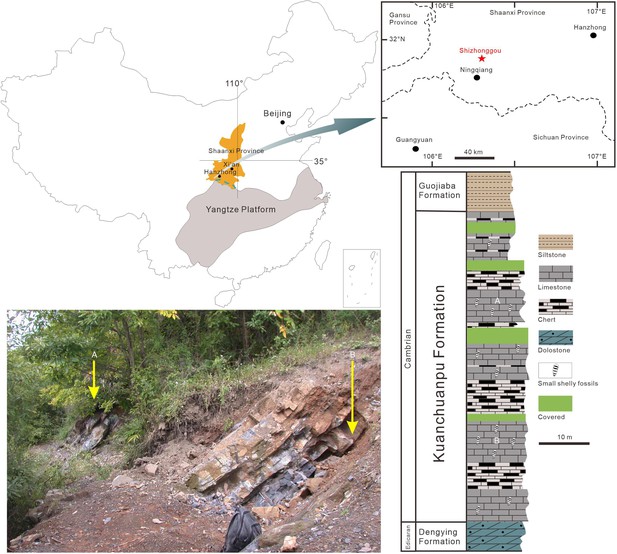
Origin of the fossil material.
Location map (Ningqiang area, Southern Shaanxi Province, China), photograph of outcrop (Shizhonggou Section; see red star) and stratigraphic position of fossil-bearing rock samples (A), (B) within the Kuanchuanpu Formation. Modified from Steiner et al., 2014.
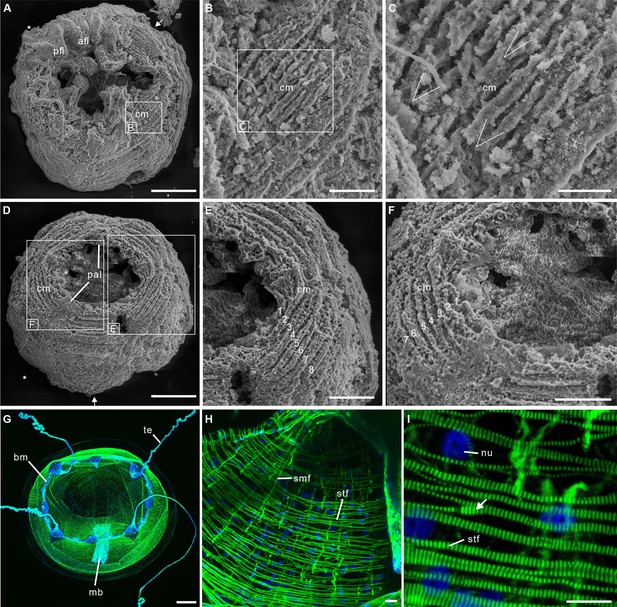
Post-embryonic stage of Olivooides sp. from the early Cambrian Kuanchuanpu Formation (ca. 535 Ma; South China), showing exposed muscle fibers by SEM.
(A–F) and myoepithelial muscle network in extant hydrozoan jellyfish by fluorescence microscopy (G–I). (A–C) ELISN052-33. General view of oral side and details of apertural circular muscle fibers and the V-shaped interconnection between the fibers in (C). (D–F) ELISN061-19. General view of oral side and details of apertural, circular muscles fibers. (G–I) Eirene sp. (Hydrozoa) young medusa, general oral view, circular muscles along subumbrella and details of striated fibers; white arrow (I) indicates bifurcating fibers. Green and blue in (G–I) correspond to actin (phalloidin) and DNA (Hoechst) staining. Abbreviations: afl, adradial fold lappet; bm, bell margin; cm, circular muscle; mb, manubrium; nu, nucleus; pal, perradial apertural lobe; pfl, perradial fold lappet; smf, smooth (radial) muscle fiber; stf, striated (circular) muscle fiber; te, tentacle *, perradii; →, interradii. Scale bars: 200 μm in (A), (D); 100 μm in (G); 50 μm in (E), (F); 20 μm in (B); 10 μm in (C), (H) and (I).

Two scanning electron micrographs of the Olivooides specimens.
ELISN115-39 (Sinaster petalon; Wang et al., 2017) and ELISN107-470 (Hanagyroia orientalis; Wang et al., 2020) from the Kuanchuanpu Formation, south China. (A) Oral view showing the external morphology of ELISN115-39. (B) Close-up view of (A) showing the possible longitudinal muscle bands marked by the two rows of giant white arrows. (C) Oral view showing the external morphology of ELISN107-470. (D) The enlarged view of (C) showing the possible rings of nematocysts on the tentacles. Abbreviations: alm?, possible longitudinal muscle bands at adradii; cm, circular muscle; eu, exumbrella; pal, perradial apertural lobe; pe, periderm; *, perradii; →, interradii. Scale bars represent: 200 μm in (A), (C); 50 μm in (B); 25 μm in (D).
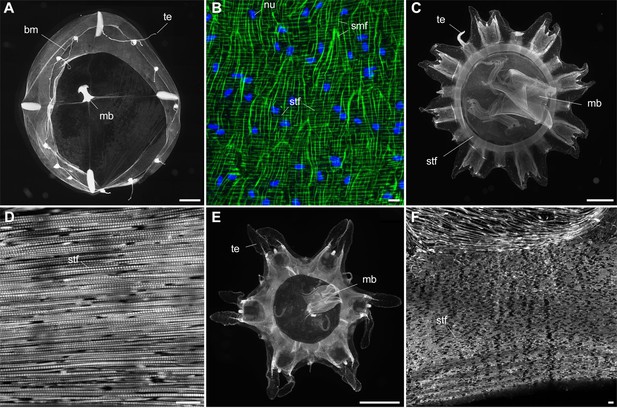
Myoepithelial muscle network in extant medusozoan cnidarians.
(A), (B) Clytia hemisphaerica (Hydrozoa) mature medusa, general oral view and details of fiber network (subumbrella). (C), (D) Pelagia noctiluca (Scyphozoa) metaephyra, general oral view and details of closely-packed circular striated muscle fibers (subumbrella). (E), (F) Chrysaora colorata (Scyphozoa) metaephyra, general oral view and details of circular striated muscle fibers. Green and blue colors (B) correspond to actin (phalloidin) and DNA (Hoechst) staining. Specimens represented by black and white images (A) and (C–F) were stained for actin (phalloidin). Abbreviations are as follows: bm, bell margin; mb, manubrium; nu, nucleus; smf, smooth (radial) muscle fiber; stf, striated (circular) muscle fiber; te, tentacle. Scale bars: 1000 µm in (A), (C) and (E); 10 µm in (B), (D) and (F).
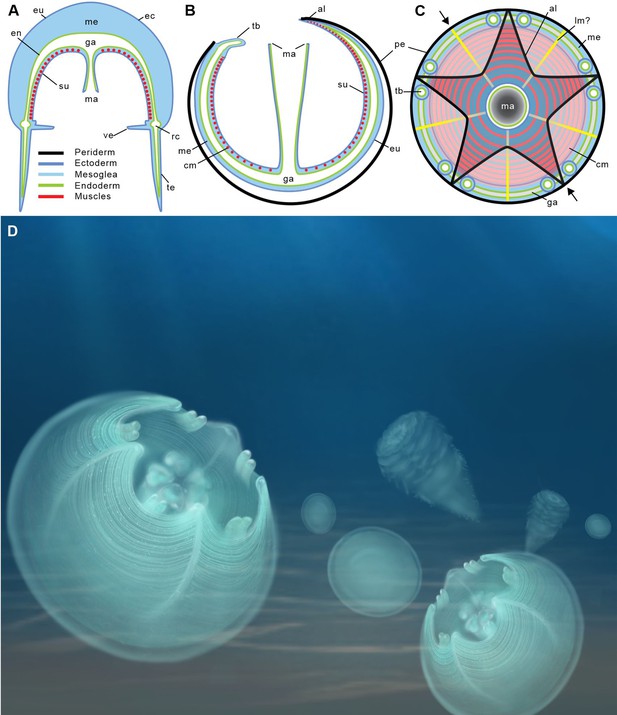
Location of epithelial muscles in extant hydromedusae (A) and early Cambrian Olivooidae medusozoans (B, C).
(A), (B) Simplified radial sections through body. (C) In oral view. (D) Artistic reconstruction of 535-million-year-old olivooid cnidarians showing eggs (the prehatched, background), post-embryonic (foreground) and polyp (background) stages. The circular muscle system is visible through the translucent periderm. The location of section (B) is indicated in (C) by small black arrows. Abbreviations: al, apertural lobe; cm, circular (coronal) muscle; en, endoderm; eu, exumbrella; ga, gastrovascular cavity; lm?, possible longitudinal muscle; ma, manubrium; me, mesoglea; pe, periderm; rc, radial canal; su, subumbrella; tb, tentacular bud; te, tentacle; ve, velum. Not to scale.
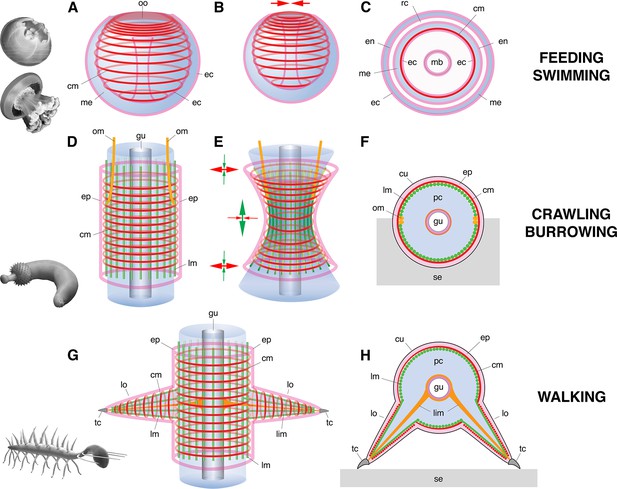
Basic muscle systems in early Cambrian animals and their main functions.
(A–C) Contractile epithelial muscles (MEC, myoepithelial, predominantly circular) and antagonistic mesoglea exemplified by olivooiids and free-swimming jellyfish; idealized relaxed (A) and contracted (B) states and simplified transverse section (C). (D–F) Grid-like network of circular and longitudinal subepidermal muscle fibers (HMS) around a cylindrical body filled with antagonistic internal fluid (hydroskeleton) exemplified by scalidophoran worms: idealized relaxed state (D); peristaltic contractions along body (E) and transverse section (F). (G–H) Longitudinal, circular muscles and extrinsic retractor muscles in lobopodians: idealized relaxed state (G); transverse section (H). Images (from top to bottom) represent an olivooid cnidarian, an extant jellyfish, the scalidophoran worm Ottoia (see Vannier, 2012) and the lobopodian Hallucigenia (see Chen and Zhou, 1997). Drawings and images not to scale. Abbreviations: cm, circular muscle; cu, cuticle; ec, ectoderm; en, endoderm; ep, epidermal layer; gu, gut; lc, lobopod claw; lim, limb muscle; lm, longitudinal muscle; lo, lobopod (soft leg); mb, manubrium; me, mesoglea; om, oblique muscle; oo, oral opening (bell margin); pc, primary cavity filled with fluid; rc, radial canal; se, sediment; tc, terminal claw.
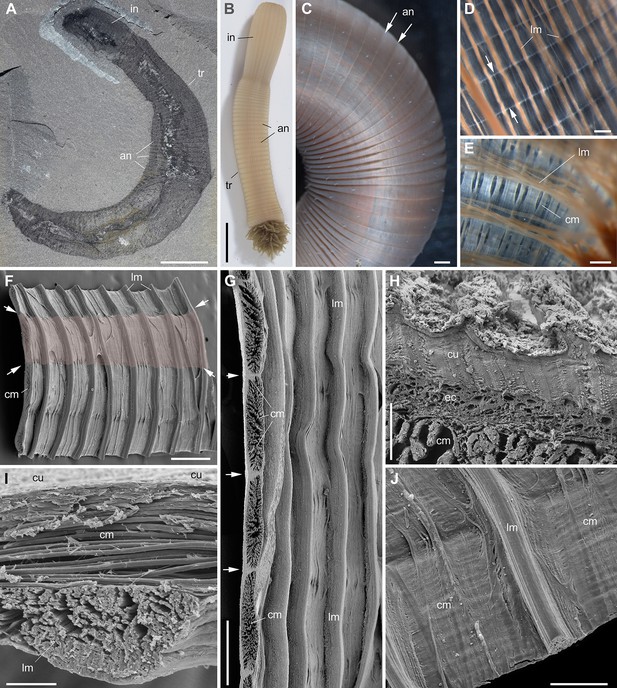
Muscle system in priapulid worms (Ecdysozoa).
(A) Ottoia prolifica from the mid-Cambrian Burgess Shale, general view showing annulated body. (B–J) Priapulus caudatus from Sweden. (B) General view of live specimen in sea water. (C) Annulated trunk. (D) Muscles (trunk). (E) Muscles (introvert). (F) Fragment of trunk showing the internal body wall with strong longitudinal muscles (pink area corresponds to one annulation). (G) Transverse section through circular muscles (one bundle per annulation). (H) Transverse section through body wall across cuticle, epithelial cells, and circular muscles. (I) Transverse section through body wall showing circular muscles overlying longitudinal ones. (J) Inner wall of body showing both circular and longitudinal muscles. Small white arrows indicate annulation boundaries. (A–E) are light photographs. (F–J) are SEM images. Abbreviations are as follows: an, annulation; cm, circular muscle; cu, cuticle; ec, epithelial cells; introvert; lm, longitudinal muscle; tr, trunk. Scale bars: 1 cm in (A), (B); 1 mm in (C); 500 µm in (D–G); 200 µm in (J); 50 µm in (I) and 10 µm in (H).
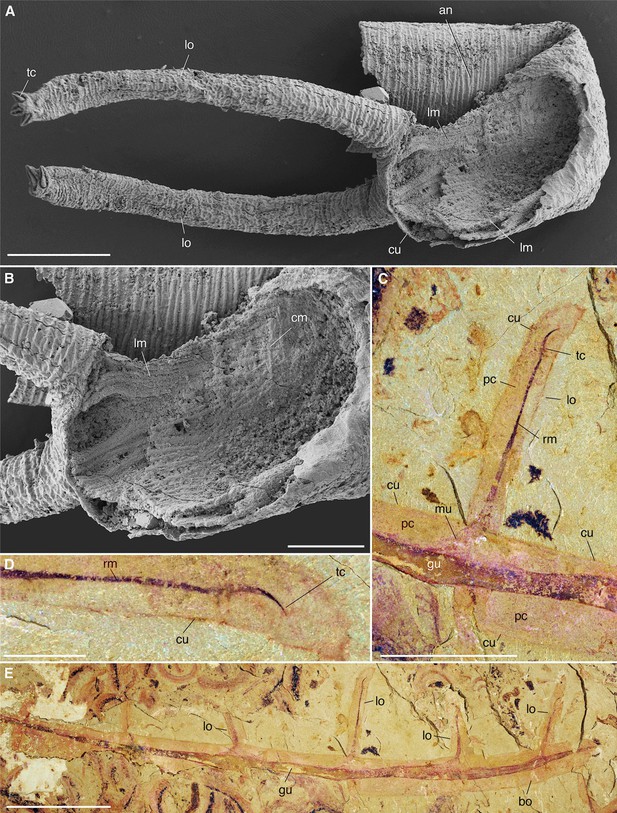
Muscle system in Cambrian lobopodians (Ecdysozoa, Panarthropoda).
(A), (B) Tritonychus phanerosarkus from the Yu’anshan Formation (Xiaotan section, Cambrian Series 2, Stage 3), Yongshan, Yunnan Province, China; general view and details of a secondarily phosphatized specimen showing a pair of walking appendages (lobopods) and the inner body wall lined with muscle fibers (see Zhang et al., 2016). (C–E) Paucipodia haikouensis from the Chengjiang Lagerstätte, ELI-JS0001a (see Vannier and Martin, 2017); close-up of lobopod and terminal claw, and general view; note muscle bridging claw to gut area. (A), (B) are SEM images (Courtesy Prof. Xiguang Zhang; see Zhang et al., 2016). Abbreviations: an, annuli; cm, circular muscle; cu, cuticle; gu, gut; lm, longitudinal muscle; lo, lobopod; mu, muscle; pc, primary cavity; rm, retractor muscle; tc, terminal claw. Scale bars: 10 mm in (E), 5 mm in (C), 1 mm in (D), 200 µm in (A) and 100 µm in (B).





