Host casein kinase 1-mediated phosphorylation modulates phase separation of a rhabdovirus phosphoprotein and virus infection
Figures
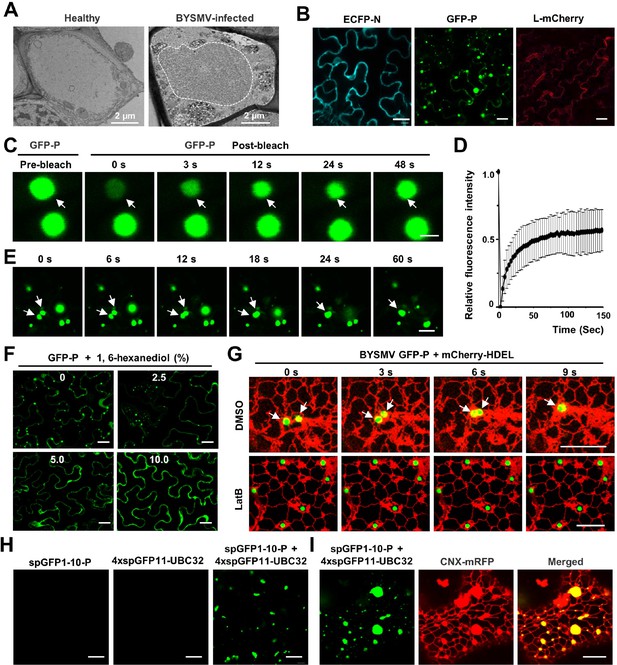
Barley yellow striate mosaic virus (BYSMV) phosphoprotein (P) protein forms liquid-like granules through liquid-liquid phase separation (LLPS) in vivo.
(A) Transmission electron microscopy characterization of the BYSMV viroplasm in barley stems infected by BYSMV at 10 days post infiltration (dpi). The electron dense granular structure of the BYSMV viroplasm is highlighted by white dotted line. Healthy stems served as negative control. Scale bars, 2 μm. (B) Confocal images showing subcellular distribution of ECFP-N, GFP-P, and L-mCherry in epidermal cells of Nicotiana benthamiana leaves at 2 dpi. Scale bars, 20 μm. (C) Representative fluorescence recovery after photobleaching (FRAP) images of GFP-P granules in epidermal cells of N. benthamiana leaves at 2 dpi. Leaves were treated with 10 μM latrunculin B (LatB) to inhibit movement of GFP-P granules at 3 hr before photobleaching. Scale bar, 2 μm. (D) FRAP recovery curves of GFP-P granules. The intensity of each granule was normalized against their pre-bleach fluorescence. Data were presented as mean ± SD of 15 granules. (E) Confocal images showing fusion of two GFP-P granules in N. benthamiana leaf epidermal cells. White arrows indicate that GFP-P granules undergo fusion. Scale bar, 10 μm. (F) Representative images showing GFP-P localization after treatment with 0, 2.5%, 5.0%, 10.0% of 1,6-hexanediol for 5 min in N. benthamiana leaf epidermal cells. Scale bars, 20 μm. (G) Time-lapse confocal micrographs showing the localization of GFP-P and mCherry-HDEL expressed in N. benthamiana leaf epidermal cells at 2 dpi. Leaves were treated with DMSO or 10 μM LatB at 3 hr before imaging. White arrows indicate fusion of two GFP-P granules. Scale bars, 10 μm. (H) Confocal micrographs of N. benthamiana leaf epidermal cells expressed spGFP1-10-P, 4× spGFP11-UBC32, or both at 2 dpi. Scale bars, 20 μm. (I) Confocal micrographs of N. benthamiana leaf epidermal cells spGFP1-10-P, 4× spGFP11-UBC32, and CNX-RFP at 2 dpi. The green signals indicate the contact sites of spGFP1-10-P granules with the tubular ER network. CNX-RFP is an ER marker. Scale bar, 10 μm.
-
Figure 1—source data 1
Fluorescence intensities of the bleached droplets during the time course experiment.
(Related to Figure 1D).
- https://cdn.elifesciences.org/articles/74884/elife-74884-fig1-data1-v3.xlsx
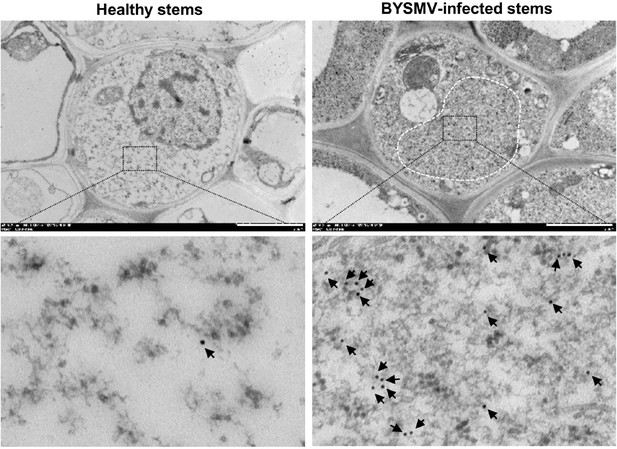
Immunoelectron microscopy detecting the barley yellow striate mosaic virus (BYSMV) phosphoprotein (P) protein in BYSMV-RFP-infected stems.
BYSMV-RFP-infected stems at 9 days post infiltration (dpi) or healthy stems were subjected to immunoelectron microscopy. P-specific antiserum showed specific labeling of BYSMV viroplasm (black arrows) in the cytoplasm. The electron dense granular structure of the BYSMV viroplasm was highlighted by white dotted line. Scale bars, 2 μm.
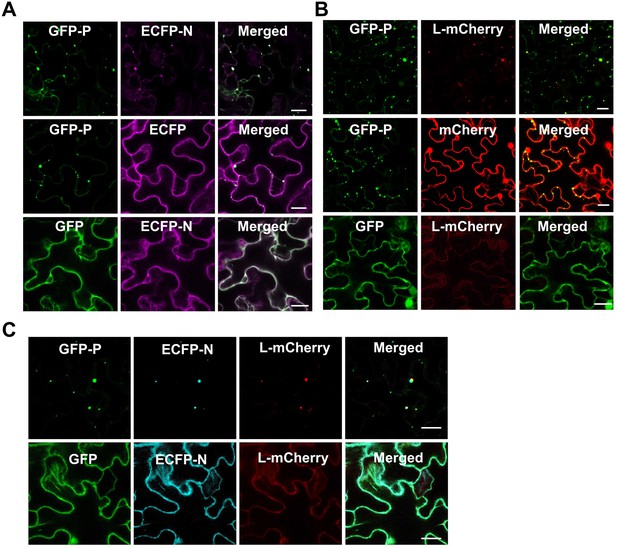
Barley yellow striate mosaic virus (BYSMV) phosphoprotein (P) forms spherule granule recruiting nucleotide (N) and polymerase (L) proteins.
(A) Confocal images showing subcellular localization of GFP-P/GFP and ECFP-N/ECFP transiently expressed in different combinations in epidermal cells of Nicotiana benthamiana leaves. Scale bars, 20 μm. (B) Confocal images showing subcellular localization of GFP-P/GFP and L-mCherry/mCherry transiently expressed in different combinations in epidermal cells of N. benthamiana leaves. Scale bars, 20 μm. (C) Confocal images showing subcellular localization of CFP-N and L-mCherry co-expressed with GFP or GFP-P in epidermal cells of N. benthamiana leaves at 2 days post infiltration (dpi). Scale bars, 20 μm.
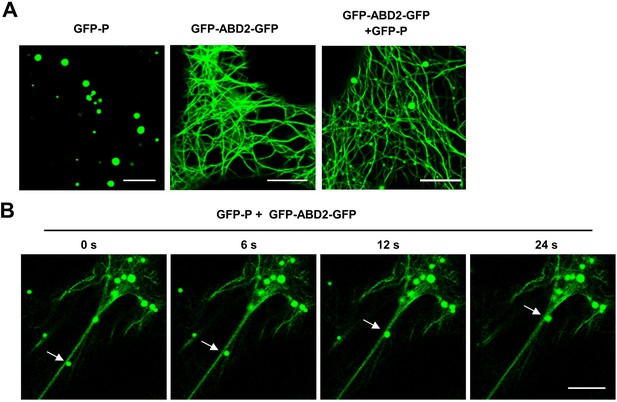
GFP-P moved along the actin filaments.
(A) Subcellular localization of GFP-P or GFP-ABD2-GFP expressed alone or together in Nicotiana benthamiana leaf epidermal cells at 2 days post infiltration (dpi). Scale bars, 10 μm. (B) Subcellular localization of GFP-P granules with GFP-ABD2-GFP-labeled actin filaments at 2 dpi. White arrows indicate that GFP-P granules moved along the actin filaments. Scale bar, 10 μm.
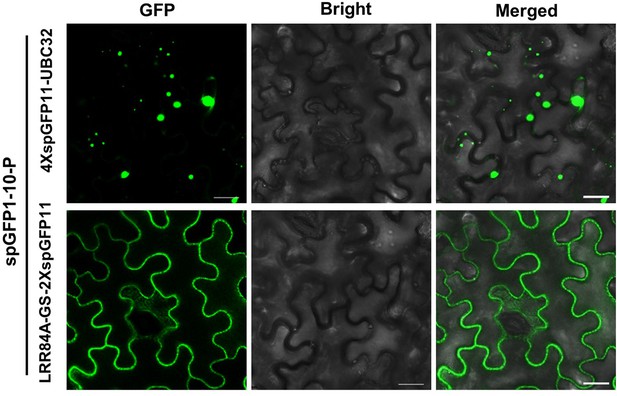
GFP-P P forms liquid structures on ER network.
Confocal micrographs of Nicotiana benthamiana leaf epidermal cells expressing spGFP1-10-P with 4× spGFP11-UBC32 or LRR84A-GS-2× spGFP11 at 2 days post infiltration (dpi). Scale bars, 20 μm.
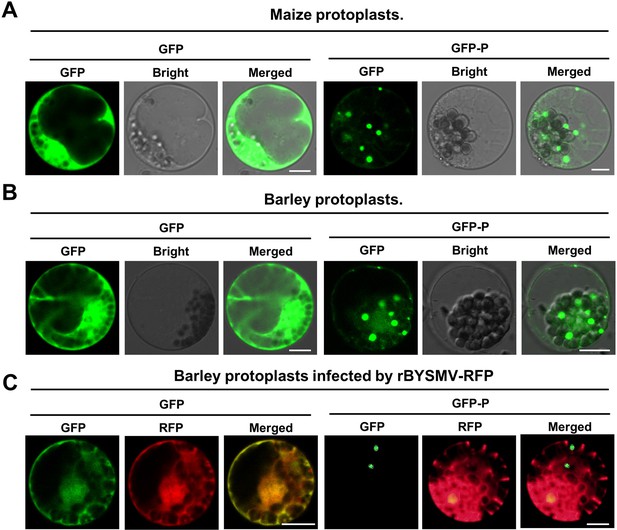
Phase separation of phosphoprotein (P) protein in maize and barley protoplasts.
(A) Confocal images showing subcellular distribution of GFP and GFP-P in maize protoplasts. Images were captured at 12–18 hr post transfection. Scale bars, 10 μm. (B) Confocal images showing subcellular distribution of GFP and GFP-P in barley protoplasts. Images were captured at 12–18 hr post transfection. Scale bars, 10 μm. (C) Confocal images showing subcellular distribution of GFP and GFP-P in barley protoplasts infected by recombinant barley yellow striate mosaic virus (rBYSMV)-RFP. Images were captured at 12–18 hr post transfection. Scale bars, 10 μm.

Northern cereal mosaic virus (NCMV) phosphoprotein (P) forms liquid structures in vivo.
(A) Confocal images showing subcellular distribution of NCMV GFP-P transiently expressed in Nicotiana benthamiana leaf epidermal cells at 2 days post infiltration (dpi). Scale bar = 20 μm. (B) Representative fluorescence recovery after photobleaching (FRAP) images of NCMV GFP-P granules transiently expressed in epidermal cells of N. benthamiana leaves at 2 dpi. Leaves were treated with 10 μM latrunculin B (LatB) to inhibit traffic of NCMV GFP-P granules at 3 hr before FRAP imaging. Scale bars, 2 μm. (C) Representative FRAP recovery curves of NCMV GFP-P granules as shown in panel B. The intensity of each body was normalized against their pre-bleach fluorescence. Data were expressed as the mean ± SD of 15 foci. (D) Time-lapse confocal micrographs showing the localization of NCMV GFP-P and mCherry-HDEL transiently expressed in N. benthamiana leaf epidermal cells at 2 dpi. Leaves were treated with DMSO or 10 μM LatB at 3 hr before imaging. White arrows indicate that GFP-P granules undergo fusion. Scale bars, 10 μm.
-
Figure 1—figure supplement 6—source data 1
Fluorescence intensities of the bleached droplets during the time course experiment.
(Related to Figure 1—figure supplement 6C).
- https://cdn.elifesciences.org/articles/74884/elife-74884-fig1-figsupp6-data1-v3.xlsx
Trafficking and fusion video of barley yellow striate mosaic virus (BYSMV) GFP-P graunles in epidermal cells of Nicotiana benthamiana leaves at 2 days post infiltration (dpi).
Representative fluorescence recovery after photobleaching (FRAP) video of barley yellow striate mosaic virus (BYSMV) GFP-P granules transiently expressed in epidermal cells of Nicotiana benthamiana leaves at 2 days post infiltration (dpi).
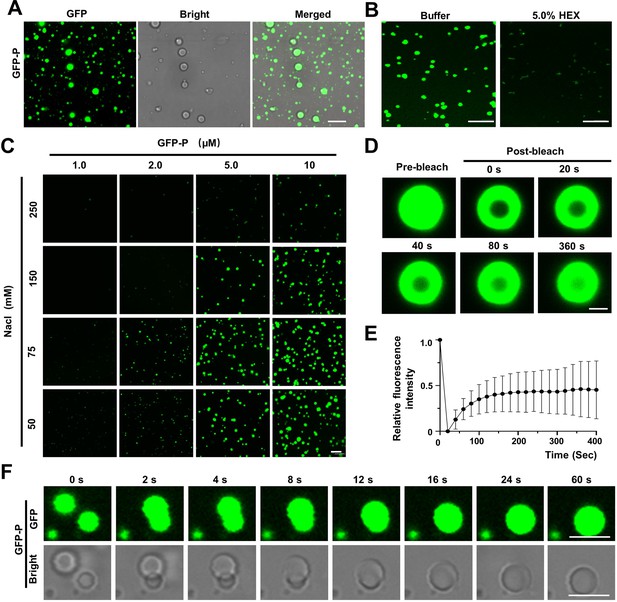
Barley yellow striate mosaic virus (BYSMV) phosphoprotein (P) undergoes phase separation in vitro.
(A) Confocal images showing that GFP-P formed droplets at the concentration of 10 μM in 125 mM NaCl. Scale bars, 10 μm. (B) Representative confocal images showing GFP-P droplets before or after treatment with 5.0% of 1,6-hexanediol for 1 min. Scale bars, 10 μm. (C) Phase separation of GFP-P at different concentrations of GFP-P and NaCl. Scale bar, 20 μm. (D) Representative fluorescence recovery after photobleaching (FRAP) of GFP-P droplets in vitro at the concentrations of 10 μM in 125 mM NaCl. Scale bar, 1 μm. (E) FRAP recovery curve of GFP-P droplets. Data are shown as the mean ± SD of 12 droplets. (F) Representative images showing fusion of two GFP-P droplets in vitro at the concentration of 15 μM in 150 mM NaCl. Scale bars, 5 μm.
-
Figure 2—source data 1
Fluorescence intensities of the bleached droplets during the time course experiment.
(Related to Figure 2E).
- https://cdn.elifesciences.org/articles/74884/elife-74884-fig2-data1-v3.xlsx
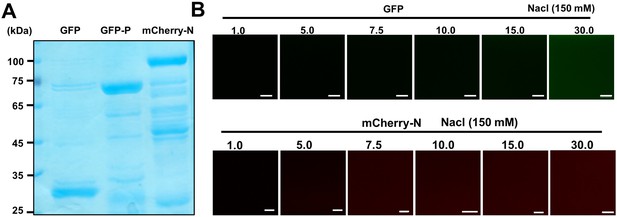
GFP and barley yellow striate mosaic virus (BYSMV) mCherry-N are deficient in forming liquid droplets in vitro.
(A) SDS-PAGE showing purified GFP, GFP-P, and mCherry-N proteins. (B) Confocal images showing that neither GFP nor mCherry-N formed droplets at the concentration of 1–30 μM in 150 mM NaCl. Scale bars, 20 μm.
-
Figure 2—figure supplement 1—source data 1
SDS-PAGE showing purified GFP, GFP-P, and mCherry-N proteins (Related to Figure 2—figure supplement 1A).
- https://cdn.elifesciences.org/articles/74884/elife-74884-fig2-figsupp1-data1-v3.tif
Representative fluorescence recovery after photobleaching (FRAP) video of barley yellow striate mosaic virus (BYSMV) GFP-P droplets in vitro.
Representative video showing fusion of barley yellow striate mosaic virus (BYSMV) GFP-P droplets in vitro.
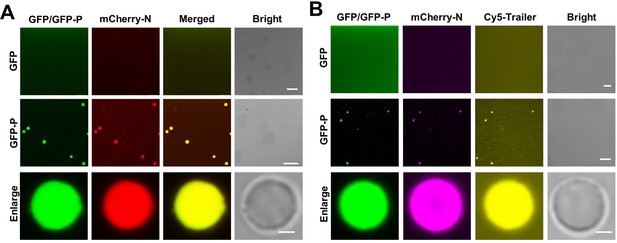
P-formed droplets recruit the N protein and genomic RNA in vitro.
(A) Confocal images showing incorporation of mCherry-N into GFP-P droplets. Free GFP was unable to form droplets to recruit mCherry-N. Scale bars, 20 μm. Scale bars (enlarge panel), 1 μm. (B) Confocal images showing incorporation of mCherry-N and Cy5-Trailer of barley yellow striate mosaic virus (BYSMV) genome into GFP-P droplets. In contrast, free GFP was unable to form droplets or recruit mCherry-N and Cy5-labeled trailer. Scale bars, 20 μm. Scale bar (enlarge panel), 1 μm.
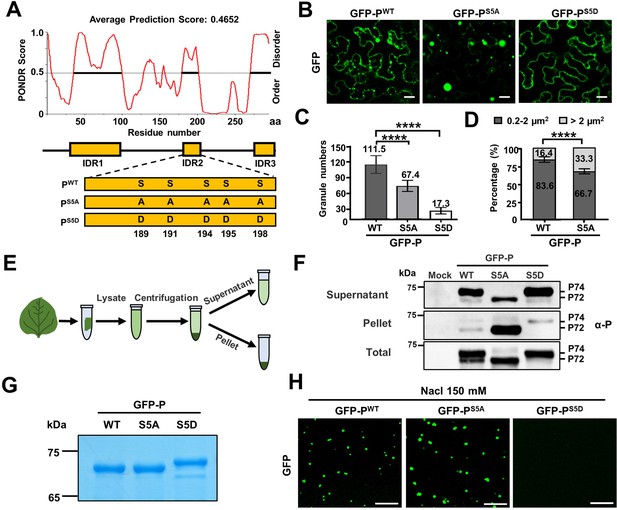
Phase separation of barley yellow striate mosaic virus (BYSMV) phosphoprotein (P) is inhibited by P protein phosphorylation.
(A) The predicted intrinsically disordered regions (IDRs) of BYSMV P and schematic representation of its phosphorylation mutants (PS5A and PS5D). IDRs were predicted according to the online tool PONDR and indicated by yellow boxes. (B) Confocal images showing subcellular distribution of GFP-PWT, GFP-PS5A, and P-GFPS5D in Nicotiana benthamiana leaf epidermal cells at 2 days post infiltration (dpi). Scale bars, 20 μm. (C) Statistical analyses of GFP granule numbers (>0.2 μm2) in a field (175 μm × 175 μm) of N. benthamiana leaves expressing GFP-PWT, GFP-PS5A, or GFP-PS5D. Error bars indicate SD of eight representative fields. ****p < 0.0001 (Student’s t-test). (D) Statistical diameter analyses of GFP-PWT and GFP-PS5A granules with different sizes (n > 500). (E) Workflow showing granule sedimentation assays using N. benthamiana leaves expressing GFP-PWT, GFP-PS5A, or P-GFPS5D at 2 dpi. (F) Western blotting analyses of supernatant, pellet, and total proteins isolated in panel E. (G) SDS-PAGE showing purified GFP-PWT, GFP-PS5A, or P-GFPS5D purified from Escherichia coli. (H) Confocal images showing droplet formed by GFP-PWT, GFP-PS5A, or P-GFPS5D in vitro. Scale bar, 10 μm.
-
Figure 4—source data 1
Statistical analyses of GFP-PWT, GFP-PS5A, or GFP-PS5D granule numbers and diameter (Related to Figure 4C, D).
- https://cdn.elifesciences.org/articles/74884/elife-74884-fig4-data1-v3.zip

Confocal images showing subcellular distribution of GFP-PWT, GFP-PS5A, and P-GFPS5D in Nicotiana benthamiana leaves at 2 days post infiltration (dpi).
Scale bars, 20 μm.

Fluorescence recovery after photobleaching (FRAP) experiment analysis liquid quality of GFP-PS5A granules.
(A) Representative FRAP images of GFP-PS5A granules in epidermal cells of Nicotiana benthamiana leaves at 2 days post infiltration (dpi). Leaves were treated with 10 μM latrunculin B (LatB) to inhibit movement of GFP-PS5A granules at 3 hr before photobleaching. Scale bar, 1 μm. (B) FRAP recovery curves of GFP-PS5A granules. The intensity of each granule was normalized against their pre-bleach fluorescence. Data were presented as mean ± SD of 15 granules.
-
Figure 4—figure supplement 2—source data 1
Fluorescence intensity of the bleached droplets during the time course experiment.
(Related to Figure 4—figure supplement 2B).
- https://cdn.elifesciences.org/articles/74884/elife-74884-fig4-figsupp2-data1-v3.xlsx

Phase separation of GFP-PWT and GFP-PS5A.
(A) Confocal images showing that GFP-PWT and GFP-PS5A formed droplets at different concentrations and 125 mM NaCl. Scale bars, 20 μm. (B) Turbidity assays (OD600) using 12 μM GFP, GFP-PWT, and P-GFPS5A in buffer of 200 mM NaCl and 20% PEG4000. The tubes were shown in left and OD600 values were analyzed. Error bars indicate SD (n = 3). Scale bars, 1 cm. ****p < 0.0001, ***p < 0.001 (Student’s t-test).
-
Figure 4—figure supplement 3—source data 1
Turbidity assays (OD600) of GFP, GFP-PWT, and P-GFPS5A (Related to Figure 4—figure supplement 3B).
- https://cdn.elifesciences.org/articles/74884/elife-74884-fig4-figsupp3-data1-v3.xlsx
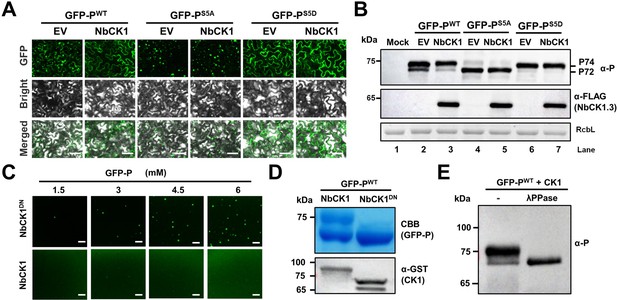
Host casein kinase 1 (CK1) inhibits phase separation of barley yellow striate mosaic virus (BYSMV) phosphoprotein (P) in vivo and in vitro.
(A) Confocal images showing subcellular distribution of GFP-PWT, GFP-PS5A, and P-GFPS5D co-expressed with empty vector (EV) or NbCK1.3 in Nicotiana benthamiana leaf epidermal cells at 2 days post infiltration (dpi). Scale bars, 50 μm. (B) Western blotting detecting accumulation of GFP-PWT, GFP-PS5A, and P-GFPS5D in the leaves as shown in panel A. (C) Confocal images showing droplet formation of GFP-PWT purified from Escherichia coli co-expressing NbCK1.3 or NbCK1.3DN. GFP-PWT was diluted to different concentration and 125 mM NaCl. Scale bars, 20 μm. (D) SDS-PAGE showing purified GFP-PWT, GFP-PS5A, or GFP-PS5D in the samples of panel C. Expression of GST-tagged NbCK1.3 or NbCK1.3DN was examined by Western blotting analyses with anti-GST antibodies. (E) Western blot detecting GFP-PWT treated with lambda protein phosphatase (λ-PPase) or mock buffer (-) with anti-P antibodies.
-
Figure 5—source data 1
Soure images (Rlated to Figure 5B, D, E).
- https://cdn.elifesciences.org/articles/74884/elife-74884-fig5-data1-v3.zip
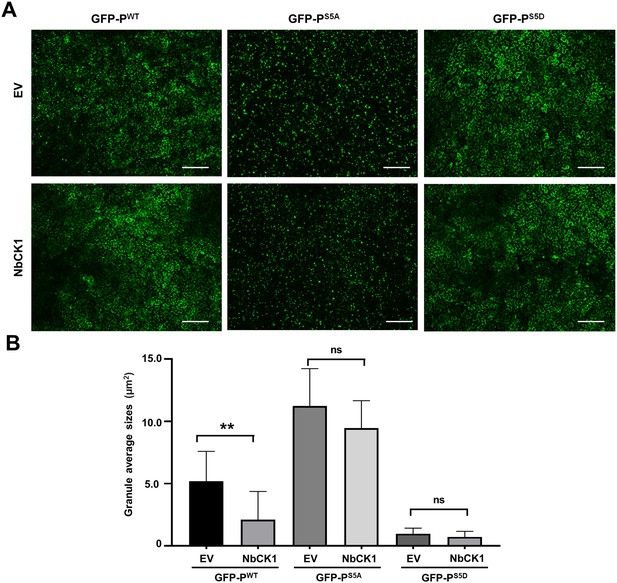
Host casein kinase 1 (CK1) inhibits phase separation of barley yellow striate mosaic virus (BYSMV) phosphoprotein (P) in vivo.
(A) Confocal images showing subcellular distribution of GFP-PWT, GFP-PS5A, and P-GFPS5D co-expressed with empty vector (EV) or NbCK1 in Nicotiana benthamiana leaves at 2 days post infiltration (dpi). Scale bars, 200 μm. (B) Statistical analyses of GFP granules from eight epidermal cells of N. benthamiana leaves expressing GFP-PWT, GFP-PS5A, and GFP-PS5D co-expressed with EV or NbCK1. Error bars indicate SD. **p < 0.01 (Student’s t-test).
-
Figure 5—figure supplement 1—source data 1
Statistical analyses of GFP-PWT, GFP-PS5A and GFP-PS5D granules co-expressed with empty vector (EV) or NbCK1 (Related to Figure 5—figure supplement 1B).
- https://cdn.elifesciences.org/articles/74884/elife-74884-fig5-figsupp1-data1-v3.xlsx

Host casein kinase 1 (CK1) inhibits phase separation of barley yellow striate mosaic virus (BYSMV) phosphoprotein (P) in vivo.
(A) Confocal images showing subcellular distribution of GFP-PWT co-expressed with empty vector (EV), NbCK1, or NbCK1DN in Nicotiana benthamiana leaf epidermal cells at 2 days post infiltration (dpi). Scale bars, 100 μm. (B) Western blotting detecting accumulation of GFP-PWT in the leaves as shown in panel A.
-
Figure 5—figure supplement 2—source data 1
Soure images (Rlated to Figure 5—figure supplement 2B).
- https://cdn.elifesciences.org/articles/74884/elife-74884-fig5-figsupp2-data1-v3.tif
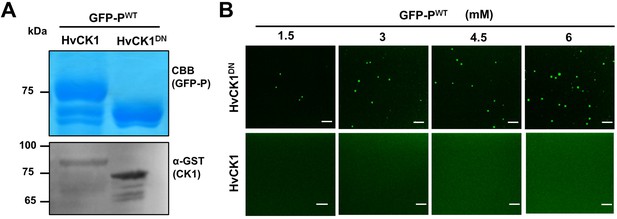
Phase separation of barley yellow striate mosaic virus (BYSMV) phosphoprotein (P) is inhibited by P protein phosphorylation.
(A) SDS-PAGE showing purified GFP-PWT purified from Escherichia coli co-expressing HvCK1.2 or HvCK1.2DN. Accumulation of GST-tagged HvCK1.2 or HvCK1.2DN was examined by Western blotting analyses with anti-GST antibodies. (B) Confocal images showing droplet formation of GFP-PWT purified from E. coli co-expressing HvCK1.2DN. GFP-PWT was diluted to different concentration in 125 mM NaCl. Scale bars, 20 μm.
-
Figure 5—figure supplement 3—source data 1
SDS-PAGE showing purified GFP-PWT purified from E. coli co-expressing HvCK1.2 or HvCK1.2DN (Related to Figure 5—figure supplement 3A).
- https://cdn.elifesciences.org/articles/74884/elife-74884-fig5-figsupp3-data1-v3.tif
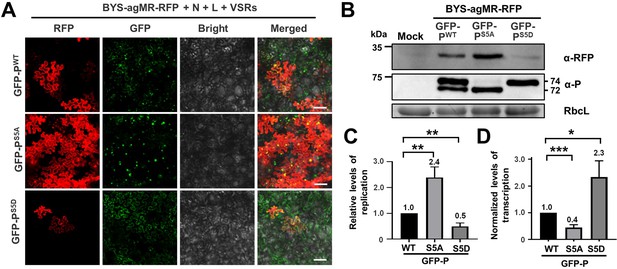
Phase separation of GFP-P modulates virus replication and transcription.
(A) RFP foci in Nicotiana benthamiana leaves infiltrated with Agrobacterium for co-expression of BYS-agMR-RFP, N, L, and VSRs with GFP-PWT, GFP-PS5A, or GFP-PS5D at 8 days post infiltration (dpi). Scale bars, 100 μm. (B) Western blotting analyzing accumulation of RFP, GFP-PWT, GFP-PS5A, and GFP-PS5D in the leaf samples of panel A. (C) Quantitative real-time PCR analyzing the relative levels of minigenome replication supported by the GFP-PWT, GFP-PS5A, and GFP-PS5D proteins. (D) Quantitative real-time PCR analyzing the relative levels of RFP mRNA in the same samples of A. In panels C and D, error bars indicate SD (n = 3). *p < 0.05, **p < 0.01, and ***p < 0.001 (Student’s t-test).
-
Figure 6—source data 1
Source data and images of Figure 6 (Related to Figure 6B-D).
- https://cdn.elifesciences.org/articles/74884/elife-74884-fig6-data1-v3.zip

Illustration of BYS-agMR-RFP infection in epidermal cells of Nicotiana benthamiana leaves.
(A) The binary vectors of the pBYS-agMR and pBYS-agMR-RFP plasmids. In the pBYS-agMR plasmid, the ORFs of barley yellow striate mosaic virus (BYSMV) nucleotide (N) and polymerase (P) were replaced with GFP and RFP, respectively, and were flanked by 3′ leader (le) and 5′ trailer (tr) sequences outside the N gene and the L gene. Base on the pBYS-agMR plasmid, the pBYS-agMR-RFP plasmid was obtained by introducing a A after the start codon of the GFP ORF. (B) Illustration of BYS-agMR-RFP replication and transcription. pGD-VSRs contain three expression cassettes to express tomato bushy stunt virus p19, the tobacco etch virus HC-Pro, and the barley stripe mosaic virus γb simultaneously. The ORFs of N, L, and GFP-PWT/GFP-PS5A/GFP-PS5D were inserted into the pGD vector for expression of N, L, and GFP-PWT/GFP-PS5A/GFP-PS5D. BYS-agMR-RFP was rescued by co-expression of pBYS-agMR-RFP, N, L, GFP-PWT/GFP-PS5A/GFP-PS5D, and VSRs in N. benthamiana leaves. After co-infiltration, the agMR-RFP was first transcribed and formed replication complexes with co-expressed N, L, and GFP-PWT/GFP-PS5A/GFP-PS5D. Then, the full-length genomic MR (gMR)-RFP was produced through replication. Subsequently, the full-length gMR-RFP serves as a template to transcribe into mRNA of GFP and RFP. Accumulation of the gMR-RFP represents replication level. The normalization of RFP mRNA levels relative to the gMR template indicates transcription level. Note that GFP mRNA cannot be translated because of A-insertion-induced frame shift.
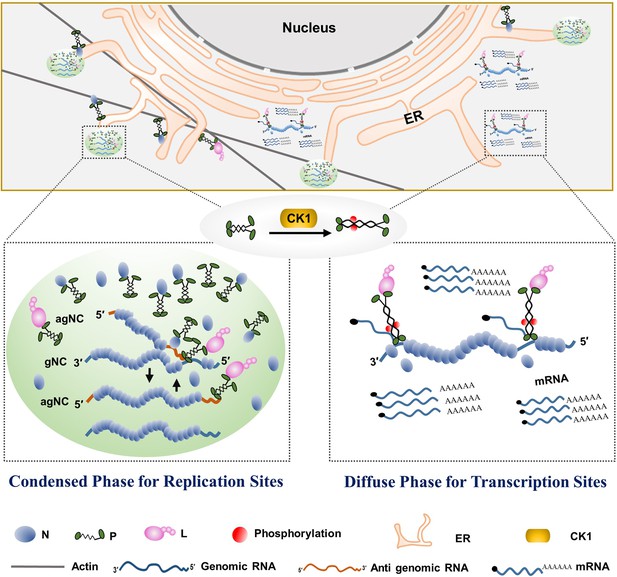
Model for phase separation of barley yellow striate mosaic virus (BYSMV) phosphoprotein (P) in modulating rhabdovirus replication and transcription.
Rhabdovirus replication requires high concentration of viral N protein for encapsidating newly synthesized genomic/antigenomic RNA. Thus, unphosphorylated BYSMV P undergoes phase separation, and then recruits the nucleotide (N) and polymerase (L) proteins, as well as genomic RNAs into membraneless condensates for optimal replication. In addition, the granules move along the ER/actin network and fuse with each other. In the transcription sites, the serine-rich (SR) motif in the middle intrinsically disordered region (IDR) of BYSMV P is phosphorylated by casein kinase 1 (CK1), and the resulting hyper-phosphorylated P is unable to undergo phase separation, which facilitates virus transcription and viral mRNA release for viral protein translation.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Escherichia coli) | BL21 | Thermo Fisher | Cat#C600003 | |
| Strain, strain background (Agrobacterium tumefaciens) | EHA105 | Weidibiotechnology | Cat#AC1012 | |
| Antibody | Anti-GST (Rabbit polyclonal) | Abcam | Cat#ab9085 | WB(1:5000) |
| Antibody | Anti-FLAG (Mouse monoclonal) | Sigma | Cat#F1804 | WB(1:5000) |
| Antibody | Anti-RFP (Rabbit polyclonal) | Fang et al., 2019 | WB(1:3000) | |
| Antibody | Anti-BYSMV-P (Rabbit polyclonal) | Fang et al., 2019 | WB(1:3000) IEM(1:500) | |
| Antibody | Anti-Mouse lgG (H + L)-HRP Conjugate (Goat polyclonal) | Bio-Rad | Cat#170–6516 | WB(1:20,000) |
| Antibody | Anti-Rabbit lgG (H + L)-HRP Conjugate (Goat polyclonal) | EASYBIO | Cat#BE0101 | WB(1:20,000) |
| Antibody | Anti-Rabbit IgG-gold Conjugate (Goat polyclonal) | Sigma | Cat#G7402 | IEM(1:50) |
| Commercial assay or kit | RiboMAXTM Large Scale RNA Production System-T7 | Promega | Cat#P1300 | |
| Chemical compound, drug | Latrunculin B | Abcam | Cat#ab144291 | |
| Software, algorithm | GraphPad Prism 8 | PMID:22434839 | RRID:SCR_002798 | |
| Software, algorithm | ImageJ | PMID:22930834 | RRID:SCR_003070 |
Additional files
-
Supplementary file 1
Primers used in this study.
- https://cdn.elifesciences.org/articles/74884/elife-74884-supp1-v3.docx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/74884/elife-74884-transrepform1-v3.docx






