Intracranial human recordings reveal association between neural activity and perceived intensity for the pain of others in the insula
Figures
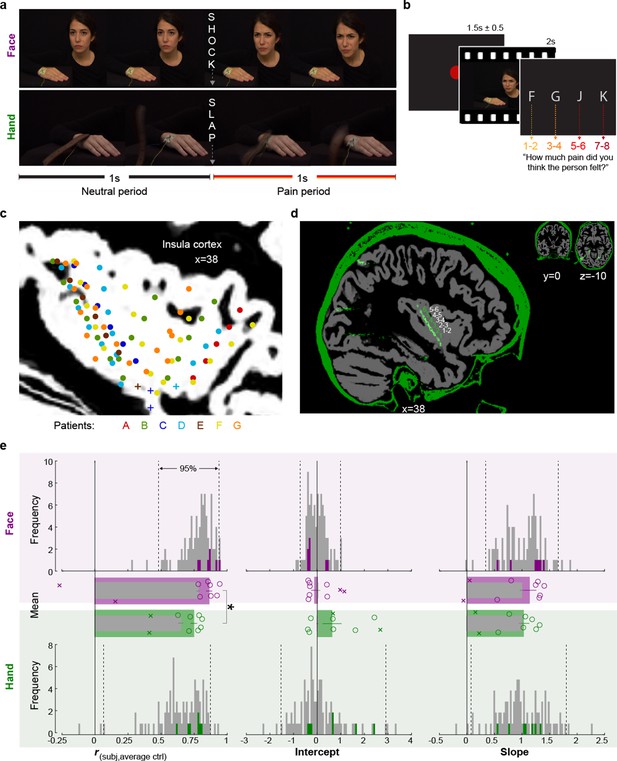
Experimental design, recording site locations, and behavioral pain ratings.
(a) Frames extracted from a Hand and a Face movie. For the Face, the first second of each movie showed a neutral facial expression, the second, the facial reaction to the shock. For the Hand, the movie started with the belt resting on the hand. The first second showed the belt lifting and coming down again, to hit the hand at the 1 s mark exactly. The hand then reacted to the force of the belt. Both the slap and the shock delivery happened in the middle of the movies, splitting them into a 1 s neutral and a 1 s pain period. (b) Single-trial structure diagram. After the presentation of each video, patients expressed their choice at their pace using the keyboard keys f, g, j, and k for pain intensities 1–2, 3–4, 5–6, and 7–8, respectively. ITI started with participant’s response. (c) Position (i.e., the midpoint between two adjacent electrodes) of the 85 bipolar macroelectrode recording sites shown as dots and of the microelectrode locations shown as pluses, color-coded by patient. Data from the two hemispheres and all lateromedial coordinates are projected here onto a single sagittal slice of the insula taken at X = 38 from the brain of one of the patients. For a list of all MNI coordinates, see Figure 1—source data 1. (d) Graphical illustration of how a bipolar recording for one patient and one insular electrode was computed. In green, the CT, and in gray, the T1 scan from patient C. The annular structures along the electrode shaft in the CT correspond to individual macroelectrode contacts (green 1, 2, 3 …). Recordings from adjacent pairs of contacts along the electrode were subtracted to calculate bipolar recordings (white 1–2, 2–3 …). (e) From left to right, Spearman’s correlation coefficient r, intercept, and slope values from the linear regression for Hand (green) and Face (purple). Histograms: values for the control group illustrate the similarity between the ratings of each participant in the control group and the average of the other controls, and are shown as gray; the similarity between each of the seven patients with the average of the control group is shown in colors. Dotted lines mark the 2.5 and 97.5% of the control group. Bar graphs: mean ± SEM of the controls (gray) and the seven included patients (color) with individual patients as circles. In the bar graphs, we also show as Xs the corresponding behavioral performance metrics of the two patients that were excluded due to atypical use of the response keys. These patients were not included in the mean and SEM calculations.
-
Figure 1—source data 1
Mean MNI coordinates of recording sites.
In black, the MNI coordinates resulting from the average MNI coordinates of the two adjacent electrodes used for re-referencing for all macroelectrodes. In gray, the MNI coordinates of the microelectrodes. The color coding and patient identifiers reflect those used in Figure 1c.
- https://cdn.elifesciences.org/articles/75197/elife-75197-fig1-data1-v2.xlsx
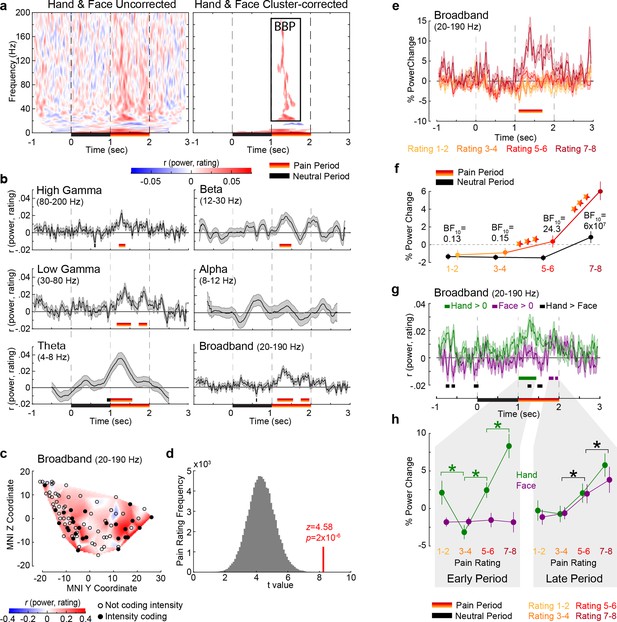
Intensity coding in the insula local field potential (LFP) activity for Hands and Faces together.
(a) For each frequency and time relative to stimulus onset, the average rS value over all insular bipolar recordings between intracranial electroencephalographic (iEEG) power and rating for Face and Hand trials together, without (left) and with (right) cluster correction for multiple comparisons. BBP: broadband power, the cluster of significant positive intensity coding frequencies (i.e., rS >0, 20–190 Hz) used throughout the article. The time–frequency decomposition per rating can be found in Figure 2—figure supplement 1. (b) Mean ± SEM time course of intensity coding in different frequencies and BBP over all insular bipolar recordings when Face and Hand trials are combined. Above the x-axis, black and yellow-to-red bars show periods of significant intensity coding after circular shift correction for multiple comparisons during the neutral and pain periods, respectively. Below the x-axis, the black bar marks the neutral and the yellow-to-red bar indicates the pain period. (c) Intensity coding in the 85 bipolar recordings is shown as significant (p1<0.05, filled black circles) or nonsignificant (p1>0.05, open circles) based on the MNI y (anterior–posterior) and z (dorsoventral) coordinates. The heatmap shows the interpolated intensity coding values between these locations. Electrodes in the right and left insula are projected onto the same sagittal representation. (d) The t-value of a t-test comparing the intensity coding of all insular 85 bipolar recordings combining Hand and Face trials within the pain period (1–2 s post-stimulus onset) in the insula against zero (red bar) was higher than the distribution of the corresponding t-values obtained when performing the same test using 85 bipolar recordings randomly selected 100,000 times from the macroelectrode contacts of our seven patients anywhere in the brain (see Figure 2—figure supplement 2 for a map of all macrocontacts and Figure 2—figure supplement 3 for the anatomical distributions of these macrocontacts). (e) Mean ± SEM time course of percent power change from baseline in BBP (20–190 Hz) over all insular bipolar recordings and over the Hand and Face conditions, but plotted separately for trials rated 1–2, 3–4, 5–6, and 7–8. (f) Mean ± SEM percent power change values over all insular bipolar recordings as a function of reported intensity separately for the neutral (black) and pain (yellow-to-red) periods when combining Hand and Face trials. BF10 values: Bayes factor quantifying evidence for H1 relative to H0 from a nonparametric t-test comparing BBP during the pain period against that during the neutral period. ***p<0.001 relative to the preceding reported intensity. See Table 2 for a complete description of the statistical values. (g) Mean ± SEM time course of intensity coding in BBP (20–190 Hz) over all insular bipolar recordings for Hands and Faces separately. rS > 0 indicated with green bars for Hands and purple bars for Faces. Black bars indicate rS_Hand > rS_Face. The early and late periods that result for Hands and Faces, respectively, are used throughout the article. Figure 2—figure supplement 4 depicts the percent power change values as a function of time for ratings 1–2, 3–4, 5–6, and 7–8 separately for Hands and Faces. (h) Mean ± SEM percent power change in the broadband frequency over all insular bipolar recordings as a function of rating for Hands and Faces in the early and late periods separately. Green *p<0.001 for Hand. Black *p<0.01 for the main effect of rating, that is, combining Hand and Face. See Table 3 for a complete description of the statistical values.
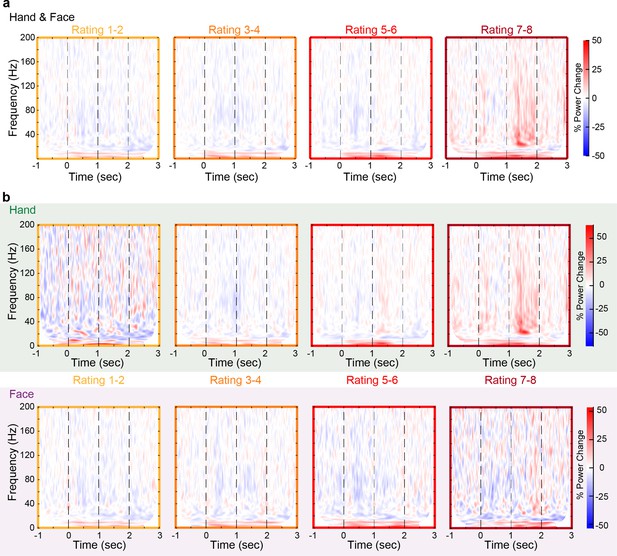
Time–frequency decomposition as a function of intensity rating.
(a) Mean percent power changes relative to the baseline period (1 s before the onset of videos) over all 85 insular bipolar recordings as a function of time and frequency for all trials rated 1–2 (first column), 3–4 (second column), 5–6 (third column), and 7–8 (fourth column) combining Hand and Face stimuli. (b) Same as (a), but separately for the Hand (top row) and Face (bottom row) trials.
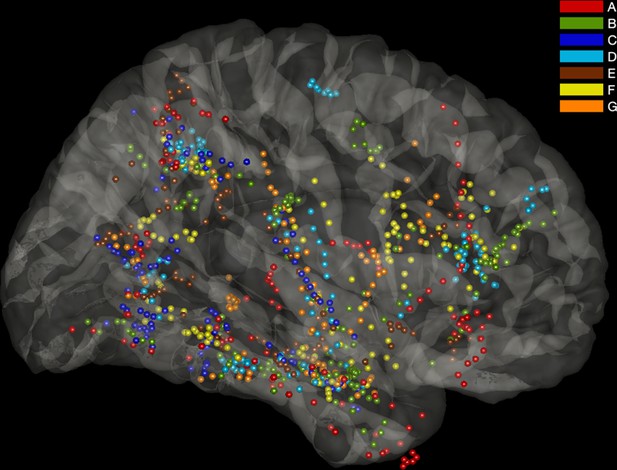
Glass brain representation of all macrocontacts available in the seven patients.
Patients are color-coded as in Figure 1c and Figure 1—source data 1. The analysis depicted in Figure 2c was performed by randomly sampling 85 electrodes 100,000 times from these macrocontacts.
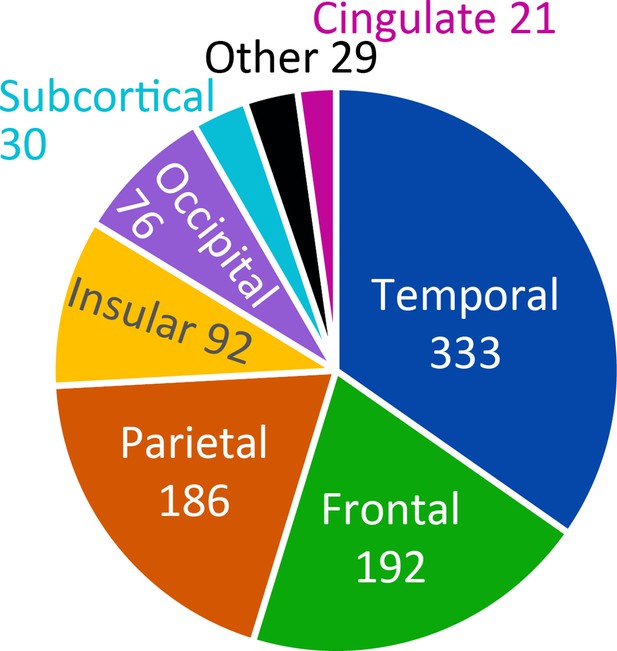
Overview of the anatomical distribution of all macrocontacts available in the seven patients.
For each electrode, the region it belongs to was determined based on the Faillenot et al., 2017 atlas by choosing the region with the highest probability of occurrence within 3 mm from the center of the selected electrode. The pie chart simply illustrates the total number of contacts falling within a particular lobe. The complete list can be found at here.
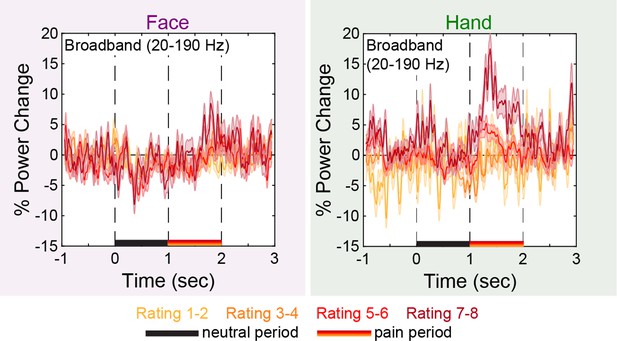
Broadband power (BBP) time course as a function of rating and stimulus.
Mean ± SEM time course of percent power change from baseline in BBP (20–190 Hz) over all insular bipolar recordings plotted separately for trials rated 1–2, 3–4, 5–6, and 7–8, and for the Hand and Face conditions.
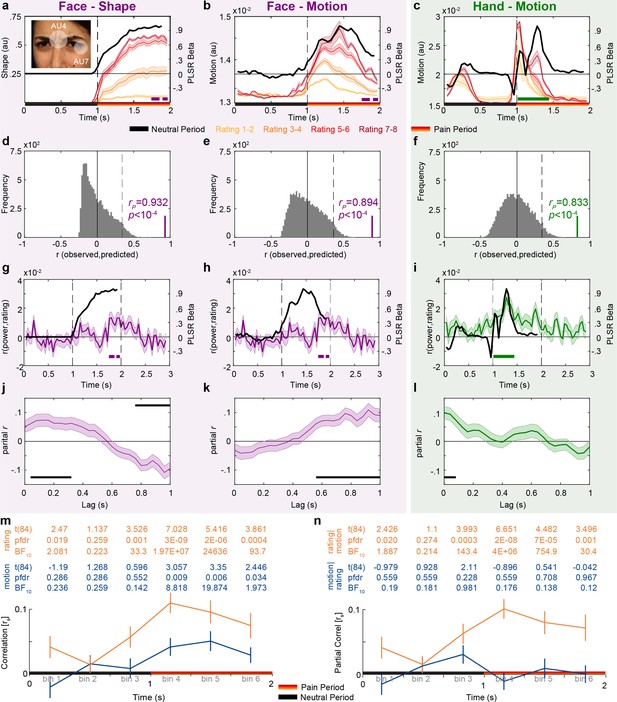
Temporal dynamics of pain rating and intensity coding in the insula broadband activity.
(a–c) Motion and shape signals as a function of time and perceived intensity for the Face and Hand videos rated as 1–2, 3–4, 5–6, and 7–8 separately. Each colored curve represents the mean ± SEM for each rating. Purple and green bars indicate the periods with significant broadband power (BBP)–rating correlations for Faces and Hands, respectively (as in Figure 2g). Black lines represent the partial least-square regression (PLSR) beta coefficients predicting perceived intensity ratings using motion (for Hand and Faces) or shape information (for Faces). The white transparent circles over the inlet figure in (a) shows the action units (AUs) 4 and 7 that were used to estimate the intensity of the shape information in Face videos. (d–f) Accuracy with which the motion or shape signal across all frames can be used to predict the intensity rating of the movie. The histogram shows the actual predictive accuracy averaged over cross-validation folds (green and purple) relative to the null distribution of shuffled ratings (gray), with the median and top 5% of the null distribution shown as full and dashed line. In all cases, the actual accuracy was higher than all 10,000 shuffling values, as indicated by p<10–4. (g–i) Mean ± SEM time courses of the correlations between BBP and pain ratings (green and purple, as in Figure 2g) superimposed with black lines from (a–c) for visualization of the temporal similarity between the two curves. (j–l) Mean ± SEM lagged correlation (left) and partial correlation coefficients (middle and right) between the temporal profile of BBP–rating correlations and that of the PLSR beta coefficients for the corresponding stimulus information. For partial correlation analyses, middle panel shows rP(BBP(t),Motion(t+lag)|Shape(t+lag)) and the right panel shows rP(BBP(t),Shape(t+lag)|Motion(t+lag)). All correlations are shown for lags from 0 to 1000 ms in steps of 40 ms. The correlation was calculated separately for each of the 85 bipolar recordings. The black bars represent periods of significant correlations, tested using a t-test of the 85 correlation values against zero followed by false discovery rate (FDR) correction at q = 0.05. (m) Mean ± SEM rs between motion energy and BBP (blue) or between subjective rating and BBP (orange) for the six consecutive bins of 333 ms during the movie. All statistics are two-tailed parametric t-tests against zero because rs values were normally distributed (all Shapiro–Wilk p>0.05). Values are indicated in the table above each panel for each time bin of ⅓ s. FDR correction is over the six bins. No rs-to-z transform was used because the rs values were in the range –0.5 < rS < 0.5 for which r and z values are extremely similar. (n) As in (m), but partial correlations: rS(BBP,motion|rating) in blue and rS(BBP,rating|motion) in orange.
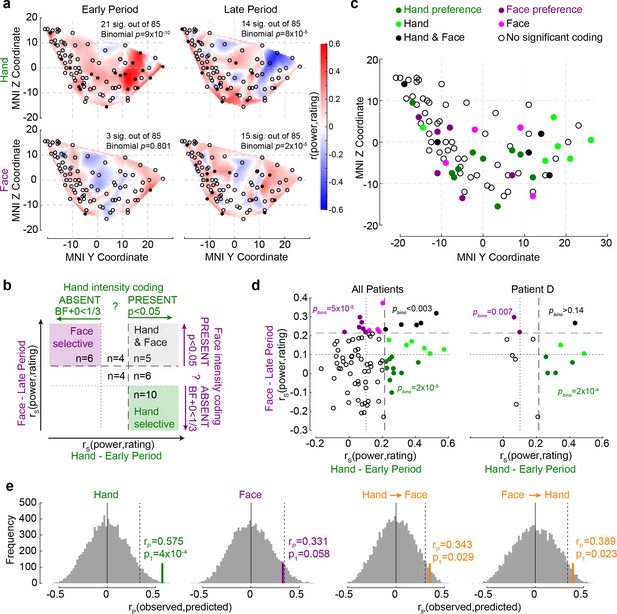
The relationship between Hand and Face intensity coding in the insula broadband activity.
(a) Topographical maps of broadband power (BBP)–rating correlation coefficients for Hands and Faces in the early and late periods. Each circle is one of the recording sites (as in Figure 1c), with filled circles indicating locations with significant correlation coefficients (p1<0.05). (b) Classification of recording locations based on their Hand (early period) and Face (late period) intensity coding. Bipolar recordings in the gray zone (n = 5) significantly co-represent intensity for Hands and Faces (both p1<0.05, i.e., beyond dashed line). Recordings in the purple (n = 6) and green (n = 10) zone represent intensity coding preference for Faces or Hands, respectively (i.e., p1<0.05 for Hands and BF+0 < ⅓ for Faces, and vice versa). (c) Location of all 85 bipolar recordings, color-coded by stimulus preference as described in (b). Note that locations Hand and Face without further specification are those with rS values for at least one of the stimulus types falling between the dashed and dotted lines, thus providing inconclusive evidence and showing neither significant dual coding, nor evidence of absence. (d) Correlation coefficients for Hands and Faces separated by coding characteristics in (b) for all patients together (left) and for an exemplary patient (right). pbino refers to the likelihood to find the observed number of locations in that quadrant using a binomial distribution as detailed in section ‘Probability of Face-but-not-Hand, Hand-but-not-Face, and dual-intensity coding LFPs’. (e) The left two panels depict the average correlation coefficients, together with corresponding resampling null distributions, as a measure of the accuracy of decoding intensity ratings using the partial least-square regression (PLSR) beta coefficients of BBP in the early period for Hands and in the late period for Faces. The right panels are similar to the left panels, but show the accuracy of cross-decoding, that is, predicting Hand ratings from the Face BBP and vice versa. The dotted lines indicate 95th percentiles of the resampling null distributions.
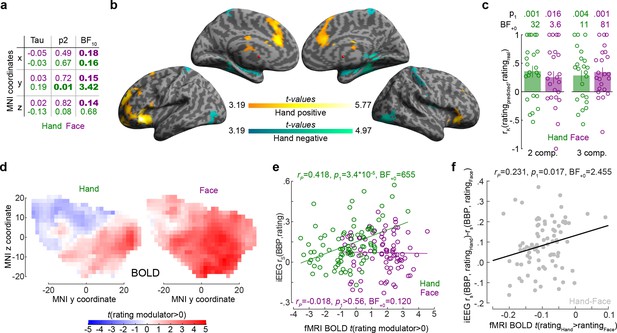
The relationship between the insula broadband and blood-oxygen-level-dependent (BOLD) activity during pain intensity ratings.
(a) Correlations (rK) between MNI coordinates and broadband power (BBP) intensity coding, separately for Hands (green) and Faces (purple). Bold numbers mark evidence for (BF10 > 3) or against (BF10 < 1/3) a significant correlation. Statistical values were obtained by correlating separately the x, y, or z coordinate of each bipolar recording with the rS(BBP,rating) of each recording over all 85 recordings. Tau refers to Kendall’s tau, p2 and BF10 the two-tailed probability and BF based on H0:tau = 0. (b) Results of the regression analysis between resting state connectivity and intensity coding for the 85 bipolar recording coordinates for Hands. Significant positive and negative regression values are indicated by warm and cold colors, respectively. Results are corrected at the cluster level at pFWE<0.05 using initial cluster cutting at punc<0.001, t(82) = 3.19, and then setting the minimum cluster size to FWEc = 772 as determined by the random field theoretical calculation in SPM. The detailed results of this analysis are provided in Figure 5—source data 1. (c) Mean ± SEM of the predictive performance of a partial least-square regression (PLSR) trained to predict ratings based on the pattern of BOLD activity across all voxels in the insula for different ratings. A leave-one-out cross-validation was used, and each circle represents the rK between the predicted and actual rating for each left-out participant, and the p1 and BF+0 values then test these n = 23 correlation values against zero using a nonparametric test. Results are shown separately for Hand and Face trials and using two or three PLSR components separately. (d) Topography of intensity coding for the Hand (left) and Face (right), as assessed at the group level, by the parametric modulator capturing changes in BOLD activity that correlate with trial-by-trial differences in participant’s ratings. t-values testing the parametric modulator >0 at the group level are shown as a function of y and z coordinate in the insula mask. For each coordinate, the maximum value across all x coordinates within the two insulae is indicated. (e) Correlation (rP because of normality) between the t-value of the parametric modulator for the rating in the fMRI BOLD responses (x-axis) and the BBP intensity coding (computed in the early period for the Hand, green; late period for the Face, purple) in the intracranial electroencephalographic (iEEG) signal (y-axis) for each of the 85 contact locations. Note that for the fMRI signal the value is taken from the voxel closest to the MNI coordinates of the corresponding contact in the iEEG signal. (f) Same as (e) but for the difference between Hand and Face coding, calculated as the Hand–Face difference in the correlation between BBP and rating for the iEEG, and the t-value of the paired comparison between the parametric rating modulator for Hand–Face in the fMRI data.
-
Figure 5—source data 1
Resting state connectivity results.
The table indicates for each significant cluster the size in number of voxels of the cluster; the number of voxels of that clusters that have been assigned to cytoarchitectonic areas based on the Anatomy toolbox for SPM (http://www.fz-juelich.de/ime/spm_anatomy_toolbox); the number of voxels assigned expressed in percentage; the hemisphere covered by the cluster (L = left, R = right); the cytoarchitectonic area those voxels have been assigned to when the information is available, or the anatomical description of the area voxels fell in; the percentage of the cytoarchitectonic area covered by the assigned voxels; the t-values of the identified peaks of activity with the respective MNI coordinates.
- https://cdn.elifesciences.org/articles/75197/elife-75197-fig5-data1-v2.xlsx
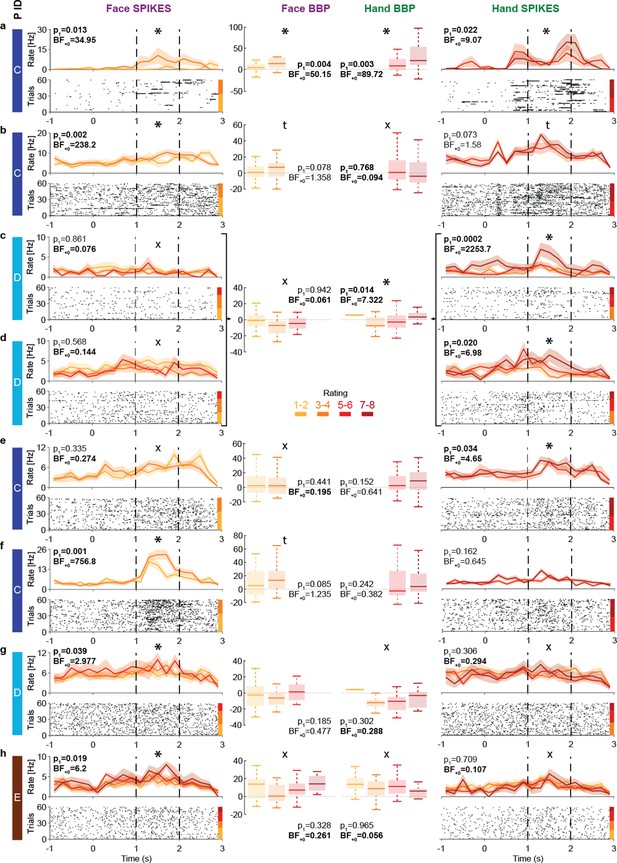
Intensity coding in the insula single units and the corresponding broadband activity.
(a–h) Left (Face) and right (Hand) columns display, for each single unit, the rastergrams and peri-stimulus time histograms (PSTH) for eight cells that showed intensity coding for at least one stimulus type. For the PSTH, each curve represents the mean ± SEM of the firing rate in each bin for trials with the corresponding rating. Not all patients gave all possible ratings in each condition. For the rastergram, trials are sorted in order of rating, with the highest ratings upmost. The color bar next to the rastergram indicates the rows corresponding to each rating. p1 and BF+0 values result from a one-tailed test of the Kendall’s tau between rating and spike count in the pain period (marked by the dashed lines). *Significant intensity coding (p1<0.05), t: trend (p1<0.1), X: evidence of absence for a positive intensity coding (BF+0 < 1/3). The x-axis (time) is relative to the movie onset. The color bar on the leftmost side indicates from which patient the data is taken. Middle columns show the broadband power (BBP) averaged over the pain period for the microelectrode (from which the corresponding single unit was extracted) as a function of rating where boxplots show variance across trials. The box and whiskers represent the quartiles across trials, and the p1 and BF+0, the Kendall’s tau test of the association of rating and BBP. Note that cells c and d were taken from the same microwire, and therefore have only one BBP graph.
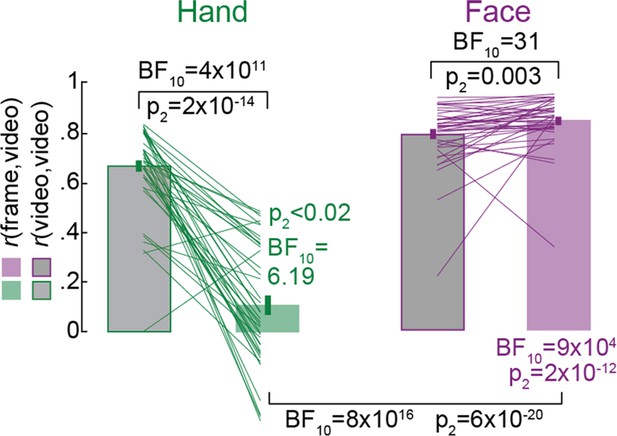
Rating from shape information alone.
Mean ± SEM correlation coefficients between each participant’s ratings in the online frame rating task and the average ratings of the other participants in the online video rating task (rS(frame,average_video), green and purple) compared against that between participant’s ratings in the online video rating task and the average ratings of the other participants in the same task (rS(video,average_video), gray) for Hands and Faces separately. Black statistics above the bars compare the respective frame and video ratings, the colored statistics compare the frame ratings against zero. The black statistics under the bars compare the frame ratings between Hands and Faces.
Tables
Pain ratings in patients and controls.
Left: the percentage of trials (out of the 60 Hand and 60 Face trials for patients, or 30 and 30 for controls) per rating per participant for the Hand and Face conditions. For the age- and gender-matched control group, only the average across the 93 controls is shown. Middle: mean (M) rating for the Hand or Face. Our patients reported slightly higher pain intensity ratings for our Hand than Face stimuli (t(6) = 2.60, p2=0.041, BF10 = 2.31), the same was true for the age- and gender-matched controls (n = 93, W = 2738.5, p2=0.001, BF10 = 39.40). This was somewhat surprising because the Hand and Face stimuli were rated as similarly intense in a validation study that preceded stimulus selection (Gallo et al., 2018). Right: standard deviation of the ratings for each participant. Because the efficiency of a regression depends on the standard deviation of the predictor, and much of our results depend on the relation between rating and intracranial electroencephalographic (iEEG) responses, we calculated the standard deviation for each participant and condition. The standard deviations were normally distributed (all Shapiro–Wilk p>0.25), we then used a t-test to compare them across the two conditions. We found no significant difference amongst the patients (t(6) = 1.44, p2=0.199, BF10 = 0.75). Differences we find in the correlations between rating and iEEG across Hand and Face stimuli therefore cannot be due to difference in the efficiency of these two estimations.
| Rating | M (rating) | SD (rating) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hand | Face | Hand | Face | Hand | Face | ||||||||
| 1–2 | 3–4 | 5–6 | 7–8 | 1–2 | 3–4 | 5–6 | 7–8 | ||||||
| Patient | A | 1.67 | 38.33 | 43.33 | 16.67 | 40.00 | 28.33 | 25.00 | 6.67 | 2.75 | 1.98 | 0.75 | 0.97 |
| B | 0.00 | 16.67 | 50.00 | 33.33 | 36.67 | 35.00 | 13.33 | 15.00 | 3.17 | 2.07 | 0.69 | 1.06 | |
| C | 0.00 | 0.00 | 43.33 | 56.67 | 56.67 | 43.33 | 0.00 | 0.00 | 3.57 | 1.43 | 0.50 | 0.50 | |
| D | 1.67 | 38.33 | 38.33 | 21.67 | 35.00 | 41.67 | 23.33 | 0.00 | 2.80 | 1.88 | 0.80 | 0.76 | |
| E | 28.33 | 28.33 | 31.67 | 11.67 | 35.00 | 20.00 | 28.33 | 16.67 | 2.27 | 2.27 | 1.01 | 1.12 | |
| F | 25.00 | 40.00 | 25.00 | 10.00 | 38.33 | 31.67 | 25.00 | 5.00 | 2.20 | 1.97 | 0.94 | 0.92 | |
| G | 38.33 | 23.33 | 26.67 | 11.67 | 36.67 | 28.33 | 25.00 | 10.00 | 2.12 | 2.08 | 1.06 | 1.01 | |
| Mean ± SEM | 13.57 ± 5.74 | 26.43 ± 5.10 | 36.90 ± 3.29 | 23.10 ± 5.90 | 39.76 ± 2.68 | 32.62 ± 2.85 | 20.00 ± 3.50 | 7.62 ± 2.33 | 2.70 | 1.95 | 0.82 | 0.91 | |
| Control | Mean ± SEM | 33.01 ± 2.13 | 38.89 ± 1.30 | 21.25 ± 1.73 | 6.85 ± 1.12 | 44.55 ± 1.69 | 32.76 ± 1.05 | 16.99 ± 1.19 | 5.70 ± 0.93 | 2.02 | 1.84 | 0.74 | 0.81 |
Post-hoc comparisons of Figure 2f.
To follow up on the repeated-measures ANOVA (rmANOVA) on the 1 s broadband power (BBP) with factors period (neutral, pain) × rating (1–2, 3–4, 5–6, 7–8), the table reports, for each contrast of interest indicated over the first two left columns: the average (SEM) of % power change, the W (if normality was violated) or t (when the data was normal) test values, and the two-tailed p and BF10 values for the tested comparison.
| Period | Pain rating | % power change | W | t(84) | p2 | BF10 |
|---|---|---|---|---|---|---|
| Neutral vs. pain period | 1–2 | –1.34 (0.35) vs. –1.16 (0.56) | 1903 | 0.742 | 0.13 | |
| 3–4 | –1.43 (0.32) vs. –0.92 (0.55) | 1801 | 0.909 | 0.15 | ||
| 5–6 | –1.48 (0.35) vs. 0.36 (0.67) | 3.42 | 0.001 | 24.31 | ||
| 7–8 | 0.84 (0.67) vs. 6.07 (1.05) | 7.29 | 2 × 10–10 | 6 × 107 | ||
| Pain period | 1–2 vs. 3–4 | –1.16 (0.56) vs. –0.92 (0.55) | 1966 | 0.545 | 0.12 | |
| 3–4 vs. 5–6 | –0.92 (0.55) vs. 0.36 (0.67) | 1065 | 8 × 10–4 | 15.21 | ||
| 5–6 vs. 7–8 | 0.36 (0.67) vs. 6.07 (1.05) | 309 | 3 × 10–11 | 950,944 |
Post-hoc comparisons of Figure 2h.
To follow up on the two significant stimulus (Hand, Face) × rating repeated-measures ANOVAs (rmANOVAs), one for the early, one for the late period, the table reports, for each contrast of interest indicated over the first three left columns: the average (SEM) of % power change, the W (if normality was violated) or t (when the data was normal) test values, and the two-tailed p and BF10 values for the tested comparison. The degrees of freedom were 47, as n = 48 since all possible rating options were only used by four patients with a total of 48 electrodes. Patients who used only some of the ratings are included in analyses using r(BBP,rating), but cannot be included in this rmANOVA approach.
| Period | Stimulus | Pain rating | % power change | W | t(84) | p2 | BF10 |
|---|---|---|---|---|---|---|---|
| Early period | Hand | 1–2 vs. 3–4 | 2.11 (1.59) vs. –3.16 (0.94) | 1014 | 3 × 10–6 | 847.14 | |
| 3–4 vs. 5–6 | –3.16 (0.94) vs. 2.44 (1.03) | 5.97 | 3 × 10–7 | 51,110 | |||
| 5–6 vs. 7–8 | 2.44 (1.03) vs. 8.32 (1.61) | 188 | 2 × 10–5 | 764.63 | |||
| Face | 1–2 vs. 3–4 | –1.81 (0.6) vs. –1.75 (0.6) | 0.1 | 0.92 | 0.16 | ||
| 3–4 vs. 5–6 | –1.75 (0.6) vs. –1.54 (0.95) | 0.25 | 0.803 | 0.16 | |||
| 5–6 vs. 7–8 | –1.54 (0.95) vs. –1.83 (1.35) | 0.23 | 0.817 | 0.16 | |||
| Late period | Hand and Face | 1–2 vs. 3–4 | –0.76 (1.01) vs. –0.73 (0.84) | 597 | 0.931 | 0.16 | |
| 3–4 vs. 5–6 | –0.73 (0.84) vs. 1.96 (1.13) | 3.46 | 0.001 | 25.15 | |||
| 5–6 vs. 7–8 | 1.96 (1.13) vs. 4.75 (1.44) | 2.9 | 0.006 | 6.29 |
Participants’ demographics and epileptic status.
Our seven patients were matched in age and gender to the online control group from which we obtained normative movie ratings. The last column indicates the postoperative status of our patients. Three patients had other brain regions than insula surgically removed, and afterward had no more attacks (marked with 1), suggesting that the foci were clearly outside the insula. One patient had a region other than the insula surgically removed because the monitoring had suggested that the foci was outside of the insula; however, the patient continued to have post-surgical attacks (marked with 2). For three patients, no surgery was performed because there was no clear link between electrode locations and epileptic attacks (marked with 3).
| Group | Sample size (n) | Age (mean ± SD years) | Age-matched | Gender (M/F) | Gender-matched | Epileptic location score |
|---|---|---|---|---|---|---|
| Patient | 7 | 34.3 ± 9 | Mann–Whitney U-test, p=0.7, BF01 = 3.6 | 3/4 | Multinomial test, p=0.7, BF01 = 7 .3 | A = 1, B = 1, C = 3, D = 3, E = 1, F = 3, G = 2 |
| Control | 93 | 33.7 ± 9 | 38/55 | N/A |





