MKK6 deficiency promotes cardiac dysfunction through MKK3-p38γ/δ-mTOR hyperactivation
Figures
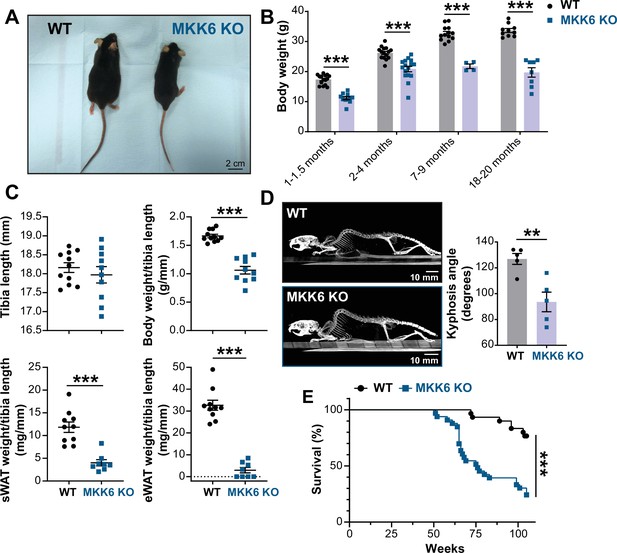
MKK6 KO mice show a reduced survival age.
(A) Representative picture of 19-month-old wild type (WT) and MKK6 KO male mice. Scale bar: 2 cm. (B) Body weight of WT (n=10–15) and MKK6 KO (n=4–16) mice over the indicated age period. Two-way ANOVA followed by Sidak’s post-test. (C) Tibia length and body weight, subcutaneous white adipose tissue (sWAT) and epidydimal white adipose tissue (eWAT) to tibia length ratio from 20-month-old WT (n=10–11) and MKK6 KO (n=8–10) mice. Unpaired t-test or Mann-Whitney U test. (D) Representative CT scan images and quantification of the column kyphosis angle of 19-month-old WT (n=5) and MKK6 KO (n=5) mice. Unpaired t-test. Scale bar: 10 mm. (E) Kaplan-Meier survival plot of age-related mortality in WT (n=30) and MKK6 KO (n=33) mice. An endpoint of 105 weeks was chosen to avoid a severe worsening of the mice’s health. Gehan-Breslow-Wilcoxon test. Data in B–D are mean ± SEM. **p<0.01; ***p<0.001.
-
Figure 1—source data 1
Raw data, statistical tests and significance, and n for Figure 1B, C and D & E.
- https://cdn.elifesciences.org/articles/75250/elife-75250-fig1-data1-v1.xlsx
Ataxia and hunched posture in MKK6 KO mice.
Video showing the movement of 18-month-old WT (left) and MKK6 KO (right) mice. WT: wild type.
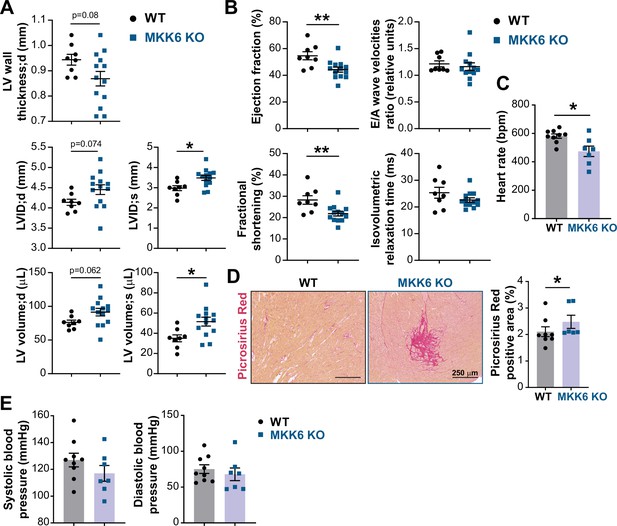
MKK6 deficiency promotes cardiac dysfunction at advanced ages.
(A and B) Echocardiography parameters related to left ventricle (LV) dimensions (A) and contractility (B) in 12–14-month-old wild type (WT) (n=8) and MKK6 KO (n=13). Each dot corresponds to an individual animal. LV wall thickness;d (LV wall thickness in diastole), LVID;d (left ventricular internal diameter in diastole), LVID;s (left ventricular internal diameter in systole), LV volume;d (left ventricular volume in diastole), and LV volume;s (left ventricular volume in systole). Unpaired t-test. (C) Heart rate in conscious 18-month-old WT (n=9) and MKK6 KO (n=7) mice. bpm (beats per minute). Unpaired t-test. (D) Picrosirius red staining and quantification of cardiac fibrosis in 23–24-month-old WT (n=8) and MKK6 KO (n=6) mice. Mann-Whitney U test. Scale bars: 250 µm. (E) Systolic and diastolic blood pressure measured in conscious 18-month-old WT (n=9) and MKK6 KO (n=7) mice. Unpaired t-test. Data in A–E are mean ± SEM. *p<0.05; **p<0.01.
-
Figure 2—source data 1
Raw data, statistical tests and significance, and n for Figure 2.
- https://cdn.elifesciences.org/articles/75250/elife-75250-fig2-data1-v1.xlsx
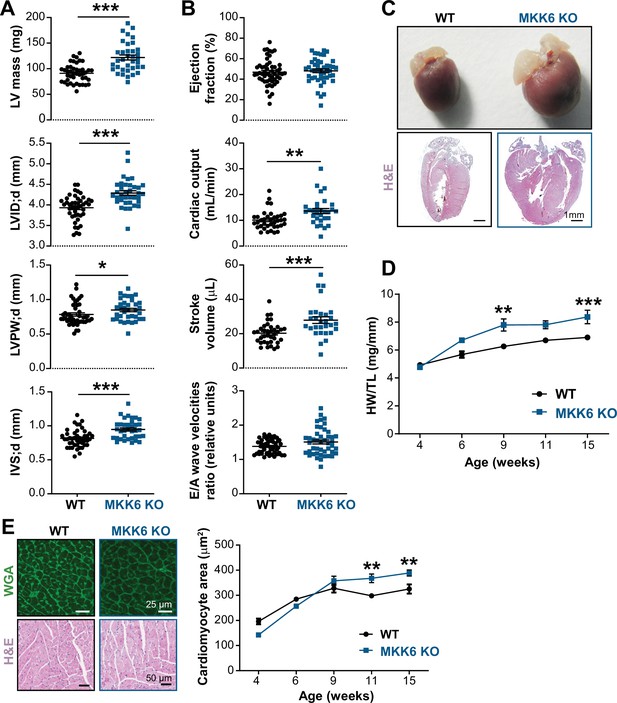
Young MKK6-deficient hearts are hypertrophic with preserved cardiac function.
(A and B) Echocardiography parameters related to left ventricle (LV) dimensions (A) and contractility (B) in 9-week-old wild type (WT) (n=37–54) and MKK6 KO (n=29–46) mice. Each dot corresponds to an individual mouse. Mean ± SEM are shown as well. LV mass (left ventricular mass), LVID;d (left ventricular internal diameter in diastole), LVPW;d (left ventricular posterior wall in diastole), and IVS;d (inter-ventricular septum in diastole). Unpaired t-test or Mann-Whitney U test. (C) Representative whole hearts and cardiac longitudinal sections stained with hematoxylin and eosin (H&E) from 9-week-old WT and MKK6 KO mice. Scale bars: 1 mm. (D) Heart weight to tibia length ratio (HW/TL) of WT (n=4–15) and MKK6 KO (n=5–14) over the indicated age period. Two-way ANOVA followed by Sidak’s post-test. (E) Top: representative Fluorescein isothiocyanate (FITC) wheat germ agglutinin (FITC-WGA)-stained heart sections from 9-week-old WT and MKK6 KO mice and quantification of cardiomyocyte cross-sectional area over time (right graph, WT n=4–5; MKK6 KO n=4–6, and two-way ANOVA followed by Sidak’s post-test). Scale bars: 25 µm. Bottom: representative H&E-stained heart sections. Scale bars: 50 µm. Data in A, B, D, and E are mean ± SEM. *p<0.05; **p<0.01; ***p<0.001.
-
Figure 3—source data 1
Raw data, statistical tests and significance, and n for Figure 3A, B and D&E and Figure 3—figure supplement 1.
- https://cdn.elifesciences.org/articles/75250/elife-75250-fig3-data1-v1.xlsx
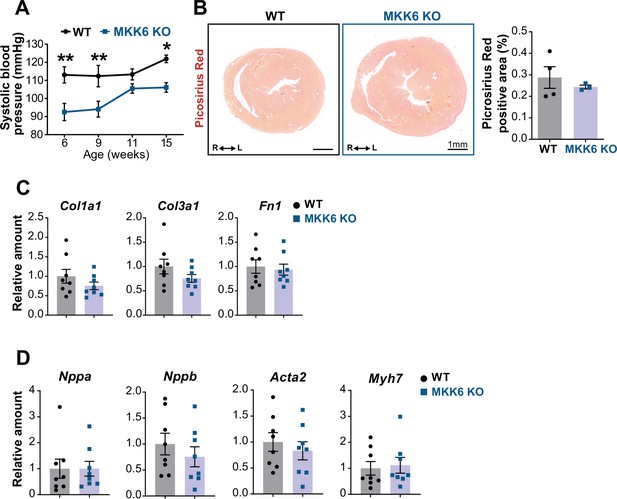
Evaluation of hallmarks of pathological cardiac hypertrophy in young MKK6 KO mice.
(A) Systolic blood pressure in wild type (WT) (n=5) and MKK6 KO (n=4–7) mice at the indicated times after birth. Two-way ANOVA followed by Sidak’s post-test. (B) Picrosirius red staining and quantification of cardiac fibrosis in 9-week-old WT (n=4) and MKK6 KO (n=3) mice. Unpaired t-test. Scale bars: 1 mm. (C–D) Cardiac gene expression of fibrosis (C) and cardiac stress (D) markers in 9-week-old WT (n=8) and MKK6 KO (n=8). Unpaired t-test or Mann-Whitney U test. Data in A–D are mean ± SEM. *p<0.05. **p<0.001.
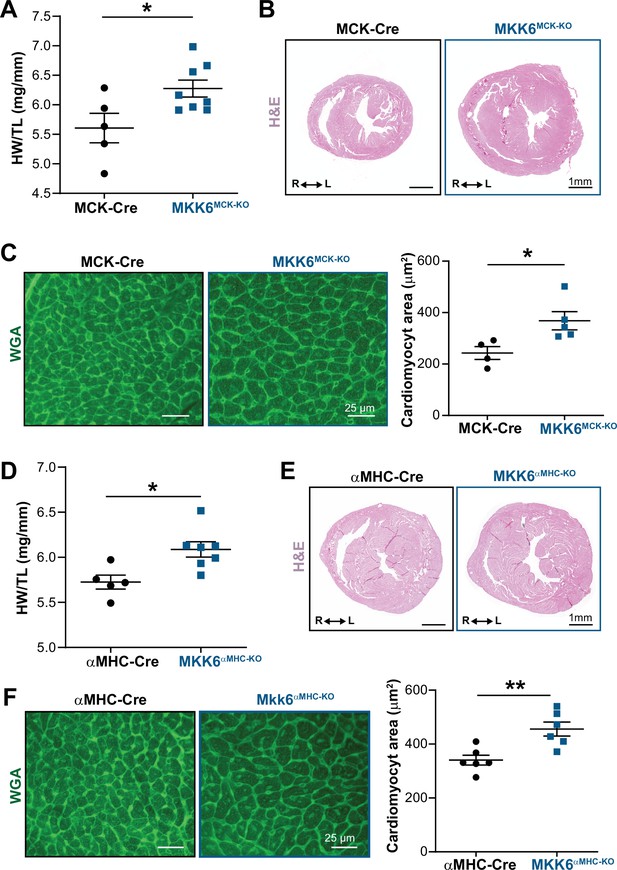
Cardiac MKK6 controls postnatal heart growth.
(A) Heart weight to tibia length ratio (HW/TL) in 9-week-old MCK-Cre (n=5) and MKK6MCK-KO (n=8) mice. Unpaired t-test. (B) Hematoxylin and eosin (H&E)-stained transverse cardiac sections from control (MCK-Cre) and MKK6MCK-KO mice. Scale bars: 1 mm. (C) Representative FITC wheat germ agglutinin (FITC-WGA) staining and corresponding quantification of cardiomyocyte cross-sectional area in MCK-Cre (n=4) and MKK6MCK-KO (n=5) mice. Unpaired t-test. Scale bars: 25 µm. (D) HW/TL in 9-week-old αMHC-Cre (n=5) and MKK6αMHC-KO (n=7) mice. Unpaired t-test. (E) H&E-stained transverse heart sections from αMHC-Cre and MKK6αMHC-KO mice. Scale bars: 1 mm. (F) Representative FITC-WGA staining and corresponding quantification of cardiomyocyte cross-sectional area in αMHC-Cre (n=6) and MKK6αMHC-KO (n=6) mice. Unpaired t-test. Scale bars: 25 µm. Data in A, C, D, and F are mean ± SEM. *p<0.05; **p<0.01.
-
Figure 4—source data 1
Raw data, statistical test and significance, and n for Figure 4A, C, D and F and Figure 4—figure supplement 2.
- https://cdn.elifesciences.org/articles/75250/elife-75250-fig4-data1-v1.xlsx
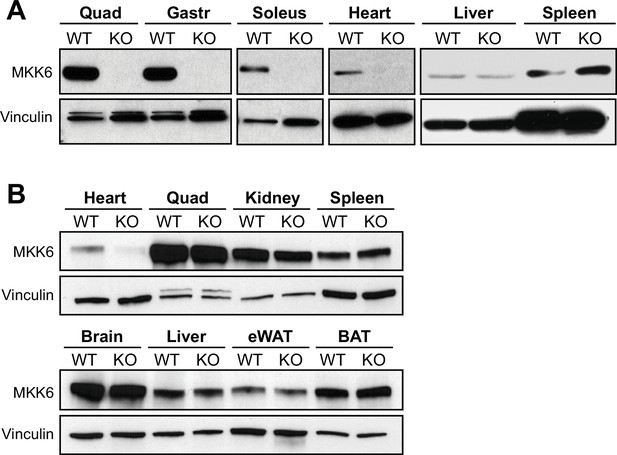
Tissue-specific MKK6 deletion in MKK6MCK-KO and MKK6αMHC-KO mice.
(A) Confirmation of MKK6 deletion in tissue lysates from MKK6MCK-KO (KO) mice in quadriceps (Quad), gastrocnemius (Gastr), soleus, and heart, but not in spleen and liver, and not in MCK-Cre (wild type [WT]) tissues. (B) Confirmation of specific MKK6 deletion in the heart of MKK6αMHC-KO, but not in lysates of αMHC-Cre (WT) and MKK6αMHC-KO (KO) Quad, kidney, spleen, brain, liver, epididymal white adipose tissue (eWAT), and brown adipose tissue (BAT).
-
Figure 4—figure supplement 1—source data 1
Raw blots for Figure 4—figure supplement 1.
- https://cdn.elifesciences.org/articles/75250/elife-75250-fig4-figsupp1-data1-v1.pdf
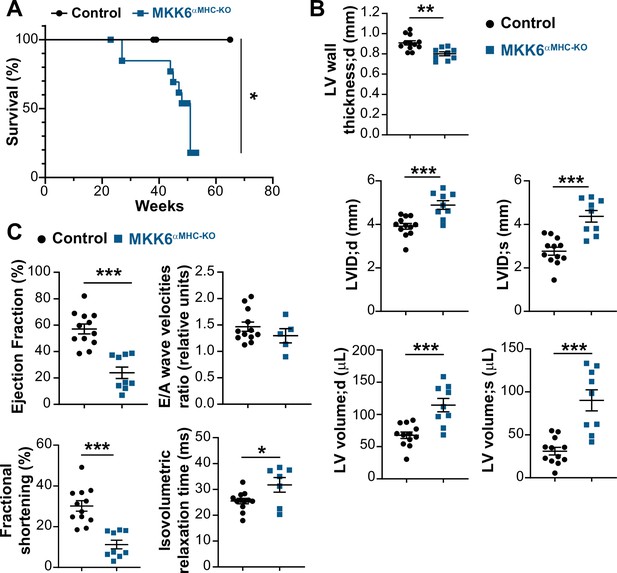
MKK6αMHC-KO mice show a reduced lifespan and associated cardiac dysfunction.
(A) Kaplan-Meier survival plot of age-related mortality in controls (n=19) and MKK6αMHC-KO (n=16) mice. Gehan-Breslow-Wilcoxon test. *p<0.05. (B and C) Echocardiography parameters related to left ventricle (LV) dimensions (B) and contractility (C) in 8–10-month-old controls (n=12) and MKK6αMHC-KO (n=5–9). LV wall thickness;d (left ventricle wall thickness in diastole), LVID;d (left ventricular internal diameter in diastole), LVID;s (left ventricular internal diameter in systole), LV volume;d (left ventricular volume in diastole), and LV volume;s (left ventricular volume in systole). Unpaired t-test or Mann-Whitney U test. *p<0.05; **p<0.01; ***p<0.001. Data are mean ± SEM.
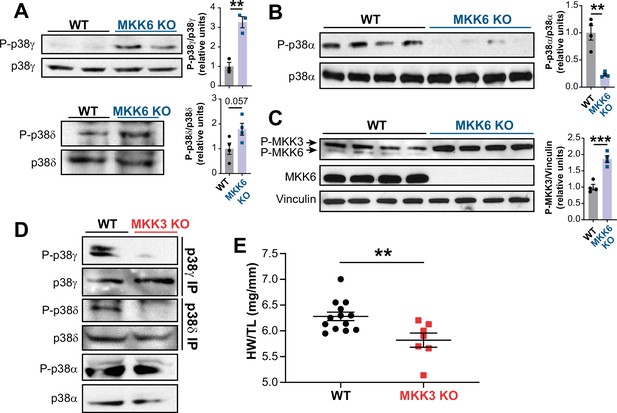
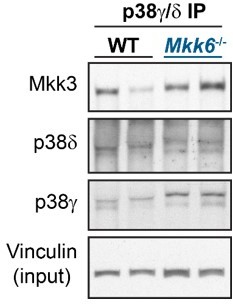
MKK6 is necessary for p38α phosphorylation and MKK3 for p38γ and p38δ phosphorylation in the heart.
(A) Western blot analysis of the phosphorylation and amount of p38γ and δ immunoprecipitated from heart lysates from 9-week-old wild type WT (n=3–4) and MKK6 KO (n=3–4) mice. Unpaired t-test. (B) Immunoblot analysis of p38α phosphorylation and protein amount in WT (n=4) and MKK6 KO (n=4) mice. Unpaired t-test. (C) Phosphorylation and protein levels of MKK3 and MKK6 in heart lysates from WT (n=4) and MKK6 KO (n=4) mice. Unpaired t-test. (D) Immunoprecipitation and western blot analysis of the phosphorylation and protein amounts of p38α, p38γ, and p38δ isoforms in heart lysates from 9-week-old WT and MKK3 KO mice. (E) Heart weight to tibia length ratio in WT (n=13) and MKK3 KO (n=7) mice at 9 weeks of age. Data in E are mean ± SEM. (n=3–13). **p<0.01; ***p<0.001.
-
Figure 5—source data 1
Raw blots for Figure 5A–D.
- https://cdn.elifesciences.org/articles/75250/elife-75250-fig5-data1-v1.pdf
-
Figure 5—source data 2
Raw data, statistical test and significance, and n for Figure 5, Figure 5—figure supplement 1 and Figure 5—figure supplement 2.
- https://cdn.elifesciences.org/articles/75250/elife-75250-fig5-data2-v1.xlsx
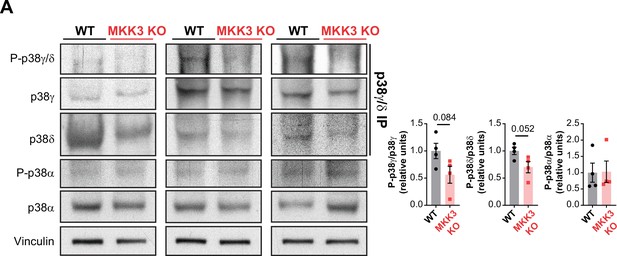
MKK3 deficiency promotes a reduced phosphorylation of p38γ/δ in the heart.
(A) Immunoprecipitation and western blot analysis of the phosphorylation and protein amounts of p38α, p38γ, and p38δ isoforms in heart lysates from 9-week-old wild type (WT) (n=4) and MKK3 KO (n=4) mice. Unpaired t-test or Mann-Whitney U test. Data are mean ± SEM (n=4).
-
Figure 5—figure supplement 1—source data 1
Raw blots for Figure 5—figure supplement 1.
- https://cdn.elifesciences.org/articles/75250/elife-75250-fig5-figsupp1-data1-v1.pdf
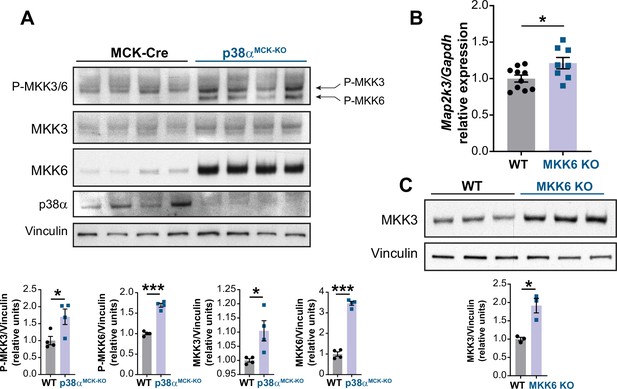
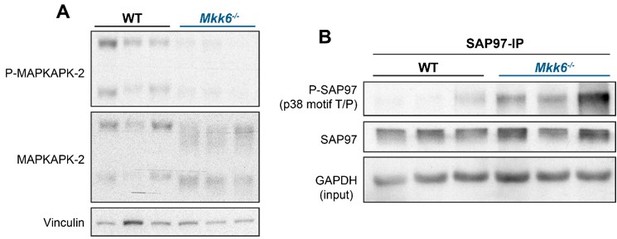
p38α negatively regulates MKK3 expression and phosphorylation in the heart.
(A) Immunoblot analysis of phosphorylation and protein levels in heart lysates from 12-week-old MCK-Cre (n=4) and p38αMCK-KO (n=4) mice. Unpaired t-test or Mann-Whitney U test. (B) mRNA levels of Map2k3 (MKK3) in 9-week-old wild type (WT) (n=10) and MKK6 KO (n=8) hearts. Map2k3 mRNA levels were relativized to Gapdh mRNA. Unpaired t-test. (C) Immunoblot evaluation of MKK3 protein levels in hearts from 9-week-old WT (n=3) and MKK6 KO (n=3) mice. Unpaired t-test. Data are mean ± SEM. (n=3–10). *p<0.05; ***p<0.001.
-
Figure 5—figure supplement 2—source data 1
Raw blots for Figure 5—figure supplement 2.
- https://cdn.elifesciences.org/articles/75250/elife-75250-fig5-figsupp2-data1-v1.pdf
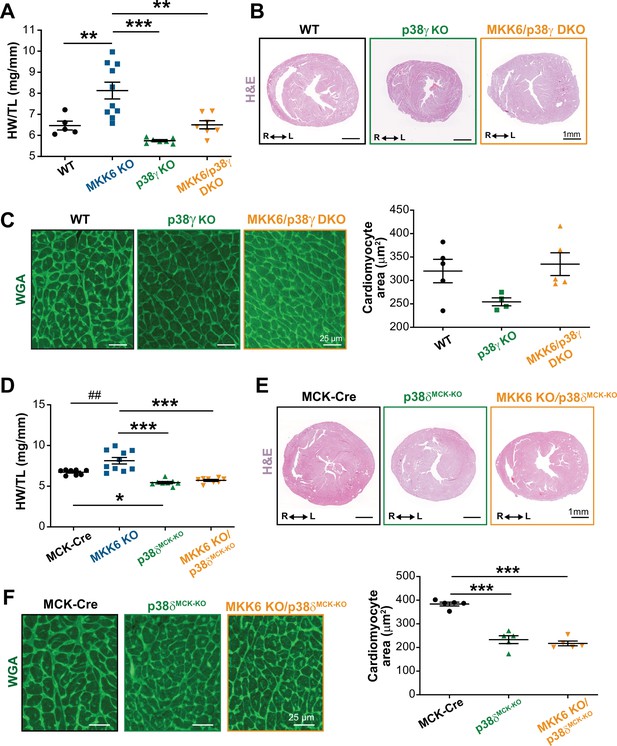
Loss of p38γ/δ in cardiomyocytes rescues the cardiac hypertrophy induced by MKK6 deficiency.
All phenotypes shown come from 9-week-old mice. (A) Heart weight to tibia length ratio in wild type (WT) (n=5), MKK6 KO (n=10), p38γ KO (n=7), and MKK6/p38γ DKO (n=7) mice. One-way ANOVA followed by Tukey’s post-test. (B) Representative hematoxylin and eosin (H&E)-stained transverse heart sections from WT, p38γ KO, and MKK6/p38γ DKO mice. Scale bars: 1 mm. (C) Representative FITC wheat germ agglutinin (FITC-WGA) staining and corresponding quantification of cardiomyocyte cross-sectional area in WT (n=5), p38γ KO (n=4), and MKK6/p38γ DKO (n=5) mice. Scale bars: 25 µm. One-way ANOVA followed by Tukey’s post-test. (D) Heart weight to tibia length ratio in MCK-Cre (n=8), MKK6 KO (n=10), p38δMCK-KO (n=8), and MKK6 KO/p38δMCK-KO (n=8) mice. Kruskal-Wallis test with Dunn’s post-test (##p<0.01 Mann-Whitney U test). (E) Representative H&E-stained transverse heart sections from MCK-Cre, p38δMCK-KO, and MKK6 KO/p38δMCK-KO mice. Scale bars: 1 mm. (F) Representative FITC-WGA staining and corresponding quantification of cardiomyocyte cross-sectional area in MCK-Cre (n=5), p38δMCK-KO (n=5), and MKK6 KO/p38δMCK-KO (n=5) mice. One-way ANOVA followed by Tukey’s post-test. Scale bars: 25 µm. The same data from MKK6 KO mice were used in (A) and (D). Means ± SEM is shown. *p<0.05; **p<0.01; ***p<0.001; ##p<0.01.
-
Figure 6—source data 1
Raw data, statistical test and significance, and n for Figure 6A, C and D & F.
- https://cdn.elifesciences.org/articles/75250/elife-75250-fig6-data1-v1.xlsx
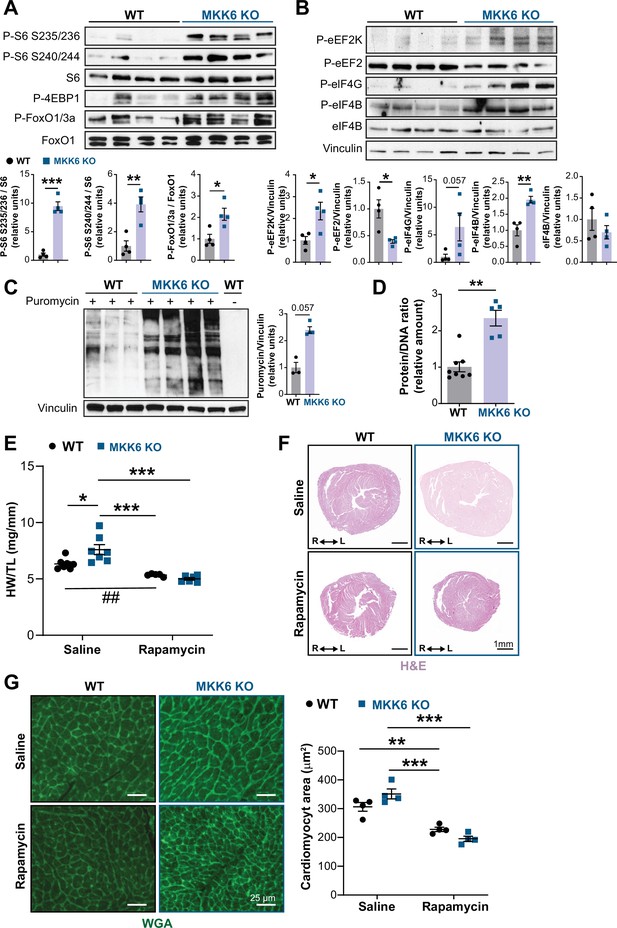
Hyperactivation of mammalian target of rapamycin (mTOR) signaling drives cardiac hypertrophy in MKK6 KO mice.
(A and B) Immunoblot analysis of mTOR signaling pathway activity (A) and activation status of translation factors (B) in heart lysates from 9-week-old wild type (WT) (n=4) and MKK6 KO (n=4) mice. Unpaired t-test or Mann-Whitney U test. (C) In vivo measurement of protein synthesis in 9-week-old WT (n=) and MKK6 KO (n=4) mice. Mice were injected intraperitoneally with 0.040 μmol g−1 puromycin dissolved in 100 μl PBS. Exactly 30 min after injection, tissues were extracted and frozen in liquid N2 for immunoblot analysis with an anti-puromycin antibody. Mann-Whitney U test. (D) Protein content of WT (n=8) and MKK6 KO (n=5) hearts measured as the protein-DNA ratio. Mann-Whitney U test. (E) Heart weight to tibia length ratio in WT (n=5–7) and MKK6 KO (n=6–7) mice after rapamycin treatment. Mice received daily intraperitoneal injections with rapamycin (2 mg kg−1 per day) or vehicle from weeks 4 to 9 after birth. Two-way ANOVA followed by Tukey’s post-test (## p<0.01 unpaired t-test). (F) Representative hematoxylin and eosin (H&E)-stained transverse heart sections after treatment. Scale bars: 1 mm. (G) Representative FITC wheat germ agglutinin (FITC-WGA) staining and corresponding quantification of cardiomyocyte cross-sectional area from WT (n=4) and MKK6 KO (n=4) mice hearts after rapamycin treatment. Two-way ANOVA followed by Tukey’s post-test. Scale bars: 25 µm. Data are mean ± SEM. *p<0.05; **p<0.01; ***p<0.001.
-
Figure 7—source data 1
Raw blots for Figure 7A–C.
- https://cdn.elifesciences.org/articles/75250/elife-75250-fig7-data1-v1.pdf
-
Figure 7—source data 2
Raw data, statistical test and significance, and n for Figure 7 and Figure 7—figure supplement 1, Figure 7—figure supplements 3 and 4.
- https://cdn.elifesciences.org/articles/75250/elife-75250-fig7-data2-v1.xlsx
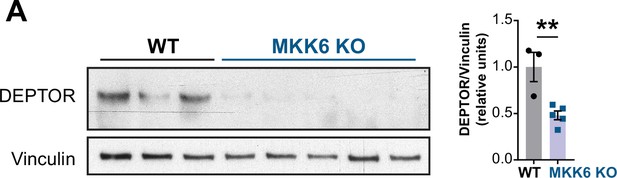
Mammalian target of rapamycin (mTOR)-inhibitory protein DEPTOR levels are reduced in MKK6-deficient hearts.
(A) Immunoblot analysis of DEPTOR protein levels in heart lysates from 9-week-old wild type (WT) (n=3) and MKK6 KO (n=5) mice. Unpaired t-test. Data are mean ± SEM. **p<0.01.
-
Figure 7—figure supplement 1—source data 1
Raw blots for Figure 7—figure supplement 1.
- https://cdn.elifesciences.org/articles/75250/elife-75250-fig7-figsupp1-data1-v1.pdf
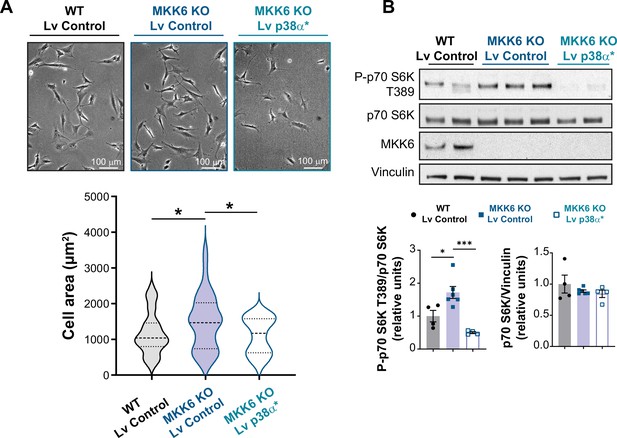
Mutant active p38α expression reverts mammalian target of rapamycin (mTOR) hyperactivation and mTOR-induced cell growth in MKK6 KO mouse embryonic fibroblasts (MEFs).
(A) Representative images and cell area quantification of immortalized MEFs from wild type (WT) or MKK6 KO mice infected with control or a mutant active form of p38α (p38α*) lentivirus (Lv). WT Lv control n=39; MKK6 KO Lv control n=40; MKK6 KO Lv p38α* n=41. Unpaired t-test or Mann-Whitney U test. (B) Evaluation of mTOR pathway activity by immunoblot analysis p70 S6 kinase phosphorylation in MEF cell lysates infected with control or a mutant active form of p38α. WT Lv control n=4; MKK6 KO Lv control n=6; MKK6 KO Lv p38α* n=4. One-way ANOVA followed by Tukey’s post-test. * p<0.05; *** p<0.001.
-
Figure 7—figure supplement 2—source data 1
Raw blots for Figure 7—figure supplement 2.
- https://cdn.elifesciences.org/articles/75250/elife-75250-fig7-figsupp2-data1-v1.pdf
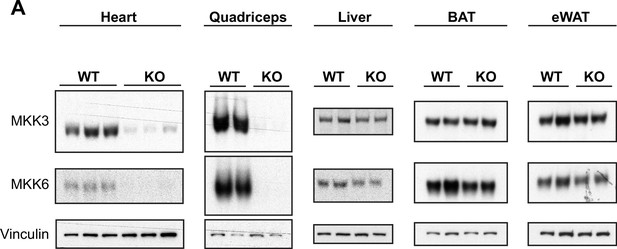
Tissue-specific MKK3 and MKK6 deletion in MKK3/6MCK-KO mice.
(A) Confirmation of MKK3 and MKK6 deletion in tissue lysates from 9-week-old MKK3/6MCK-KO (KO) mice in heart and quadriceps, but not in liver, brown adipose tissue (BAT) or epidydimal white adipose tissue (eWAT), and not in MCK-Cre (wild type [WT]) tissues.
-
Figure 7—figure supplement 3—source data 1
Raw blots for Figure 7—figure supplement 3.
- https://cdn.elifesciences.org/articles/75250/elife-75250-fig7-figsupp3-data1-v1.pdf
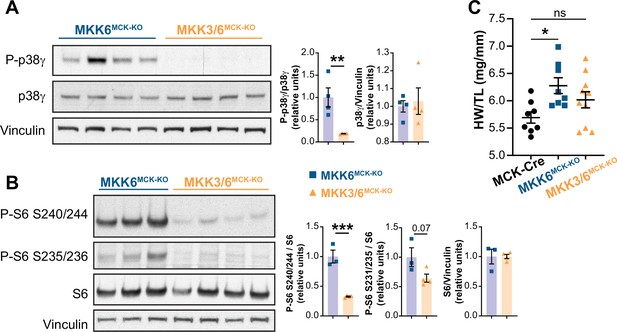
MKK3 deletion suppresses p38γ and mammalian target of rapamycin (mTOR) hyperactivation in MKK6-deficient hearts.
(A) Immunoblot analysis of p38γ phosphorylation in hearts from 9-week-old MKK6MCK-KO (n=4) and MKK3/6MCK-KO mice (n=4). Unpaired t-test. (B) Evaluation of mTOR pathway activity by immunoblot analysis of S6 ribosomal protein phosphorylation in hearts from 9-week-old MKK6MCK-KO (n=3) and MKK3/6MCK-KO mice (n=4). Unpaired t-test. (C) Heart weight to tibia length ratio in hearts from MCK-Cre (n=8), MKK6MCK-KO (n=8), and MKK3/6MCK-KO (n=10) mice at 9 weeks of age. The data for MKK6MCK-KO mice are the same as in Figure 6A (one-way ANOVA followed by Tukey’s post-test). Data are mean ± SEM. *p<0.05; **p<0.01; **p<0.001.
-
Figure 7—figure supplement 4—source data 1
Raw blots for Figure 7—figure supplement 4.
- https://cdn.elifesciences.org/articles/75250/elife-75250-fig7-figsupp4-data1-v1.pdf
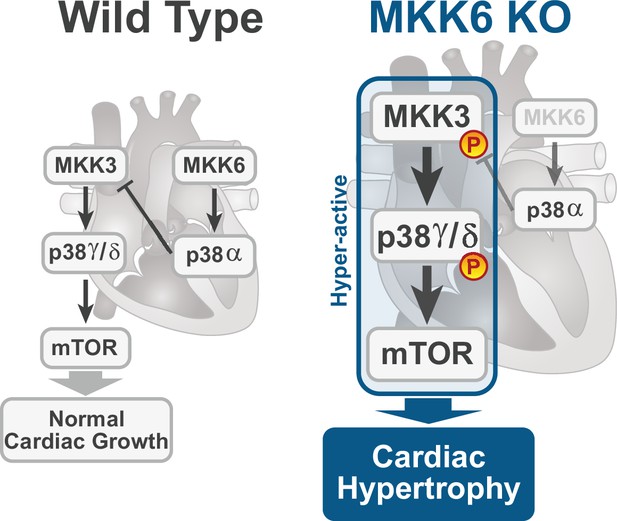
Model for p38γ/δ activation mediated cardiac hypertrophic growth.
In a physiological context, MKK3-p38γ/δ pathway promotes normal cardiac growth through the activation of mammalian target of rapamycin (mTOR) signaling pathway. MKK6 deficiency stimulates the hyperactivation of MKK3-p38γ/δ and the consequent increase in mTOR activity, which drives increased cardiac hypertrophy.
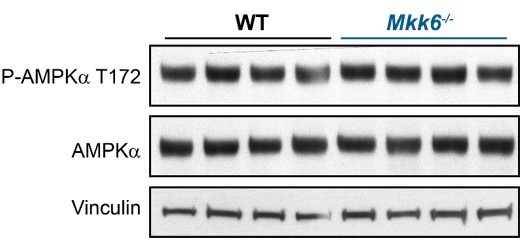
AMPK phosphorylation is not altered in MKK6-deficient hearts.
Immunoblot analysis of AMPKa phosphorylation and protein levels in hearts from 9-week-old WT and Mkk6-/- mice.
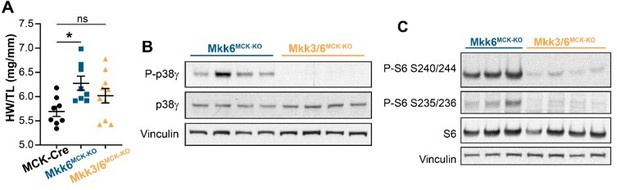
MKK3 deletion attenuates p38g and mTOR activation and cardiac hypertrophy in MKK6-deficient hearts.
(A) Heart weight to tibia length ratio in 9-week-old MCK-Cre (n=8), Mkk6MCK-KO (n=10) and Mkk3/6MCK-KO (n=10). 1-way ANOVA followed by Tukey’s multiple comparison test. *P<0.05; ns: non-significant. Data are mean± SEM. (B) Immunoblot analysis of p38g phosphorylation and protein levels in heart lysates from 9-week-old Mkk6MCK-KO and Mkk3/6MCK-KO mice. (C) Immunoblot analysis of mTOR pathway activation by phosphorylation evaluation of its downstream target S6 in hearts from 9-week-old Mkk6MCK-KO and Mkk3/6MCK-KO mice.