Making memories last using the peripheral effect of direct current stimulation
Figures
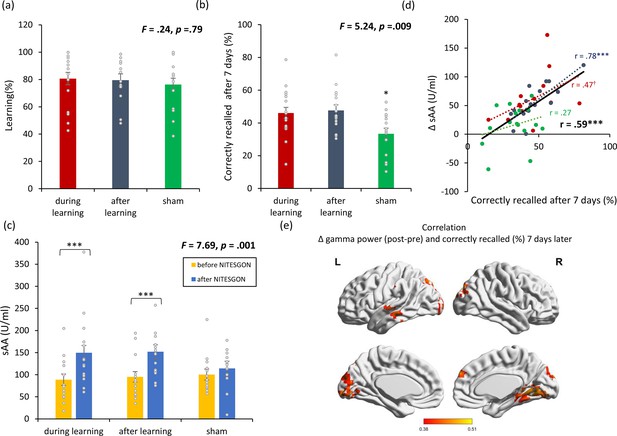
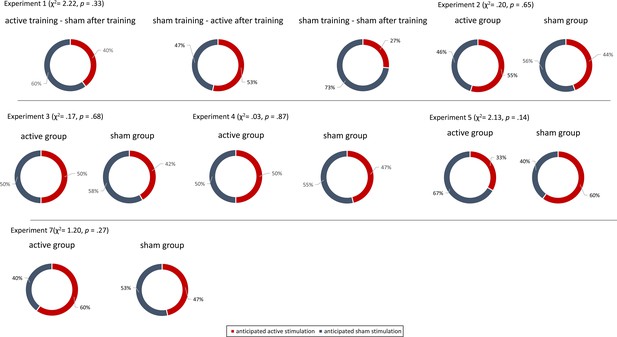
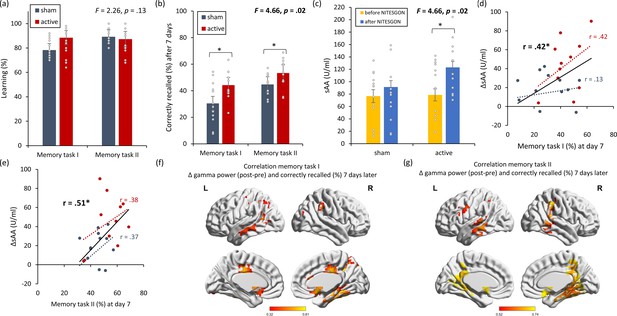
Non-invasive transcutaneous electrical stimulation of the greater occipital nerve (NITESGON) immediately after training can enhance memory with Figure 1—figure supplement 1.
(a) No difference was observed in the cumulative learning rate between active and sham NITESGON during or immediately after the study phase of the word-association memory task. (b) NITESGON during or immediately after the word-association memory task can improve memory recall 7 days after the study phase for the active relative to the sham group. (c) After NITESGON salivary α-amylase (sAA) levels increase for both active groups, but not for sham NITESGON. (d) Memory recall 7 days later correlates with the difference in sAA levels during the first visit (pre vs. post study phase). (e) Improved memory recall 7 days after stimulation is associated with increased activity in the medial temporal lobe as well as anterior and posterior cingulate cortex immediately after NITESGON for the gamma frequency band. Error bars, standard error of the mean (s.e.m.). Asterisks represent significant differences (*p < 0.05; **p < 0.01; *** p < 0.001), ΔsAA levels are the subtraction of sAA levels before NITESGON from sAA levels immediately after NITESGON.
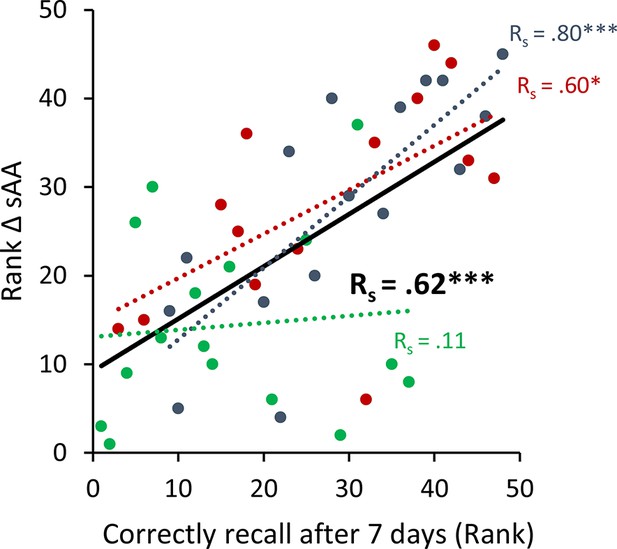
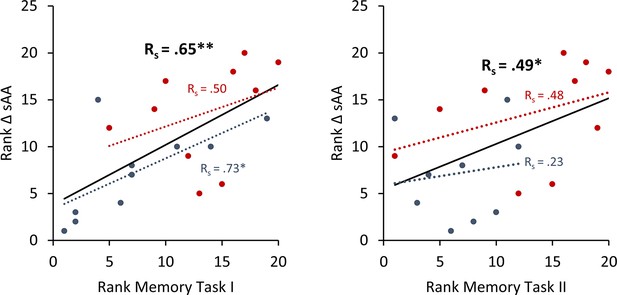
To avoid the potential outliers would drive the Pearson correlation between a memory recall 7 days after learning the task and the difference in salivary α-amylase (sAA) levels (difference between before and after stimulation on day 1), a Spearman rank correlation was calculated to cross validate our findings.
Spearman correlation for Experiment 1 the rank of the difference of sAA levels and the rank for memory recall 7 days after learning the task. Asterisks represent significant differences (*p < 0.05; **p < 0.01; ***p < 0.001), ΔsAA levels are the subtraction of sAA levels before non-invasive transcutaneous electrical stimulation of the greater occipital nerve (NITESGON) from sAA levels immediately after NITESGON.
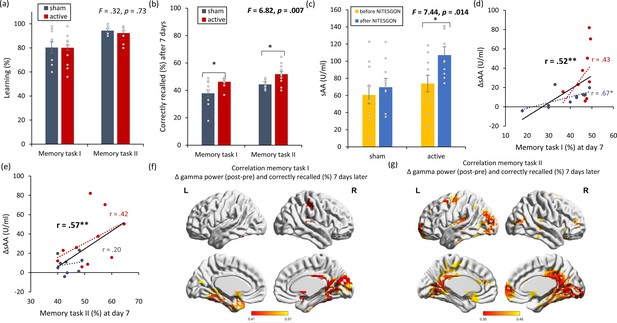
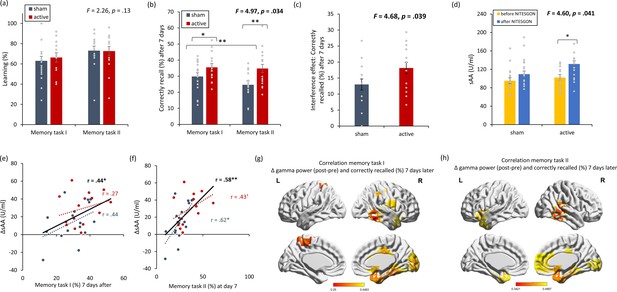
Non-invasive transcutaneous electrical stimulation of the greater occipital nerve (NITESGON) has a retroactive memory effect – NITESGON during the second task memory with Figure 2—figure supplement 1.
(a) No difference was observed in the cumulative learning rate between active and sham NITESGON after the study phase for the first task (i.e., word-association task) or second task (i.e., object-location task). (b) NITESGON can improve memory recall 7 days after the study phase for the active relative to the sham group for both the first and second tasks. (c) After NITESGON salivary α-amylase (sAA) levels increase for active group, but not for sham NITESGON. (d, e) Memory recall 7 days later correlates with the difference in sAA levels during the first visit (pre vs. post study phase) for the first and second tasks. (f, g) Improved memory recall 7 days after stimulation is associated with increased activity in the medial temporal lobe immediately after NITESGON for the gamma frequency band. Error bars, standard error of the mean (s.e.m.). Asterisks represent significant differences (*p < 0.05; **p < 0.01). ΔsAA levels are the subtraction of sAA levels before NITESGON from sAA levels immediately after NITESGON.
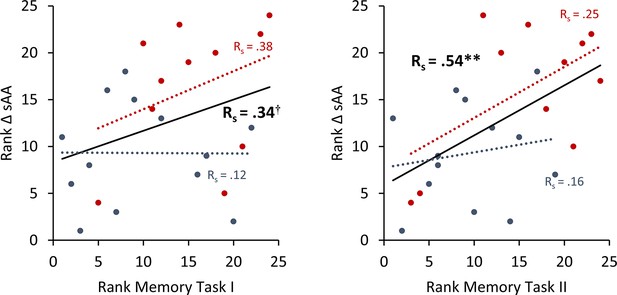
To avoid the potential outliers would drive the Pearson correlation between a memory recall 7 days after learning the task and the difference in salivary α-amylase (sAA) levels (difference between before and after stimulation on day 1), a Spearman rank correlation was calculated to cross validate our findings.
Spearman correlation for Experiment 2 the rank of the difference of sAA levels and the rank for memory recall 7 days after learning the task. Asterisks represent significant differences (*p < 0.05; **p < 0.01). ΔsAA levels are the subtraction of sAA levels before non-invasive transcutaneous electrical stimulation of the greater occipital nerve (NITESGON) from sAA levels immediately after NITESGON.
Non-invasive transcutaneous electrical stimulation of the greater occipital nerve (NITESGON) has a proactive memory effect – NITESGON during the first task memory with Figure 3—figure supplement 1.
(a) No difference was observed in the cumulative learning rate between active and sham NITESGON after the study phase for the first task (i.e., word-association task) or second task (i.e., object-location task). (b) NITESGON can improve memory recall 7 days after the study phase for the active relative to the sham group for both the first and second tasks. (c) After NITESGON salivary α-amylase (sAA) levels increase for active group, but not for sham NITESGON. (d, e) Memory recall 7 days later correlates with the difference in sAA levels during the first visit (pre vs. post study phase) for the first and second tasks. (f, g) Improved memory recall 7 days after stimulation is associated with increased activity in the medial temporal lobe immediately after NITESGON for the gamma frequency band. Error bars, standard error of the mean (s.e.m.). Asterisks represent significant differences (*p < 0.05; **p < 0.01), ΔsAA levels are the subtraction of sAA levels before NITESGON from sAA levels immediately after NITESGON.
To avoid the potential outliers would drive the Pearson correlation between a memory recall 7 days after learning the task and the difference in salivary α-amylase (sAA) levels (difference between before and after stimulation on day 1), a Spearman rank correlation was calculated to cross validate our findings.
Spearman correlation for Experiment 3 the rank of the difference of sAA levels and the rank for memory recall 7 days after learning the task. Asterisks represent significant differences († p < 0.10; **p < 0.01), ΔsAA levels are the subtraction of sAA levels before non-invasive transcutaneous electrical stimulation of the greater occipital nerve (NITESGON) from sAA levels immediately after NITESGON.
Non-invasive transcutaneous electrical stimulation of the greater occipital nerve (NITESGON) reduces the interference effect memory with Figure 4—figure supplement 1.
(a) No difference was observed in the cumulative learning rate between active and sham NITESGON after the study phase for the first task (i.e., word-association task) or second task (i.e., word-association task). (b) NITESGON can improve memory recall 7 days after the study phase revealing improve in memory for the active relative to the sham group for both the first and second tasks. (c) The interference effect is less present for the active relative to the sham group. (d) After NITESGON salivary α-amylase (sAA) levels increase for the active group, but not for sham NITESGON. (e, f) Memory recall 7 days later correlates with the difference in sAA levels during the first visit (pre vs. post study phase) for the first and second tasks. (g, h) Improved memory recall 7 days after stimulation is associated with increased activity in the medial temporal lobe immediately after NITESGON for the gamma frequency band. Error bars, standard error of the mean (s.e.m.). Asterisks represent significant differences (*p < 0.05; **p < 0.01), ΔsAA levels are the subtraction of salivary α-amylase levels before NITESGON from salivary α-amylase levels immediately after NITESGON.
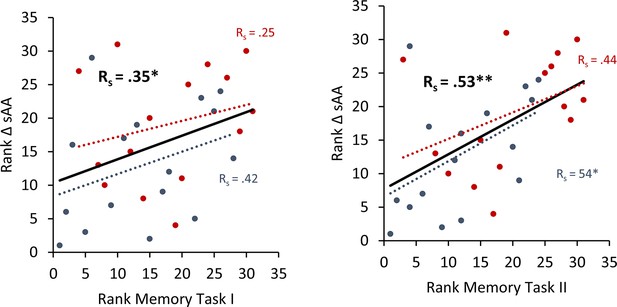
To avoid the potential outliers would drive the Pearson correlation between a memory recall 7 days after learning the task and the difference in salivary α-amylase (sAA) levels (difference between before and after stimulation on day 1), a Spearman rank correlation was calculated to cross validate our findings.
Spearman correlation for Experiment 4 the rank of the difference of sAA levels and the rank for memory recall 7 days after learning the task. Error bars, standard error of the mean (s.e.m.). Asterisks represent significant differences (*p < 0.05; **p < 0.01). ΔsAA levels are the subtraction of sAA levels before non-invasive transcutaneous electrical stimulation of the greater occipital nerve (NITESGON) from sAA levels immediately after NITESGON.
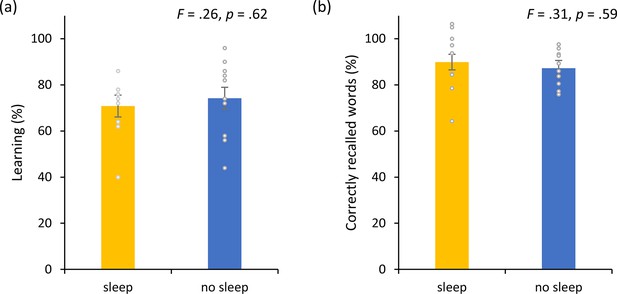
Non-invasive transcutaneous electrical stimulation of the greater occipital nerve (NITESGON) and sleep.
(a) No difference was observed in the cumulative learning rate between participants who had slept versus those who had not slept after NITESGON applied during the study phase. (b) Sleep has no effect on memory recall 12 hr after the study phase. Error bars, standard error of the mean (s.e.m.). Asterisks represent significant differences (*p < 0.05; **p < 0.01).
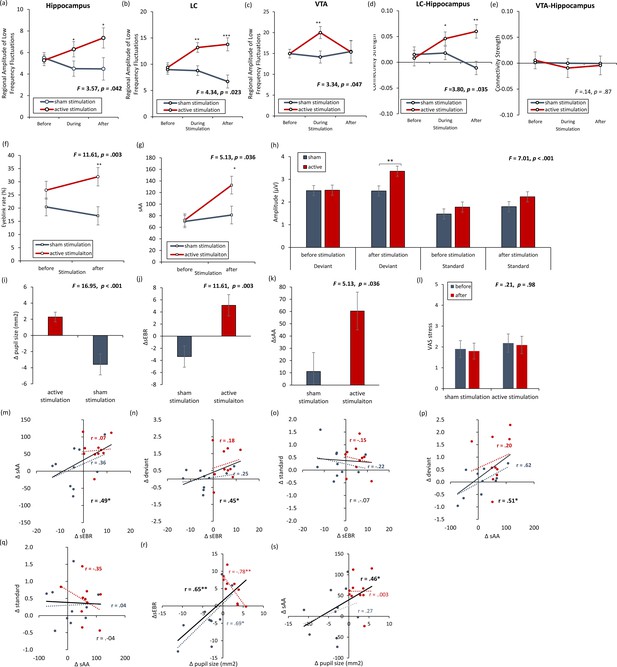
rsfMRI and physiology – locus coeruleus and dopamine with Figure 6—figure supplement 1.
(a, b) The locus coeruleus and hippocampus revealed increased activity during stimulation as well as after stimulation for the active non-invasive transcutaneous electrical stimulation of the greater occipital nerve (NITESGON) group in comparison to the sham NITESGON group. (c) The ventral tegmental area revealed increased activity during stimulation, but not after stimulation for the active NITESGON group in comparison to the sham NITESGON group. (d) Increased connectivity between the locus coeruleus and hippocampus was observed during and after stimulation for the active NITESGON group in comparison to the sham NITESGON group. (e) No significant difference in connectivity between the ventral tegmental area and hippocampus was observed when comparing the active and sham NITEGSON groups during or after stimulation. (f, g) A significant increase in spontaneous eye blink rate and salivary α-amylase (sAA) was observed after active NITESGON in comparison to sham NITESGON. (h) A significant increase in peak-to-peak amplitude over the left parietal electrode side was observed for the active group in comparison to the sham group for the deviant after stimulation. (i–l) A significant difference when subtracting pupil size, eyeblink rates, α-amylase, and the visual analogues scale for stress before NITESGON from immediately after NITESGON showed a significant increase for pupil size, eyeblink rates, α-amylase, but not for the VAS. (m,n, j) A positive correlation was observed between the difference (post–pre) in spontaneous eye blink rate and the difference in sAA as well as between the difference in spontaneous eye blink rate and the difference in peak-to-peak amplitude for the deviant. (o) No significant correlation was observed between the difference in spontaneous eye blink rate and the difference in peak-to-peak amplitude for the standard. (p) A positive correlation was observed between the difference in sAA and the difference in peak-to-peak amplitude for the deviant. (q) No correlation was observed between the difference in sAA and the difference in peak-to-peak amplitude for the standard. (r, s) A positive correlation was observed between the difference (post–pre) in spontaneous eye blink rate and the difference in pupil sizeas well as between the difference in sAA and the difference pupil size. Error bars, standard error of the mean (s.e.m.). Asterisks represent significant differences (*p < 0.05; **p < 0.01; ***p < 0.001). Δ the subtraction of rate/levels before NITESGON from sAA levels immediately after NITESGON.
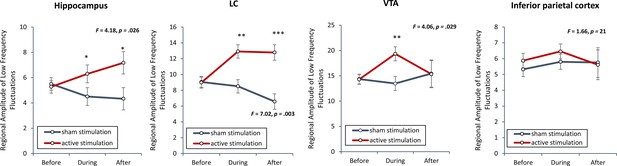
To verify if smoothing had an effect on the outcome, we recalculated the regional amplitude of low-frequency fluctuations without including a smoothing kernel was inspected to verify if non-invasive transcutaneous electrical stimulation of the greater occipital nerve (NITESGON) evoked activity changes in the locus coeruleus (LC), ventral tegmental area (VTA) and hippocampus.
Our findings replicated our previous findings (see Figure 6) and demonstrated a significant effect for the LC (F = 7.02, p = 0.003), VTA (F = 3.42, p = 0.047) and hippocampus (F = 4.18, p = 0.026) when comparing the active and control groups. For both the LC and hippocampus, a significant increase was obtained during and after (stimulation for the active group in comparison to the sham group). Before stimulation, no significant difference was obtained between the active and sham groups. For the VTA, a significant increase was obtained during stimulation for the active group in comparison to the sham group. Before or after stimulation, no significant difference was obtained between the active and sham groups. To further confirm our data, we included two control areas, the left inferior parietal cortex, where we do not expect to see any changes. Our analysis showed indeed no significant effect (F = 1.66, p = 0.21). Error bars, standard error of the mean (s.e.m.). Asterisks represent significant differences (*p < 0.05; **p < 0.01; ***p < 0.001).
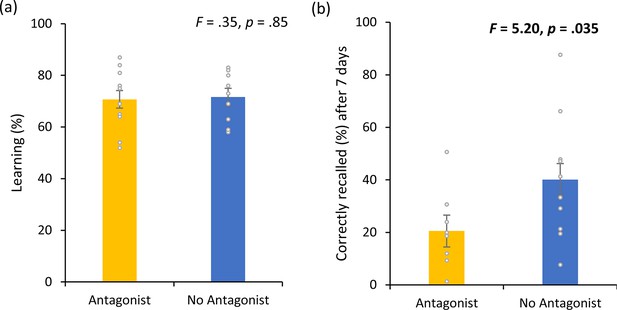
Dopamine experiment.
(a) No difference was observed in the cumulative learning rate after non-invasive transcutaneous electrical stimulation of the greater occipital nerve (NITESGON) for participants who were taking a dopamine (DA) antagonist in comparison to participants who were not taking a DA antagonist. (b) A significant difference was observed in the number of recalled words after 3 or 4 days for participants who were taking a DA antagonist in comparison to participants who were not taking a DA antagonist. Error bars, standard error of the mean (s.e.m.). Asterisks represent significant differences (*p < 0.05; **p < 0.01).