Inhibiting host-protein deposition on urinary catheters reduces associated urinary tract infections
Figures
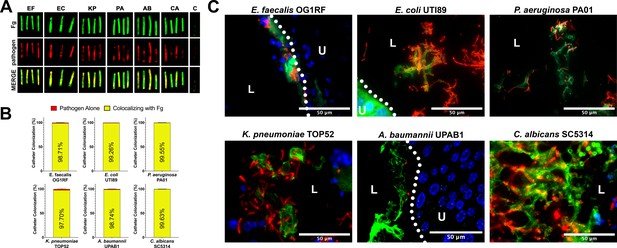
Uropathogens interact with fibrinogen (Fg) in vivo.
(A) Urinary catheters stained with immunofluorescence (IF) for Fg deposition (Fg; green) and microbe binding (respective pathogen; red). Unimplanted catheters were used as controls for autofluorescence, n = 3–4. (B) Quantification of uropathogen–Fg colocalization on catheters from panel A. (C) Representative images from a single bladder illustrating the interaction of uropathogens (red), Fg (green), and nuclei (blue) on the bladder urothelium (U) and in the lumen (L). Scale bar, 50 nm. Montages can be found in Figure 1—figure supplement 1. For all graphs error bars show the standard error of the mean (SEM). Between 3 and 5 replicates of n = 4–12 each were performed for each pathogen and condition.
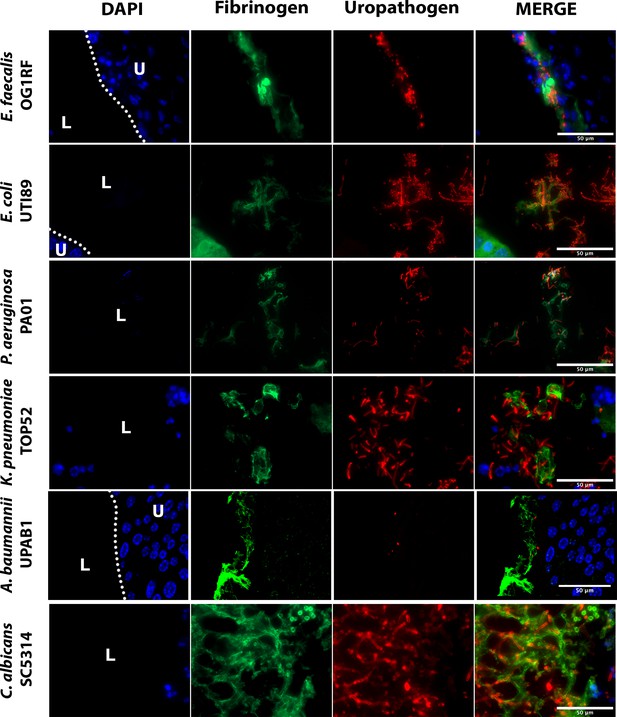
Montages of Figure 1 merged images.
Mice were implanted and infected with 1 × 106 CFU of the respective uropathogens. At 24 hpi, bladder tissues were harvested, fixed, and parafilm embedded. Bladder were subjected to immunofluorescence (IF) analysis, antibody staining was used to detect fibrinogen (anti-Fg; green), uropathogens (red), and DAPI (blue) for cell nuclei. Scale bar, 50 µm. Magnification ×100.
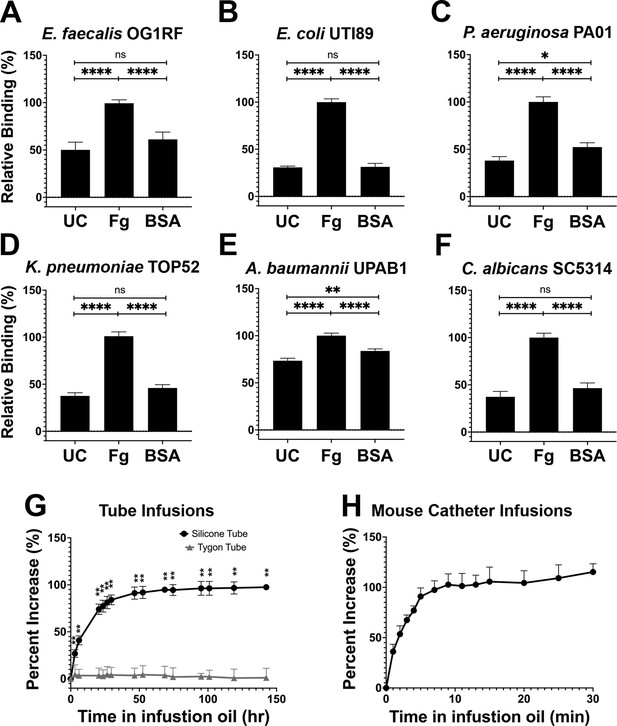
Silicone infusion and fibrinogen (Fg) enhancement of microbial surface binding.
(A–F) Uropathogens were tested for their ability to bind to protein coated and uncoated (UC) silicone catheters. For all graphs, error bars show the standard error of the mean (SEM). Between 3 and 5 replicates of n = 4–12 each were performed for each pathogen and condition. (G) Kinetics of silicone oil infusion on silicone and Tygon tubes, as well as (H) mouse silicone catheters. Differences between groups were tested for significance using the Mann-Whitney U test. *, P < 0.05; **, P < 0.005; and ****, P < 0.0001.
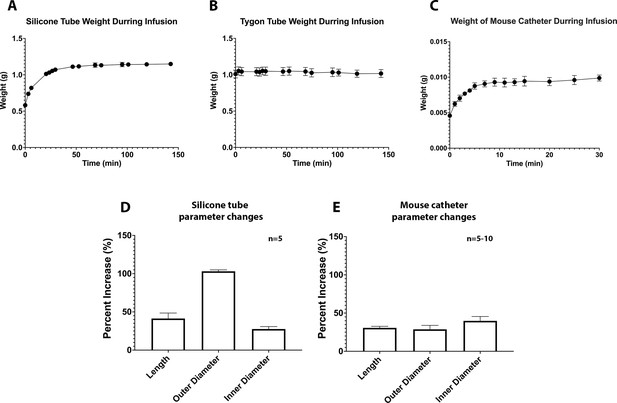
Characterization of LI tubing and mouse catheters.
(A) Weight of silicone tubes were measured in designated time points before and during silicone oil infusion, the mean ( ± standard error of the mean [SEM]) of n = 5 silicone tubes over infusion time was shown in this figure. (B) Weight of Tygon tubes were measured in designated time points before and during silicone oil infusion, the mean ( ± SEM) of n = 5 silicone tubes over infusion time was shown in this figure. (C) Weight of mouse catheters were measured in designated time points before and during silicone oil infusion, the mean ( ± SEM) of n = 5 mouse catheter over infusion time was shown in this figure. The length, outer diameter, and inner diameter of (D) silicone catheters (n = 5) or (E) mouse catheters (n = 5–10) were measured before and after infusion and the percentage change was calculated.
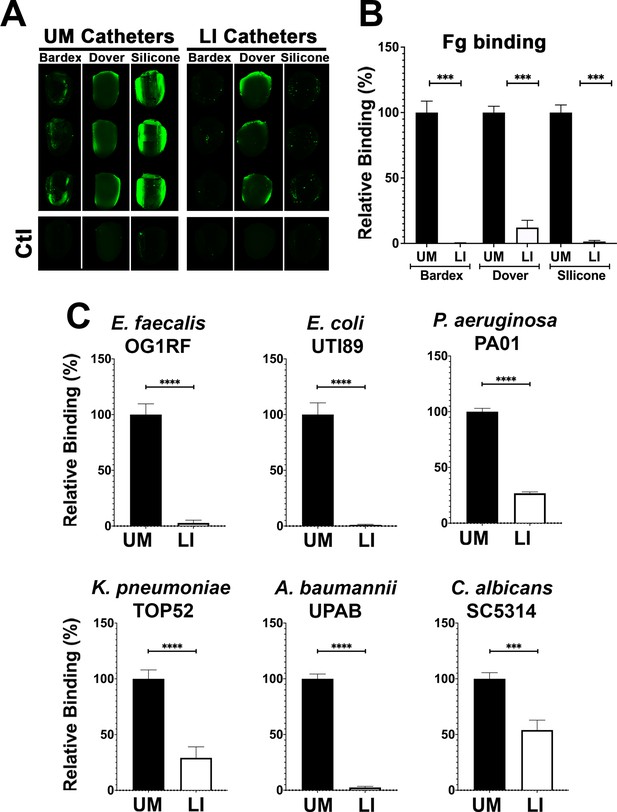
Liquid-infused silicone (LIS) modification reduces fibrinogen (Fg) deposition and microbial-binding in vitro.
(A) Visualization and (B) quantification of Fg (green) deposition on unmodified (UM)-catheter material (black bars) and LIS-catheter materials (white bars) by immunofluorescence (IF) staining. Three replicates with n = 2–3 each. (C) Microbial binding on UM and LIS. For all graphs, error bars show the standard error of the mean (SEM) and each condition had 3 replicates with n = 3 each. Differences between groups were tested for significance using the Mann-Whitney U test. ***, P < 0.0005; and ****, P < 0.0001*.
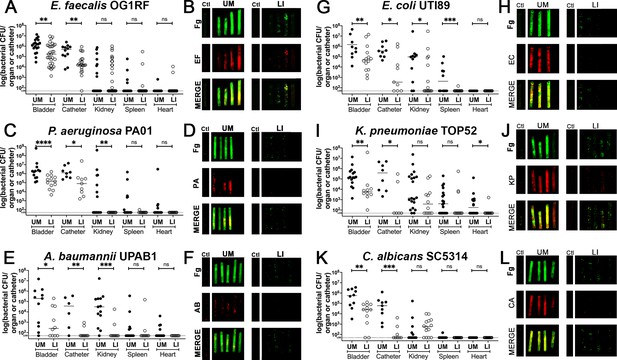
In vivo reduction of fibrinogen (Fg) deposition to restrict microbial burden.
Mice were catheterized and infected with one of six uropathogens. (A, C, E, G, I, K) Organ and catheter CFUs from mice with either an unmodified (UM)-catheter (closed circles) or liquid-infused silicone (LIS)-catheter (open circles) show the dissemination profile of the pathogen. (B, D, F, H,J,L) Imaging of catheters for Fg (green), respective uropathogen (red), and a merged image compare deposition on UM-catheters (left) with LIS-catheters (right); nonimplanted catheters as controls. Quantification of microbial colonization and colocalization on the catheters can be found in Figure 4—figure supplement 1. All animal studies for CFUs, catheter and bladder imaging had at least 10 animals per strain and catheter type. Differences between groups were tested for significance using the Mann-Whitney U test. *, P < 0.05; **, P < 0.005; ***, P < 0.0005; and ****, P < 0.0001.
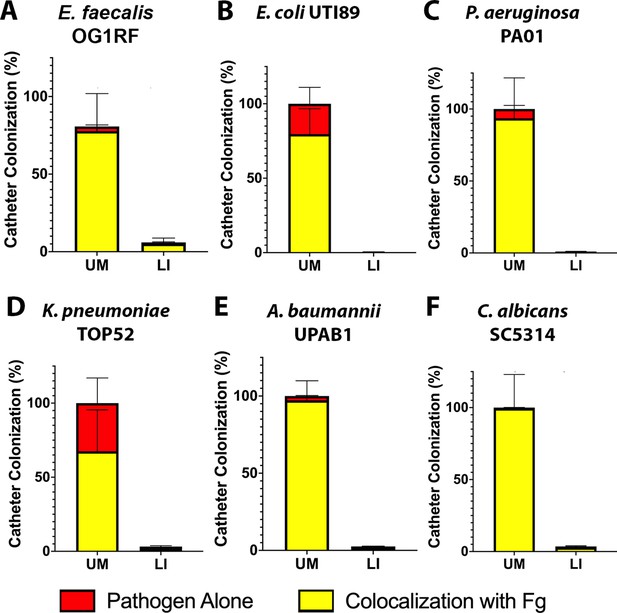
Pathogen predilection for fibrinogen (Fg).
Quantification of uropathogen–Fg colocalization on unmodified (UM)- and liquid-infused silicone (LIS)-catheters from mice catheterized and infected with (A) E. faecalis, (B) E. coli, (C) P. aeruginosa, (D) K. pneumoniae, (E) A. baumannii, and (F) C. albicans. Quantification was done using pixel color counter from Fiji where colocalization (yellow) of Fg (green) and pathogen (red) were quantified and compared to the total pathogen colonization of the catheter.
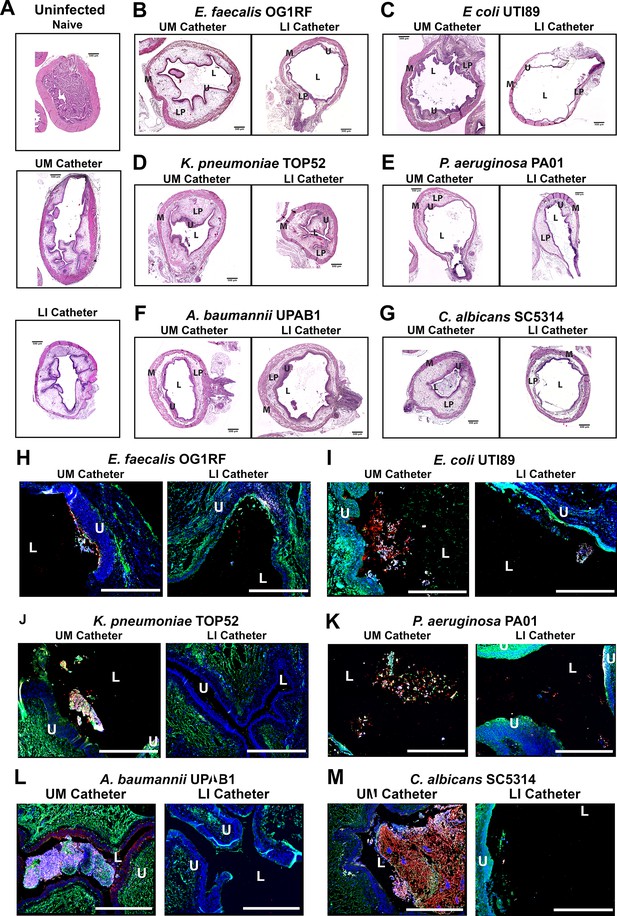
Liquid-infused silicone (LIS)-catheters reduce bladder colonization and inflammation.
Mice were catheterized and inoculated one of six strains. (A) Naive or bladders catheterized with an unmodified (UM)- or LIS-catheter were uninfected controls. (B–G) Bladder sections were stained with hematoxylin and eosin (H&E) to compare inflammation from UM-catheters (left) and LIS-catheters (right). (C–M) ×20 images of immunofluorescence (IF) stained bladders catheterized with an UM-catheter (left panels) or a LIS-catheter (right panels). Bladders stained for nuclei (blue), fibrinogen (Fg; green), respective uropathogens (red), and neutrophils (white). The urothelial/lumen boundaries are outlined in white dotted lines and labeled U (urothelium) and L (lumen) and all scale bars are 500 µm. Montages can be found in Figure 5—figure supplement 1.
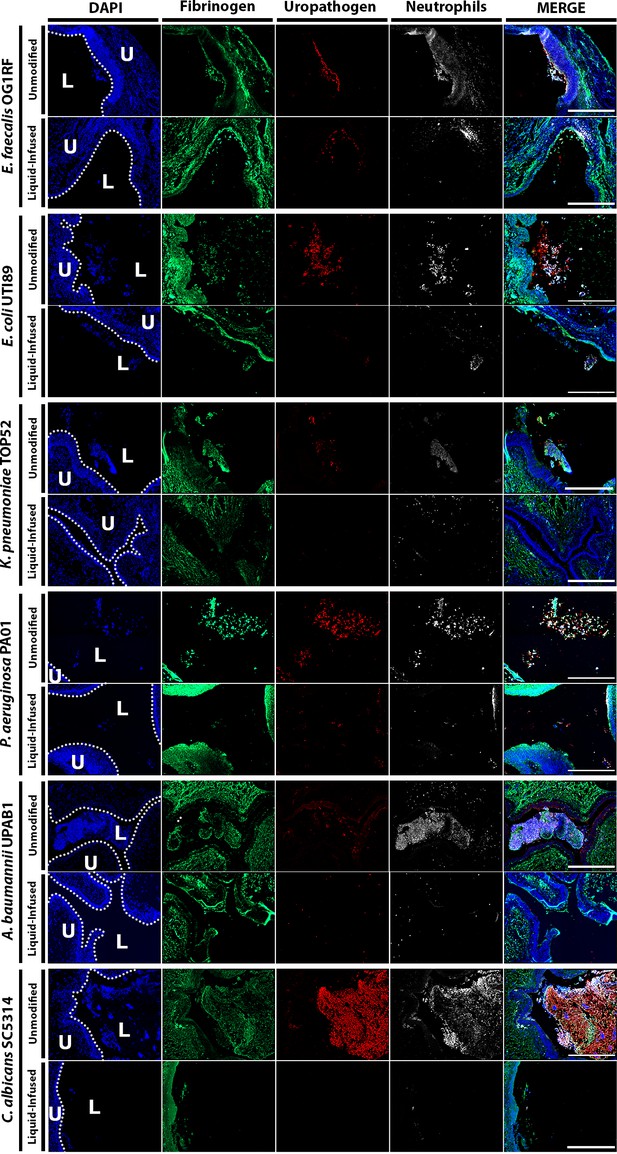
Montage of Figure 5 merged images.
Mice were implanted with either an unmodified catheter or a liquid-infused catheter and infected with 1 × 106 CFU of the respective uropathogens. At 24 hpi, bladders tissues were harvested, fixed, and parafilm embedded. Bladder were subjected to immunofluorescence (IF) analysis, antibody staining was used to detect Fg (anti-Fg; green), uropathogens (red), neutrophils (anti-Ly6G; white), and DAPI (4',6-diamidino-2-phenylindole) (blue) for cell nuclei. Scale bars, 500 µm. Images are stitched 2 × 2 tiles at ×20 magnification.
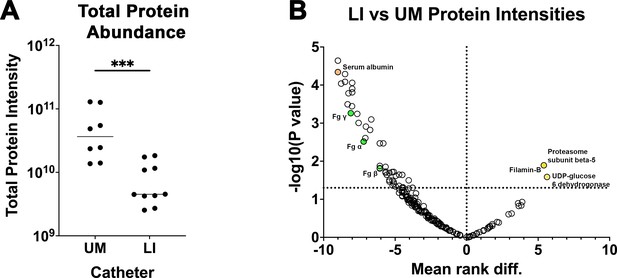
Liquid-infused silicone (LIS)-catheter reduces host-protein deposition in vivo.
A subset of unmodified (UM)- and LIS-catheters taken from mice 24 hpi with E. faecalis were assessed for protein deposition via mass spectrometry four UM-catheters and five LIS-catheters were used. (A) Intensities of the 95% most abundant proteins were summed in a total proteome approach and compared between the UM- and LIS-catheter groups. (B) A volcano plot for a subset of proteins. Negative mean rank difference indicates less protein on the LIS-catheter then on the UM-catheter and a significant difference is a −log10(p value) over 1.3. The fibrinogen (Fg) chains (α, β, and γ) are highlighted in green, serum albumin in orange, UDP-glucose 6-dehydrogenase, filamin-B, and proteasome subunit beta type-5 in yellow. Differences between groups were tested for significance using the Mann-Whitney U test. ***, P < 0.0005.
-
Figure 6—source data 1
Source data for small datasets.
Individual data from all figures involving small datasets displayed in individual tabs of this source file. This includes Figures 1B and 2A-F, Figure 3B, Figure 4, Figure 1—figure supplement 1 and Figure 2—figure supplement 1.
- https://cdn.elifesciences.org/articles/75798/elife-75798-fig6-data1-v2.zip
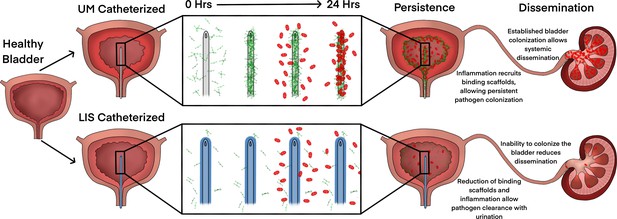
Liquid-infused silicone (LIS)-catheter reduces bladder inflammation, incidence of catheter-associated urinary tract infection (CAUTI), and dissemination.
Urinary catheter-induced inflammation promotes the release of fibrinogen (Fg) into the bladder to heal physical damage. Consequently, this Fg is deposited onto the catheter creating a scaffold for incoming pathogens to bind, establish infection, and promote systemic dissemination. However, catheterization with a LIS-catheter reduces Fg deposition onto its surface; thus, reducing the availability of a binding scaffolds for incoming pathogens. Consequently, overall bladder colonization and systemic dissemination are reduced making LIS-catheters a strong candidate for CAUTI prevention.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Enterococcus faecalis) | OG1RF | ATCC | 47,077 | |
| Strain, strain background (Escherichia coli) | UTI89 | Obtained from Dr, Scott Hultgren lab | ||
| Strain, strain background (Pseudomonas aeruginosa) | PA01 | ATCC | BAA-47 | |
| Strain, strain background (Klebsiella pneumoniae) | TOP52 1721 | Obtained from Dr. Scott Hultgren lab | ||
| Strain, strain background (Acetobacter baumannii) | UPAB1 + CUP1,2 | Obtained from Dr Mario Feldman lab Di Venanzio et al., 2019 | ||
| Strain, strain background (Candida albicans) | SC5314 | ATCC | MYA-2876 | |
| Biological sample (Homosapian female) | Urine | This study | IRB #19-04-5273 | |
| Antibody | (Goat polyclonal) antifibrinogen | Sigma-Aldrich | Cat# F8512, RRID:AB_259765 | 1:1000 |
| Antibody | (Rabbit polyclonal) anti-strep group d | From Dr. Scott Hultgren lab Flores-Mireles et al., 2014 | 1:1000 (in vitro)1:500 (IHC) | |
| Antibody | (Rabbit polyclonal) anti-E. coli serotype O/K | Invitrogen | Cat# PA1-25636 RRID:AB_780488 | 1:1000 (in vitro)1:500 (IHC) |
| Antibody | (Rabbit polyclonal) anti P. aeruginosa | Invitrogen | Cat# PA173117 RRID:AB_1018279 | 1:1000 (in vitro)1:500 (IHC) |
| Antibody | (Rabbit polyclonal) anti-K. pneumoniae polyclonal | Thermo Scientific | Cat# PA17226 RRID:AB_559816 | 1:1000 (in vitro)1:500 (IHC) |
| Antibody | (Rabbit polyclonal) anti A. baumannii | Di Venanzio et al., 2019 | 1:1000 (in vitro)1:500 (IHC) | |
| Antibody | (Rabbit polyclonal) anti C. albicans | Thermo Fisher Scientific | Cat# PA1-27158 RRID:AB_779500 | 1:1000 (in vitro)1:500 (IHC) |
| Antibody | (Rat polyclonal) anti-Ly6G | BioLegend | Cat# 127602 RRID:AB_1089180 | 1:500 |
| Antibody | (Donkey polyclonal) anti-goat 800CW | LI-COR Biosciences | Cat# 926-32214, RRID:AB_621846 | 1:5000 |
| Antibody | (Donkey polyclonal) anti-rabbit 680RD | LI-COR Biosciences | Cat# 926-68073, RRID:AB_10954442 | 1:5000 |
| Antibody | (Donkey polyclonal) anti-rat 680 | Thermo Fisher | Cat# A-21472, RRID:AB_2535875 | 1:500 |
| Antibody | (Donkey polyclonal) anti-goat 488 | Thermo Fisher | Cat# A11055 RRID:AB_2534102 | 1:500 |
| Antibody | (Donkey polyclonal) anti-rabbit 550 | Thermo Fisher | Cat# A31572 RRID:AB_162543 | 1:500 |
| Peptide, recombinant protein | Fibrinogen | Enzyme Research Laboratories | Cat# FIB 3 | Adjusted to 150 µg/ml in PBS |
| Software, algorithm | Zeiss pro Software | Carl Zeiss Microscopy | ||
| Software, algorithm | Image studio software | Licor Biosciences | ||
| Other | Silicone oil | Gelest | 63148-62-9 | 20 cst |
Additional files
-
Supplementary file 1
List of proteins found on LI and UM mouse catheters infected with E. faecalis OG1RF.
The average number of peptides for each protein found on 10 mouse catheters sorted by greatest abundance on the UM-catheter.
- https://cdn.elifesciences.org/articles/75798/elife-75798-supp1-v2.xlsx
-
Supplementary file 2
Microbe details.
List of microbial strains and their corresponding growth conditions, inoculum concentrations, and antibodies used in this study
- https://cdn.elifesciences.org/articles/75798/elife-75798-supp2-v2.xlsx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/75798/elife-75798-transrepform1-v2.docx
-
Source data 1
Proteomic data from UM- and LIS-catheters.
- https://cdn.elifesciences.org/articles/75798/elife-75798-data1-v2.xlsx





