Seipin transmembrane segments critically function in triglyceride nucleation and lipid droplet budding from the membrane
Figures
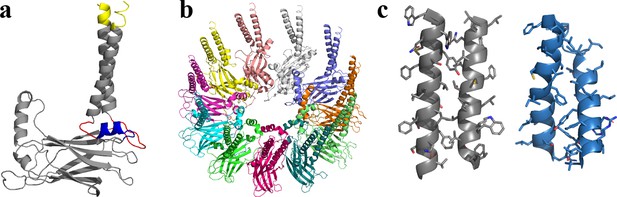
Structural model of human seipin.
(a) Structure of a seipin subunit. The structure included in the cryoelectron microscopy data is shown in gray. Red loops were modeled with Modeller (Fiser et al., 2000). The blue region was predicted using the yeast structure. The helical structures were extended (yellow). (b) Structure of the human seipin oligomer used in the simulations. Each chain is shown with different colors. (c) Comparison of transmembrane segments of human seipin used in this study (gray) and yeast seipin (blue; PDB 7OXP).
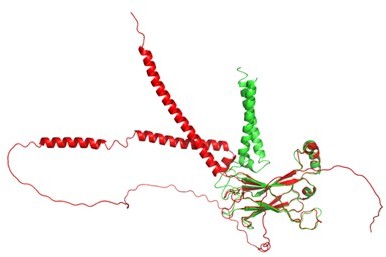
Cryoelectron microscopy of human seipin.
The cyan model includes the seipin luminal domain and partially resolved transmembrane segments. The electron density of Ser165 and Ser166 is shown with yellow. The unidentified density that interacts with Ser165 and Ser166 is shown with red.
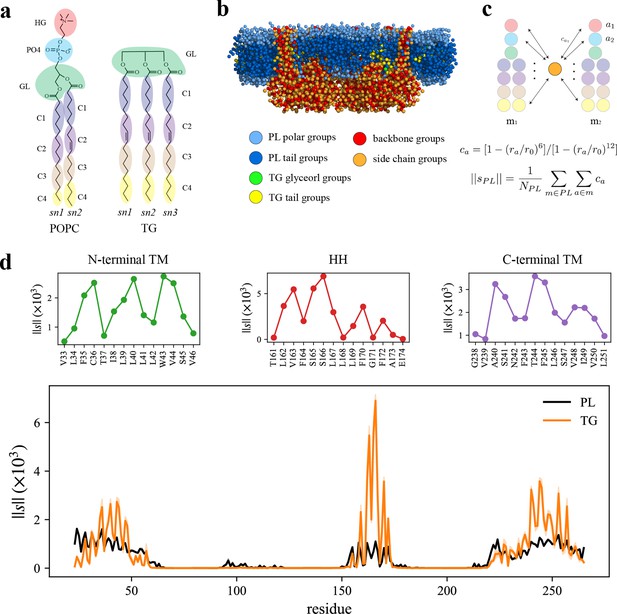
Seipin hydrophobic helix (HH) and transmembrane (TM) segments attract triacylglycerol (TG).
(a) Molecular groupings of lipids. Each protein residue was mapped onto one side chain and one backbone molecular group. (b) Initial structure of the system at the reduced resolution. The snapshot was clipped in the XZ plane. (c) Illustration of the calculation of the coordination number per molecule. A side chain group was depicted with orange circle and PL groups with other colors. (d) Interaction plot of protein residues with phospholipid (PL) (black) and TG (orange). The shaded area represents the standard error of the results of three equal-length blocks, each containing 1-µs all-atom (AA) molecular dynamics (MD) trajectory. The residues that had high interactions with TG in the N-terminal segment, HH, and C-terminal segment were shown in separate plots in the upper panel, colored with green, red, and purple lines, respectively.
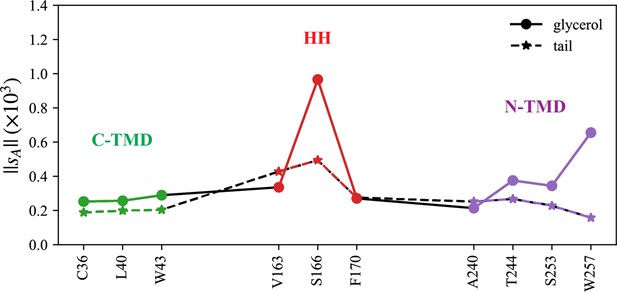
Normalized coordination number by atom.
The interactions with triacylglycerol (TG) glycerol moiety are shown with a continuous line and circle markers and those with TG tail atoms are shown with a dashed line and star markers.
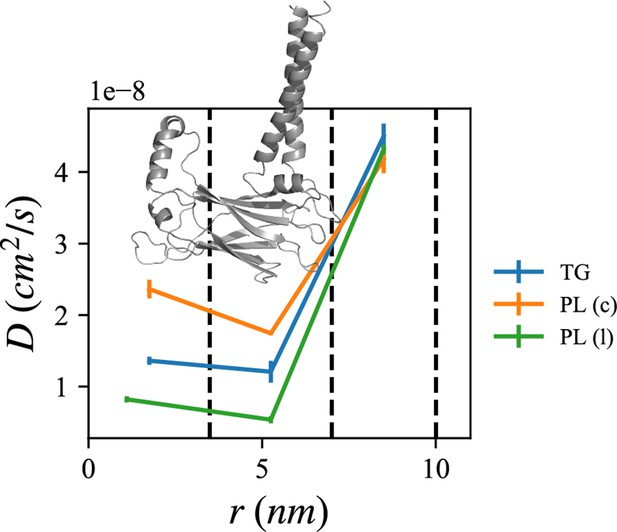
Lipid diffusion coefficients depend on location relative to the seipin oligomer.
The center of the mass of the lumenal domain of seipin is at origin. The first region (0–3.5 nm) contains the seipin hydrophobic helices and the second region (3.5–7.0 nm) the transmembrane (TM) segments. The third region (7.0–10.0 nm) is protein free. Diffusion coefficients of triacylglycerol (TG), cytosolic phospholipid (PL), and lumenal PL are shown with blue, orange, and green lines, respectively. The error bar represents the standard error of the results of three equal-length all-atom (AA) molecular dynamics (MD) blocks, each containing 1 µs.
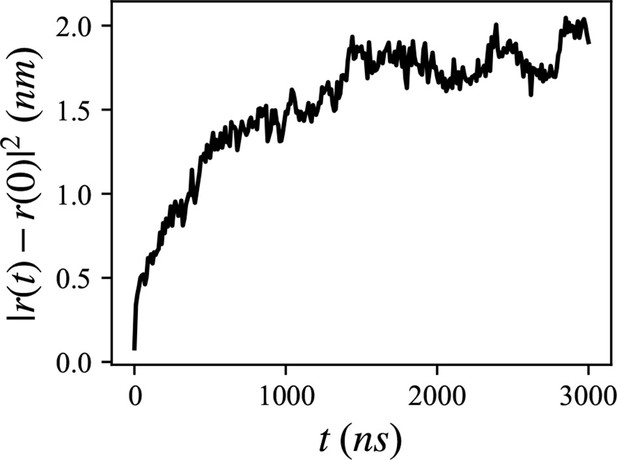
Mean squared distance of the 20 lumenal phospholipids (PLs), trapped inside the hydrophobic helix.
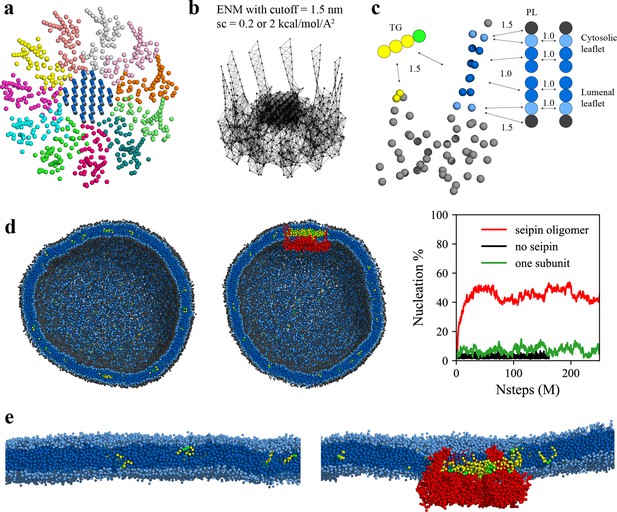
Seipin lowers the critical concentration of triacylglycerol (TG) nucleation.
(a) Coarse-grained (CG) model of human seipin oligomer. The CG atoms inside the hydrophobic helix ring represent phospholipid (PL) atoms (proteinized PLs). (b) Elastic network model (ENM) with a spring constant of 0.2 or 2 kcal/mol/Å2. (c) Scaling factors of attraction parameters between seipin–PL and seipin–TG interactions. PL head, interfacial, and tail groups are shown with black, light blue, and dark blue, respectively. TG glycerol and tail groups are shown with green and yellow, respectively. Seipin atoms that attract PL tails are shown with dark blue, and those that attract PL interfacial atoms are shown with sky blue. Two seipin atoms in the hydrophobic helix (HH), shown with yellow, and four central seipin atoms in each transmembrane (TM) segment, shown with dark blue, attract TG atoms. (d) CG molecular dynamics (MD) simulations of bilayers containing 2% TG with a diameter of 40 nm were carried out. The clipped snapshots of the last frames of the pure lipid (left) and seipin-containing systems (center) are shown. The ENM model with a spring constant of 0.2 kcal/mol/Å2 was used. PL head, interfacial, and tail atoms are shown with black, light blue, and dark blue, respectively. TG glycerol and tail atoms are shown with green and yellow, respectively. Seipin oligomer is indicated with red. The nucleation percentages of three systems with simulation steps are shown in right. (e) MARTINI CG model simulations of TG nucleation. TG does not nucleate in the bilayer without seipin (left), while it does in the bilayer with seipin, forming its distinct phase inside the seipin complex (right). Both bilayers have 2% TG, lower than the critical concentration.

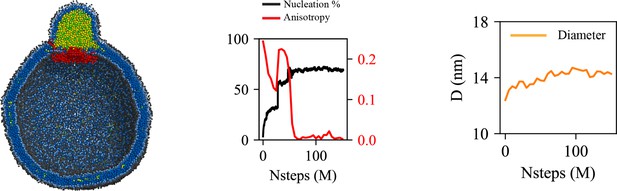
Cage-like geometry and neck formed by seipin transmembrane (TM) segments are key to modulating the morphology of a forming oil lens.
The first row shows the clipped snapshots of the equilibrated frames, and the second row shows the closeup view of the seipin. The third row shows the nucleation percentage (black) and anisotropy (red). ‘CG simulations of spherical bilayers containing 6% TG with a diameter of 40 nm were carried out’. The elastic network model (ENM) model for seipin with a spring constant of 0.2 kcal/mol/Å2 was used.
Coarse-grained (CG) simulation with a spring constant of 2 kcal/mol/Å2.
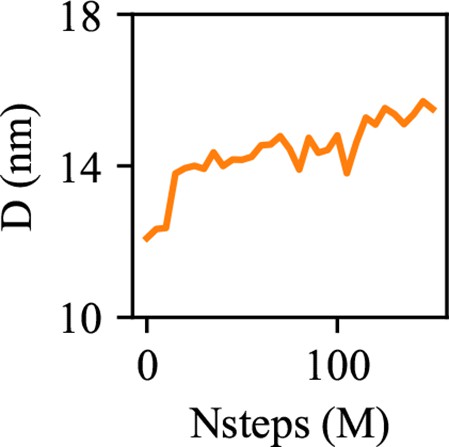
The bilayer contains 6% triacylglycerol (TG) with a diameter of 40 nm. The clipped snapshot is shown in left, the nucleation percentage (black) and anisotropy (red) are shown in middle, and the diameter of an oil lens (orange) is shown in right.
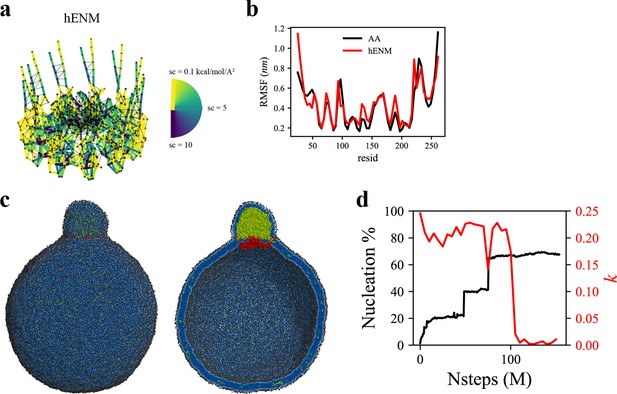
Coarse-grained (CG)-molecular dynamics (MD) shows lipid droplet (LD) growth in a large bilayer.
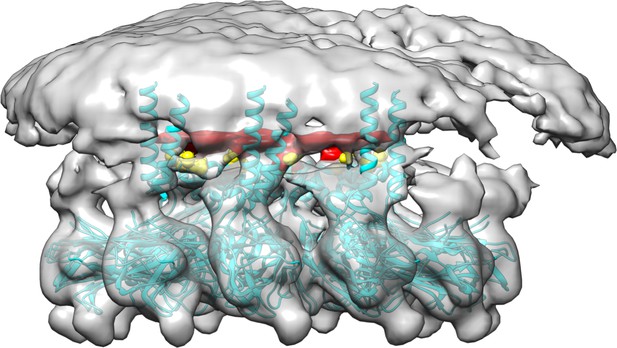
‘CG MD simulations of spherical bilayers containing 6% triacylglycerol (TG) with a diameter of 60 nm were carried out’. (a) Heterogeneous ENM (hENM) model of human seipin was constructed. Pairs of atoms in a subunit were connected with harmonic springs with their spring constants (sc) represented by their color. Additional harmonic springs (black lines) with a spring constant of 0.1 kcal/mol/Å2 were added between pairs of atoms that were not included in the hENM with a distance cutoff of 11 Å to ensure connections between subunits. (b) RMSF of a seipin subunit was compared between the all-atom (AA) trajectory and hENM in a bilayer. (c) Exterior and interior view of the last frame of the CG simulation. (d) The nucleation percentage (black line) and anisotropy (red line) are shown.
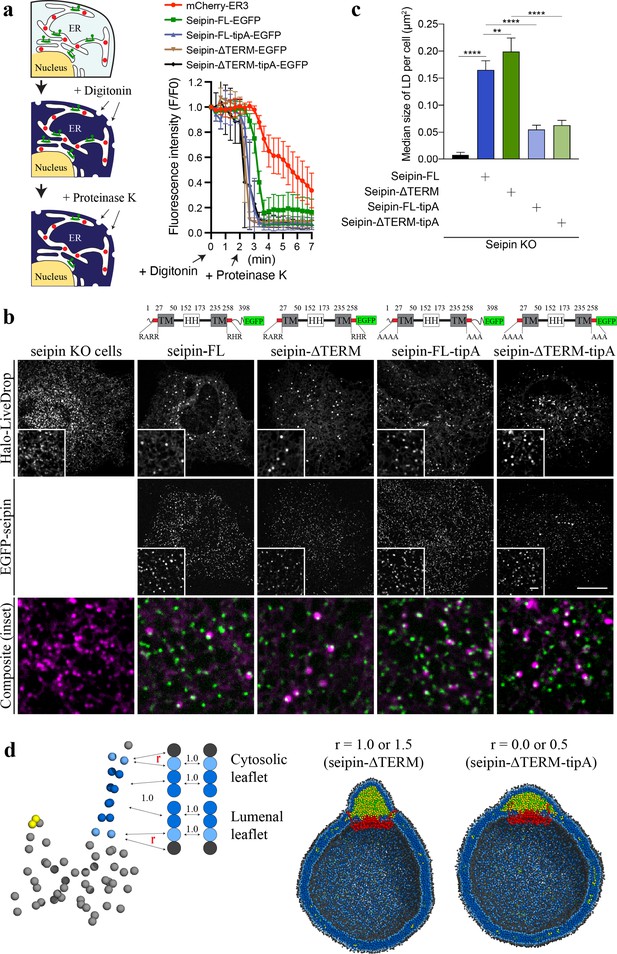
Nonconserved, cytosolic tails of human seipin are dispensable for the function, while the conserved, positively charged residues at the ends of seipin transmembrane (TM) segments are critical for lipid droplet (LD) maturation.
(a) Schematic representation of a fluorescence protease protection (FPP) assay (left). Low concentration of digitonin allows permeabilization of the plasma membrane without disrupting the endoplasmic reticulum (ER) membrane. Application of proteinase K selectively cleaves cytosolically exposed fluorescent protein (green) without affecting lumenally exposed fluorescent protein (red). Quantification of the fluorescence intensities of the whole time series of the FPP assay (right). (mean ± standard error of the mean [SEM]). (b) Confocal imaging of live seipin knockout (KO) SUM159 cells transiently transfected with various seipin constructs fused with EGFP and Halo-LiveDrop (stained with JF549). The cells were preincubated with 0.5 mM oleic acid for 1 hr prior to image acquisition. Scale bars, full-size, 15 μm; inserts, 2 μm. (c) Quantification of size of LDs per cell shown in (b) n = 4 cells. More than 300 LDs were analyzed in each sample. Median with interquartile range. ****p < 0.0001, **p < 0.01 were calculated by unpaired t-test. (d) CG molecular dynamics (MD) simulations with various attraction scaling factors (r). The spherical bilayer has a diameter of 40 nm and contains 6% mol TG. The elastic network model (ENM) model of human seipin with a spring constant of 0.2 kcal/mol/Å2 was used.
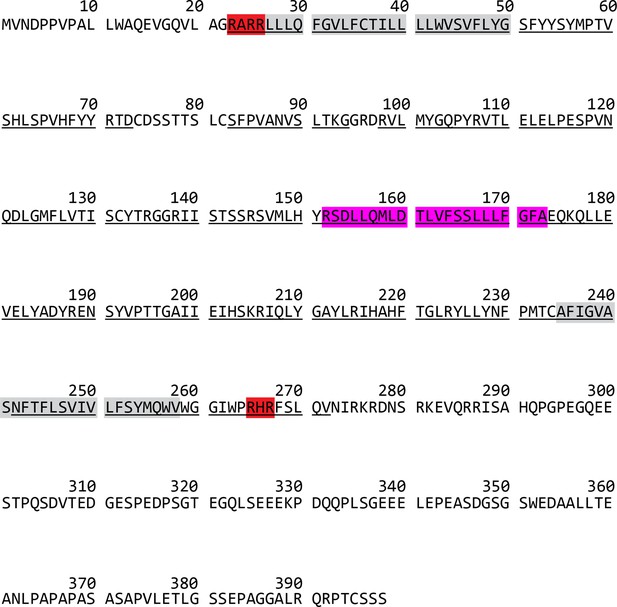
Sequence of human seipin.
The conserved residues were underlined. Residues highlighted with red are positively charged residues, located at the seipin transmembrane (TM) tips. Residues highlighted with gray or pink represent the TM segments or hydrophobic helix, respectively.
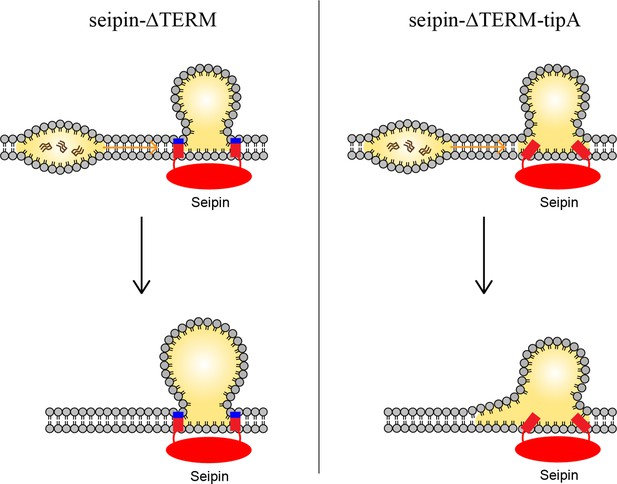
Model for the role of the charged residues at the end of seipin transmembrane (TM) segments during oil coalescence.
The left side shows the seipin TM segments maintain the bilayer thickness around the endoplasmic reticulum (ER)-lipid droplet (LD) neck structure. The right side shows the TM segments are immersed in the oil lens during oil coalescence because positively charged residues at the end of seipin TM segments (blue) are mutated to alanine.