Drosulfakinin signaling modulates female sexual receptivity in Drosophila
Figures
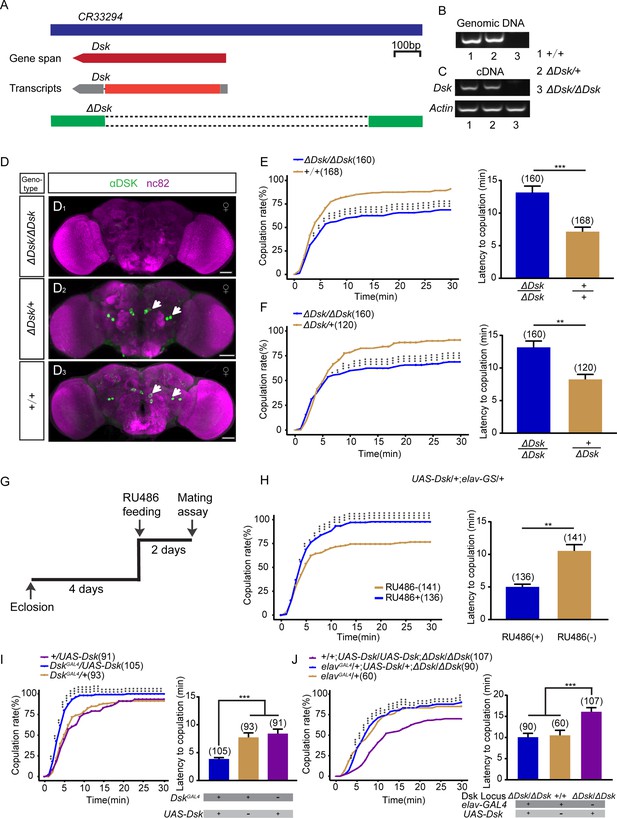
Drosulfakinin (Dsk) gene is important for female receptivity.
(A) Organization of Dsk gene and generation of ΔDsk. (B–C) Validation of ΔDsk. PCR analysis at the deletion locus on genomic DNA samples of ΔDsk/ΔDsk, +/ΔDsk, +/+. (B) RT-PCR analysis from cDNA samples of ΔDsk/ΔDsk, +/ΔDsk, +/+ (C). (D) Brain of indicated genotype, immunostained with anti-DSK antibody (green) and counterstained with nc82 (magenta). Arrows show cell bodies (green) stained with anti-DSK antibody. Scale bars, 50 μm. (E–F) Receptivity of virgin females within 30 min. Dsk mutant females reduced copulation rate and prolonged the latency to copulation compared with wild-type (E) and heterozygous females (F). (G) Schematic of experimental design. (H) Conditional overexpression of Dsk under the control of elav-GeneSwitch (elav-GS) significantly increased copulation rate and shortened the latency to copulation after feeding RU486 compared without feeding RU486. (I) Overexpression of Dsk in DSK neurons significantly increased copulation rate and shortened the latency to copulation compared with genetic controls. (J) Decreased female sexual behavior phenotypes of ΔDsk/ΔDsk were rescued by elavGAL4 driving UAS-Dsk. The number of female flies paired with wild-type males is displayed in parentheses. For the copulation rate, chi-square test is applied. For the latency to copulation, Mann-Whitney U test is applied in (E, F, and H), Kruskal-Wallis and post hoc Mann-Whitney U tests are applied in (I–J). Error bars indicate SEM. *p < 0.05, **p < 0.01, ***p < 0.001.
-
Figure 1—source data 1
Source data for Figure 1B–C, D–E and H–J.
- https://cdn.elifesciences.org/articles/76025/elife-76025-fig1-data1-v1.xlsx
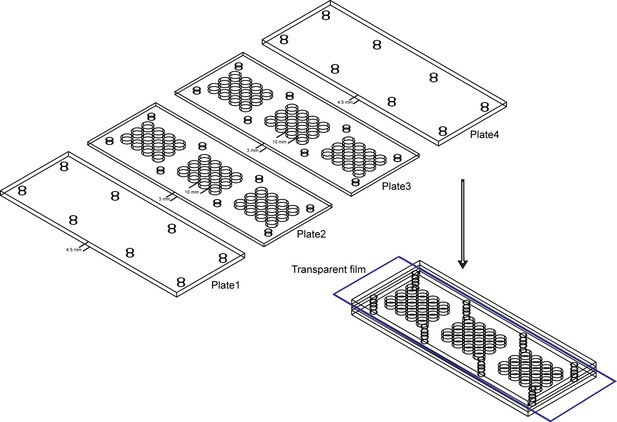
Behavior arena used in mating behavior assay.
The mating arena contains four acrylic plates. The top (Plate1) and bottom (Plate4) are made up of acrylic plates of a thickness of 4.5 mm. The middle two layers (Plate2 and Plate3) are made up of acrylic plates with 36 cylindrical arenas (diameter: 10 mm; height of each plates: 3 mm). A removable transparent film was placed between Plate2 and Plate3 to separate the two flies and the film was removed to allow the pair of a test female and a wild-type male to contact.
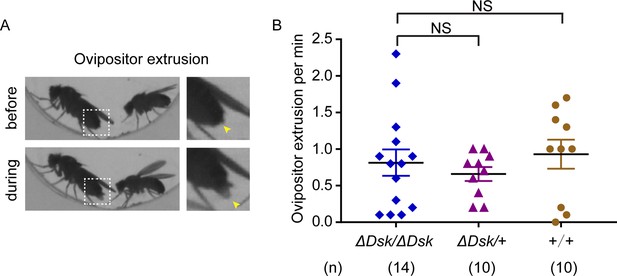
Mutation of Drosulfakinin (Dsk) in females did not alter female ovipositor extrusion.
(A) Video showed that ovipositor extrusion occurred during mating behavior. Magnification of white boxed region (left) is shown on the right and yellow arrowheads indicate ovipositor extrusion. (B) Ovipositor extrusion per minute observed in virgin females. Females for ΔDsk/ΔDsk, ΔDsk/+, +/+ were analyzed. Error bars indicate SEM. NS indicates no significant difference (Kruskal-Wallis and post hoc Mann-Whitney U tests).
-
Figure 1—figure supplement 2—source data 1
Source data for Figure 1—figure supplement 2B.
- https://cdn.elifesciences.org/articles/76025/elife-76025-fig1-figsupp2-data1-v1.xlsx
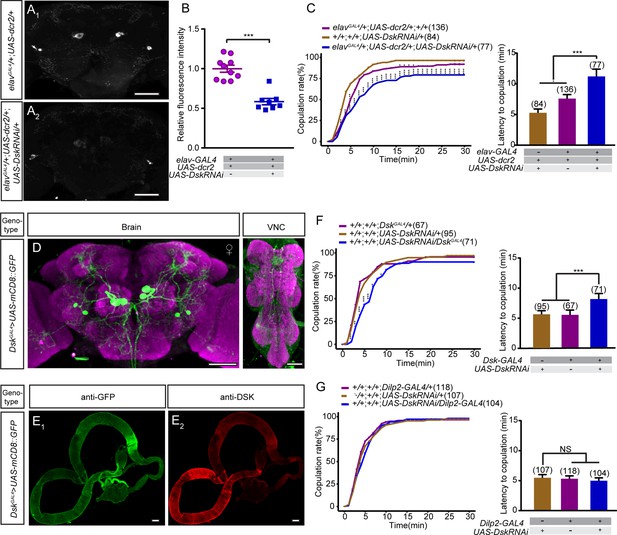
Effect of knocking down the expression of Drosulfakinin (Dsk) on female sexual behavior.
(A) Anti-DSK signals were significantly decreased in females with knocking down the expression of Dsk gene. Scale bars, 50 μm. (B) Quantification of anti-DSK signals in the brain. n = 10 and 8. Mann-Whitney U test is applied. (C) Knockdown of Dsk significantly decreased copulation rate and prolonged the latency to copulation compared with controls. UAS-DskRNAi was driven by elavGAL4;UAS-dcr2. (D) Expression pattern of the DskGAL4 in the brain and the ventral nerve cord. Scale bars, 50 μm. (E) No expression pattern was observed in the gut. Scale bars, 100 μm. (F) Knockdown of Dsk in DSK neurons significantly reduced the copulation rate and prolonged the latency to copulation compared with controls. UAS-DskRNAi was driven by DskGAL4. (G) Knocking down the expression of Dsk only in insulin-producing cells did not alter copulation rate and the latency to copulation compared with controls. UAS-DskRNAi was driven by Dilp2-GAL4. The number of female flies paired with wild-type males is displayed in parentheses. For the copulation rate, chi-square test is applied. For the latency to copulation, Kruskal-Wallis and post hoc Mann-Whitney U tests are applied. Error bars indicate SEM. *p < 0.05, **p < 0.01, ***p < 0.001.
-
Figure 1—figure supplement 3—source data 1
Source data for Figure 1—figure supplement 3B-C,F-G.
- https://cdn.elifesciences.org/articles/76025/elife-76025-fig1-figsupp3-data1-v1.xlsx
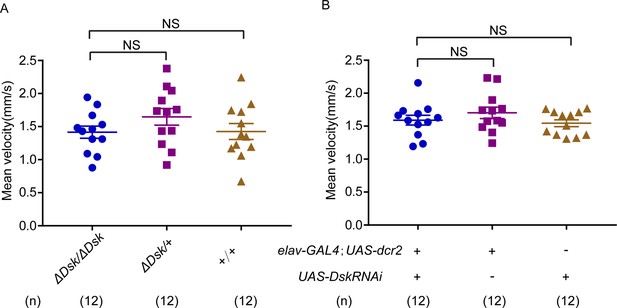
Locomotor behavior of Drosulfakinin (Dsk) mutant and Dsk RNA interference (RNAi) in females.
(A–B) Mean velocity had no significant change in Dsk mutant females (A) and Dsk RNAi females (B). Error bars indicate SEM. NS indicates no significant difference (Kruskal-Wallis and post hoc Mann-Whitney U tests).
-
Figure 1—figure supplement 4—source data 1
Source data for Figure 1—figure supplement 4.
- https://cdn.elifesciences.org/articles/76025/elife-76025-fig1-figsupp4-data1-v1.xlsx
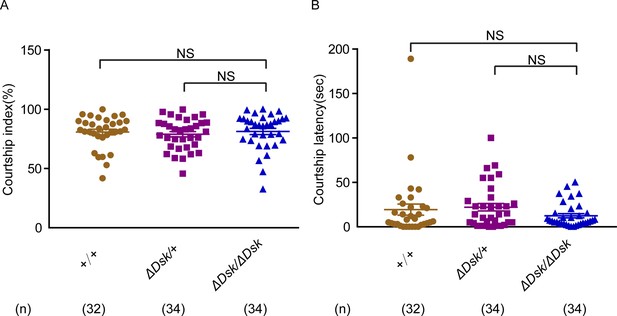
Courtship behavior of wild-type males paired with females of indicated genotypes.
(A–B) Courtship index (%) and courtship latency (s) in wild-type males paired with tested genotypes. Females for ΔDsk/ΔDsk, ΔDsk/+, +/+ were used. Error bars indicate SEM. NS indicates no significant difference (Kruskal-Wallis and post hoc Mann-Whitney U tests).
-
Figure 1—figure supplement 5—source data 1
Source data for Figure 1—figure supplement 5.
- https://cdn.elifesciences.org/articles/76025/elife-76025-fig1-figsupp5-data1-v1.xlsx
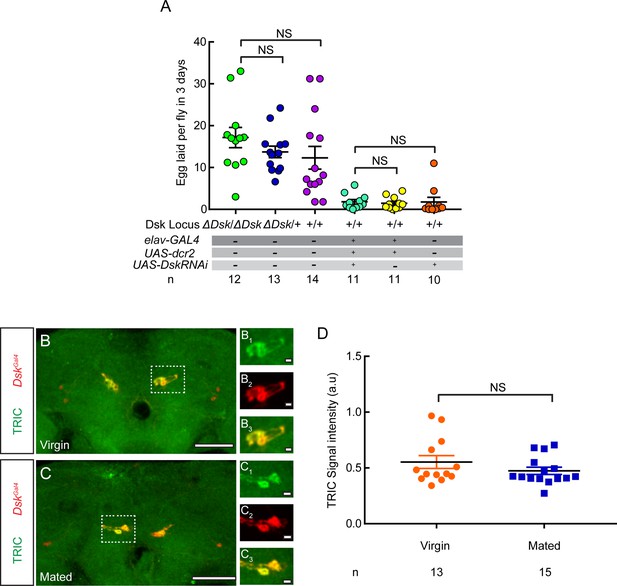
Drosulfakinin (Dsk) mutation or knockdown does not affect egg laying.
(A) The number of eggs laid by virgin females within 3 days after mutation or RNA interference (RNAi) of Dsk. (B and C) Ca2+ activity of DSK neurons in virgin and mated female brains was detected by using transcriptional reporter of intracellular Ca2+ (TRIC) method. Confocal images of TRIC labeling DSK neurons of virgin (B) and mated females (C). White boxes are shown in (B1–B3) and (C1–C3). Scale bars are 50 μm in (B and C), and 5 μm in (B1–B3) and (C1–C3). (D) The TRIC signal intensity from DSK neurons indicates intracellular Ca2+ activity in virgin and mated females. Genotype: UAS-IVS-mCD8::RFP,LexAop2-mCD8::GFP/+;nSyb-MKII::nlsLexADBD/+;DskGAL4/UAS-p65AD::CaM. For egg laying, Kruskal-Wallis and post hoc Mann-Whitney U tests are applied. For TRIC experiment, Mann-Whitney U test is applied. Error bars indicate SEM. NS indicates no significant difference.
-
Figure 1—figure supplement 6—source data 1
Source data for Figure 1—figure supplement 6A, D.
- https://cdn.elifesciences.org/articles/76025/elife-76025-fig1-figsupp6-data1-v1.xlsx
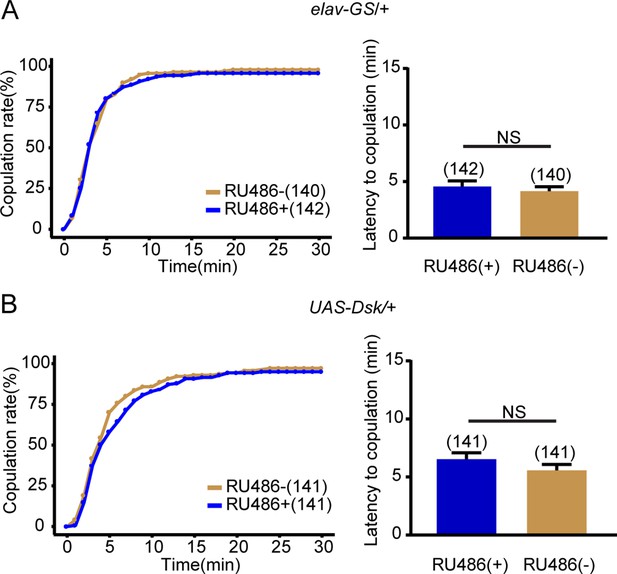
Female receptivity of control flies for Drosulfakinin (Dsk) overexpression.
(A–B) The controls with either elav-GeneSwitch (elav-GS) alone or UAS-Dsk alone showed comparable copulation rate and the latency to copulation with or without feeding RU486. The number of female flies paired with wild-type males is displayed in parentheses. For the copulation rate, chi-square test is applied. For the latency to copulation, Mann-Whitney U test is applied. Error bars indicate SEM. NS indicates no significant difference.
-
Figure 1—figure supplement 7—source data 1
Source data for Figure 1—figure supplement 7.
- https://cdn.elifesciences.org/articles/76025/elife-76025-fig1-figsupp7-data1-v1.xlsx
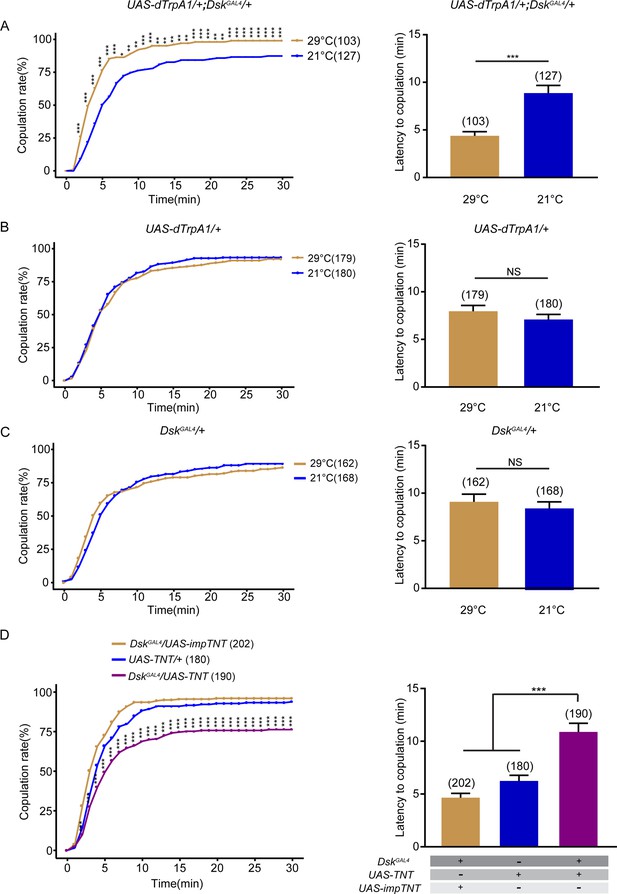
Drosulfakinin (DSK) neurons promote female receptivity.
(A) Activation of DSK neurons significantly increased copulation rate and shortened the latency to copulation at 29°C relative to 21°C. DskGAL4 driving UAS-dTrpA1 activated DSK neurons at 29°C. (B–C) The controls with either UAS-dTrpA1 alone or DskGAL4 alone did not alter the copulation rate and the latency to copulation at 29°C relative to 21°C. (D) Inactivation of DSK neurons significantly decreased copulation rate and prolonged the latency to copulation compared with controls. DskGAL4 driving UAS-TNT inactivated DSK neurons. The number of female flies paired with wild-type males is displayed in parentheses. For the copulation rate, chi-square test is applied. For the latency to copulation, Mann-Whitney U test is applied in (A–C), Kruskal-Wallis and post hoc Mann-Whitney U tests are applied in (D). Error bars indicate SEM. *p < 0.05, **p < 0.01, ***p < 0.001, NS indicates no significant difference.
-
Figure 2—source data 1
Source for Figure 2.
- https://cdn.elifesciences.org/articles/76025/elife-76025-fig2-data1-v1.xlsx
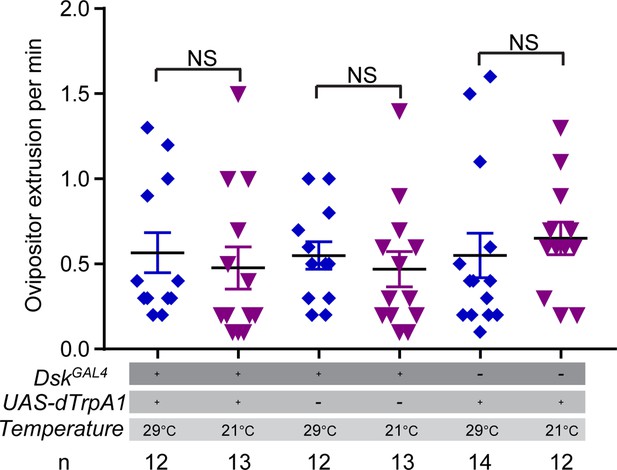
Activating Drosulfakinin (DSK) neurons in females did not affect female ovipositor extrusion.
Ovipositor extrusion per minute observed in virgin females when activating DSK neurons. Error bars indicate SEM. NS indicates no significant difference (Kruskal-Wallis and post hoc Mann-Whitney U tests).
-
Figure 2—figure supplement 1—source data 1
Source data for Figure 2—figure supplement 1.
- https://cdn.elifesciences.org/articles/76025/elife-76025-fig2-figsupp1-data1-v1.xlsx
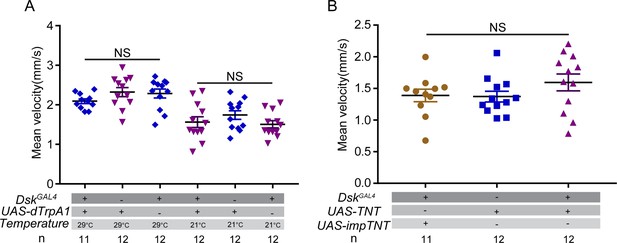
Locomotor behavior of females did not change after manipulating activity of Drosulfakinin (DSK) neurons.
(A) Mean velocity of females after activating DSK neurons. (B) Mean velocity of females after inactivating DSK neurons. Activating or inactivating DSK neurons did not change the locomotor behavior. Error bars indicate SEM. NS indicates no significant difference (Kruskal-Wallis and post hoc Mann-Whitney U tests).
-
Figure 2—figure supplement 2—source data 1
Source data for Figure 2—figure supplement 2.
- https://cdn.elifesciences.org/articles/76025/elife-76025-fig2-figsupp2-data1-v1.xlsx
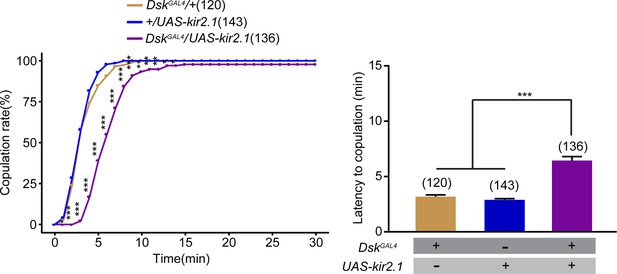
Inactivation of Drosulfakinin (DSK) neurons reduced female receptivity.
(A) Inactivation of DSK neurons significantly decreased copulation rate and prolonged the latency to copulation compared with controls. DskGAL4 driving UAS-kir2.1 inactivates DSK neurons. The number of female flies paired with wild-type males is displayed in parentheses. For the copulation rate, chi-square test is applied. For the latency to copulation, Kruskal-Wallis and post hoc Mann-Whitney U tests are applied. Error bars indicate SEM. *p < 0.05, **p < 0.01, ***p < 0.001, NS indicates no significant difference.
-
Figure 2—figure supplement 3—source data 1
Source data for Figure 2—figure supplement 3.
- https://cdn.elifesciences.org/articles/76025/elife-76025-fig2-figsupp3-data1-v1.xlsx
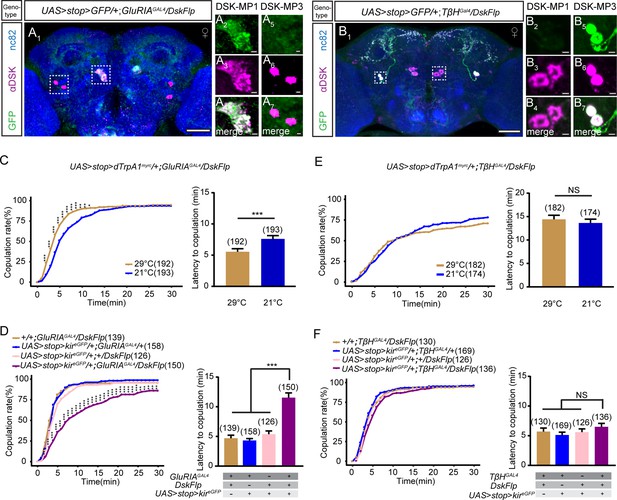
DSK-MP1 neurons play a critical role in female receptivity.
(A) Intersectional expression of Drosulfakinin (Dsk) neurons and glutamate receptor IA (GluRIA) neurons were detected by immunostaining with anti-GFP (green) and anti-DSK (magenta) antibodies in female brain and were counterstained with anti-nc82 (blue). Magnification of white boxed region in (A) is shown in (A2–A7). Genotype: UAS > stop > myr::GFP/+;GluRIAGAL4/DskFlp. (B) Intersectional expression of Dsk neurons and TβH neurons were detected by immunostaining with anti-GFP (green) and anti-DSK (magenta) antibodies in female brain and were counterstained with anti-nc82 (blue). Magnification of white boxed region in (B) is shown in (B2–B7). Genotype: UAS > stop > myr::GFP/+;TβHGAL4/DskFlp. Scale bars are 50 μm in (A1 and B1), 5 μm in (A2–A7) and (B2–B7). (C) Activation of co-expression neurons of Dsk and GluRIA significantly increased copulation rate and shortened the latency to copulation at 29°C relative to 21°C. Genotype: UAS > stop > dTrpAmyrc/+;GluRIAGAL4/DskFlp. (D) Inactivation of co-expression neurons of Dsk and GluRIA significantly decreased the copulation rate and prolonged the latency to copulation compared with controls. Genotype: UAS > stop > kireGFP/+;GluRIAGAL4/DskFlp, +/+;GluRIAGAL4/DskFlp, UAS > stop > kireGFP/+;GluRIAGAL4/+, UAS > stop > kireGFP/+;+/DskFlp. (E) Activation of co-expression neurons of Dsk and TβH did not alter the copulation rate and copulation latency at 29°C relative to 21°C. Genotype: UAS > stop > dTrpAmyrc/+;TβHGAL4/DskFlp. (F) Inactivation of co-expression neurons of Dsk and TβH did not alter the copulation rate and copulation latency compared with controls. Genotype: UAS > stop > kireGFP/+;TβHGAL4/DskFlp, UAS > stop > kireGFP/+;+/DskFlp, UAS > stop > kireGFP/+;TβHGAL4/+, +/+;TβHGAL4/DskFlp. The number of female flies paired with wild-type males is displayed in parentheses. For the copulation rate, chi-square test is applied. For the latency to copulation, Mann-Whitney U test is applied in (C and E), Kruskal-Wallis and post hoc Mann-Whitney U tests are applied in (D and F). Error bars indicate SEM. *p < 0.05, **p < 0.01, ***p < 0.001, NS indicates no significant difference.
-
Figure 3—source data 1
Source for Figure 3.
- https://cdn.elifesciences.org/articles/76025/elife-76025-fig3-data1-v1.xlsx
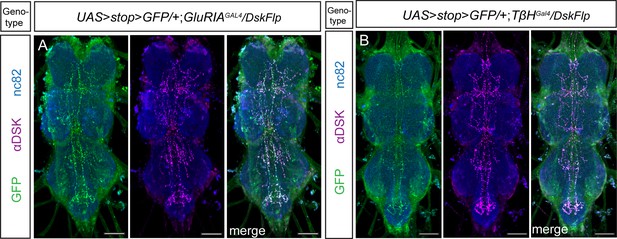
Expression pattern of Drosulfakinin (DSK) neurons targeted with genetic intersections in the ventral nerve cord.
(A) The intersectional expression between GluRIAGal4 and DskFlp in the ventral nerve cord. (B) The intersectional expression between TβHGal4 and DskFlp in the ventral nerve cord. Scale bars, 50 μm.
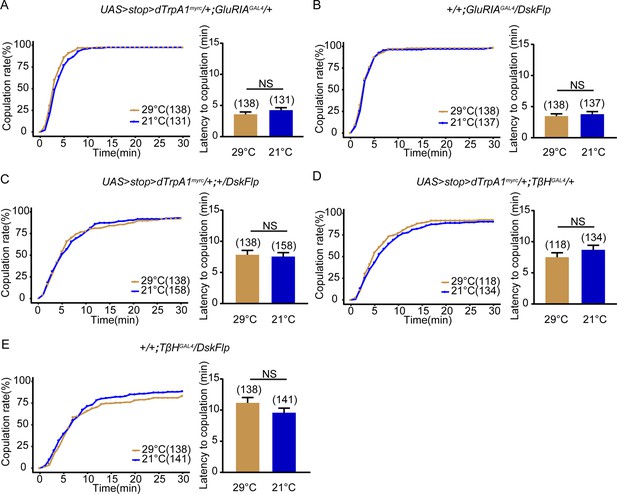
Female receptivity of control females for activation of specific Drosulfakinin (DSK) neurons.
(A–C) The controls for activation of DSK-MP1 neurons did not alter the copulation rate and the latency to copulation at 29°C relative to 21°C in the absence of DskFlp (A), UAS > stop > dTrpAmyrc (B) and GluRIAGAL4 (C). (D–E) The controls for activation of DSK-MP3 neurons also did not alter the copulation rate and the latency to copulation at 29°C relative to 21°C in the absence of TβHGAL4 (C), DskFlp (D), UAS > stop > dTrpAmyrc (E). The number of female flies paired with wild-type males is displayed in parentheses. For the copulation rate, chi-square test is applied. For the latency to copulation, Mann-Whitney U test is applied. Error bars indicate SEM. *p < 0.05, **p < 0.01, ***p < 0.001, NS indicates no significant difference.
-
Figure 3—figure supplement 2—source data 1
Source for Figure 3—figure supplement 2.
- https://cdn.elifesciences.org/articles/76025/elife-76025-fig3-figsupp2-data1-v1.xlsx
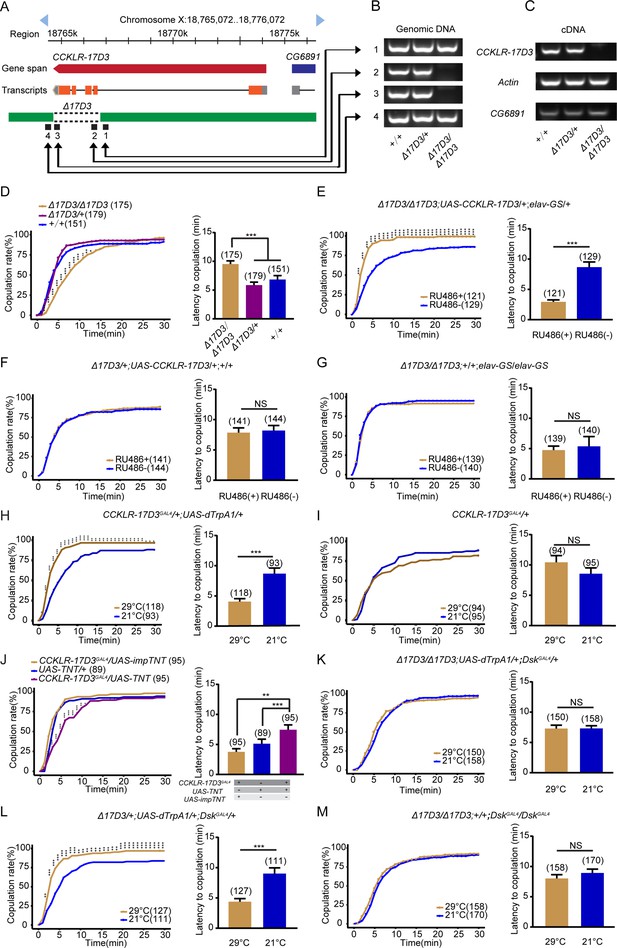
Drosulfakinin (Dsk) regulates female receptivity via CCKLR-17D3 receptor.
(A) Organization of CCKLR-17D3 and generation of Δ17D3. (B–C) Validation of Δ17D3. PCR analysis from genomic DNA samples of Δ17D3/Δ17D3, +/Δ17D3, +/+ (B). RT-PCR analysis from cDNA samples of Δ17D3/Δ17D3, +/Δ17D3, +/+ (C). (D) CCKLR-17D3 mutant females significantly decreased copulation rate and prolonged the latency to copulation compared with wild-type and heterozygous. (E) Conditional expression of UAS-CCKLR-17D3 in the Δ17D3 mutant background after feeding RU486 significantly increased copulation rate and shortened the latency to copulation compared without feeding RU486. (F–G) The controls with either UAS-CCKLR-17D3 alone or elav-GeneSwitch (elav-GS) alone did not rescue the phenotypes of Δ17D3/Δ17D3 at feeding RU486 relative to without feeding RU486. (H) Activating CCKLR-17D3 neurons significantly increased copulation rate and shortened the latency to copulation at 29°C relative to 21°C. CCKLR-17D3GAL4 driving UAS-dTrpA1 activated CCKLR-17D3 neurons at 29°C. (I) The control with CCKLR-17D3GAL4 alone did not alter the copulation rate and the latency to copulation at 29°C relative to 21°C. (J) Inactivation of CCKLR-17D3 neurons significantly decreased copulation rate and prolonged the latency to copulation compared with controls. DskGAL4 driving UAS-TNT inactivated DSK neurons. (K) The copulation rate and the latency to copulation have no difference at 29°C relative to 21°C in the case of activating DSK neurons in the Δ17D3 mutant background. (L) The positive control significantly increased copulation rate and shortened the latency to copulation at 29°C relative to 21°C. (M) The negative control did not alter the copulation rate and the latency to copulation by heating. The number of female flies paired with wild-type males is displayed in parentheses. For the copulation rate, chi-square test is applied. For the latency to copulation, Kruskal-Wallis and post hoc Mann-Whitney U tests are applied in (D and J), Mann-Whitney U test is applied in (E–I and K–M). Error bars indicate SEM. *p < 0.05, **p < 0.01, ***p < 0.001, NS indicates no significant difference.
-
Figure 4—source data 1
Source data for Figure 4.
- https://cdn.elifesciences.org/articles/76025/elife-76025-fig4-data1-v1.xlsx
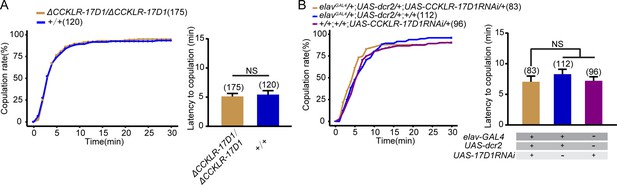
CCKLR-17D1 mutant or knockdown does not affect female receptivity.
(A) CCKLR-17D1 mutant females did not alter the copulation rate and the latency to copulation compared with wild-type females. (B) Knocking down CCKLR-17D1 did not change the copulation rate and the latency to copulation compared with controls. CCKLR-17D1RNAi was driven by elavGal4;UAS-dcr2. The number of female flies paired with wild-type males is displayed in parentheses. For the copulation rate, chi-square test is applied. For the latency to copulation, Mann-Whitney U test is applied in (A), Kruskal-Wallis and post hoc Mann-Whitney U tests are applied in (B). Error bars indicate SEM. NS indicates no significant difference.
-
Figure 4—figure supplement 1—source data 1
Source data for Figure 4—figure supplement 1.
- https://cdn.elifesciences.org/articles/76025/elife-76025-fig4-figsupp1-data1-v1.xlsx
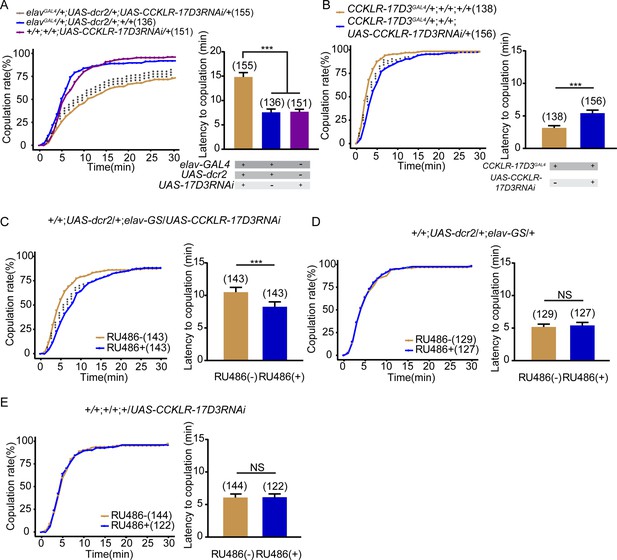
CCKLR-17D3 knockdown reduces female receptivity.
(A–B) Knockdown of CCKLR-17D3 significantly reduced copulation rate and prolonged the latency to copulation compared with controls. CCKLR-17D3RNAi was driven by elavGal4;UAS-dcr2 (A), UAS-CCKLR-17D3RNAi was driven by CCKLR-17D3GAL4 (B). (C) Conditional knockdown of CCKLR-17D3 after feeding RU486 significantly decreased copulation rate and prolonged the latency to copulation compared to females without feeding RU486. UAS-CCKLR-17D3RNAi was driven by UAS-dcr2;elav-GS. (D–E) The controls with either UAS-dcr2;elav-GS alone or UAS-CCKLR-17D3RNAi alone showed comparable copulation rate and the latency to copulation with or without feeding RU486. The number of female flies paired with wild-type males is displayed in parentheses. For the copulation rate, chi-square test is applied. For the latency to copulation, Kruskal-Wallis and post hoc Mann-Whitney U tests are applied in (A), Mann-Whitney U test is applied in (B–E). Error bars indicate SEM. *p < 0.05, **p < 0.01, ***p < 0.001, NS indicates no significant difference.
-
Figure 4—figure supplement 2—source data 1
Source data for Figure 4—figure supplement 2.
- https://cdn.elifesciences.org/articles/76025/elife-76025-fig4-figsupp2-data1-v1.xlsx
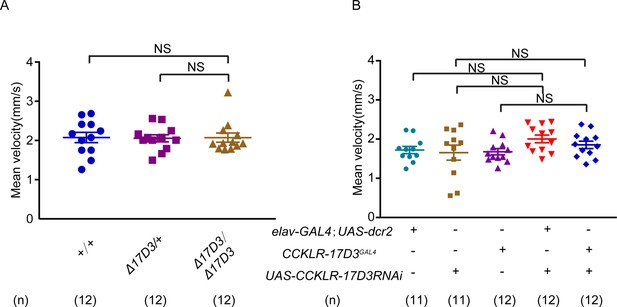
Locomotor behavior of CCKLR-17D3 mutant and knockdown females.
(A–B) Mean velocity had no significant change in CCKLR-17D3 mutant females (A) and CCKLR-17D3 RNA interference (RNAi) females (B). Error bars indicate SEM. NS indicates no significant difference (Kruskal-Wallis and post hoc Mann-Whitney U tests).
-
Figure 4—figure supplement 3—source data 1
Source data for Figure 4—figure supplement 3.
- https://cdn.elifesciences.org/articles/76025/elife-76025-fig4-figsupp3-data1-v1.xlsx
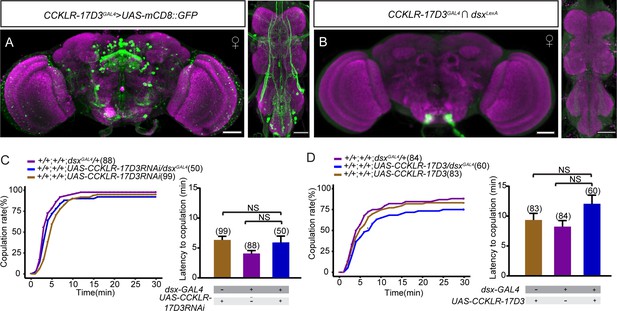
CCKLR-17D3 neurons do not overlap with doublesex (dsx) neurons.
(A) The expression pattern of CCKLR-17D3GAL4 in the brain and the ventral nerve cord. (B) Intersectional expression between CCKLR-17D3 neurons and dsx neurons. Scale bars, 50 μm. (C–D) Knocking down (C) or overexpressing (D) CCKLR-17D3 in all dsx neurons does not affect female receptivity. For the copulation rate, chi-square test is applied. For the latency to copulation, Kruskal-Wallis and post hoc Mann-Whitney U tests are applied. Error bars indicate SEM. NS indicates no significant difference.
-
Figure 4—figure supplement 4—source data 1
Source data for Figure 4—figure supplement 4C-D.
- https://cdn.elifesciences.org/articles/76025/elife-76025-fig4-figsupp4-data1-v1.xlsx
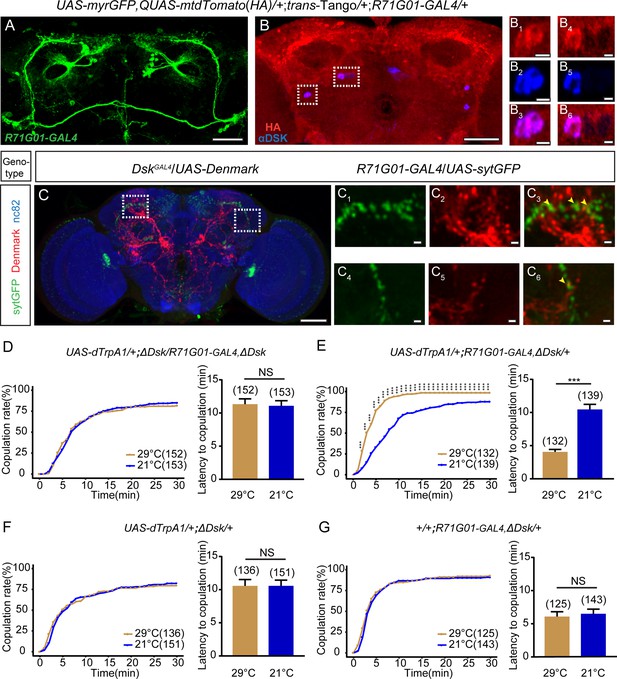
Drosulfakinin (DSK) neurons are functional targets of R71G01-GAL4 neurons in regulating mating behavior.
(A–B) Transsynaptic circuit analysis using trans-Tango confirms that Dsk-expressing neurons are postsynaptic neurons of R71G01-GAL4 neurons. In the central brain, expression of the Tango ligand in R71G01-GAL4 neurons (green) (A) induced postsynaptic mtdTomato signals (anti-HA, red) (B). Cell bodies of Dsk were stained with anti-DSK (blue) (B). Magnification of white boxed region in (B) is shown in (B1–B3) and (B4–B6). Scale bars are 50 μm in (A–B), 5 μm in (B1–B3) and (B4–B6). (C) Axons of R71G01-GAL4 neurons overlapped with dendrites of DSK neurons by anatomical registration. Magnification of white boxed region in (C) is shown in (C1–C3) and (C4–C6). Yellow arrowheads indicated the region of overlaps between R71G01-GAL4 neurons axons with DSK neurons dendrites. R71G01-GAL4-driven UAS-sytGFP expression (green), DskGAL4-driven UAS-Denmark expression (red). Scale bars are 50 μm in (C), 5 μm in (C1–C3) and (C4–C6). (D) The copulation rate and the latency to copulation had no difference at 29°C relative to 21°C in the case of activation of R71G01-GAL4 neurons in the ΔDsk mutant background. (E) The positive control significantly increased copulation rate and shortened the latency to copulation at 29°C relative to 21°C. (F–G) The negative controls did not alter the copulation rate and the latency to copulation by heating. The number of female flies paired with wild-type males is displayed in parentheses. For the copulation rate, chi-square test is applied. For the latency to copulation, Mann-Whitney U test is applied. Error bars indicate SEM. ***p < 0.001, NS indicates no significant difference.
-
Figure 5—source data 1
Source data for Figure 5.
- https://cdn.elifesciences.org/articles/76025/elife-76025-fig5-data1-v1.xlsx
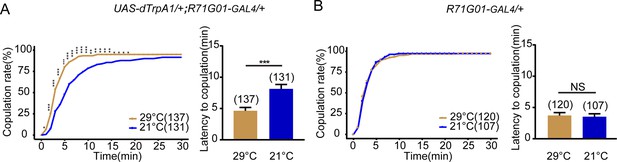
Activation of R71G01-GAL4 neurons promotes female receptivity.
(A) Activation of R71G01-GAL4 neurons significantly increased copulation rate and shortened the latency to copulation at 29°C relative to 21°C. R71G01-GAL4 driving UAS-dTrpA1 activated R71G01-GAL4 neurons at 29°C. (B) The controls with R71G01-GAL4 alone did not alter the copulation rate and the latency to copulation at 29°C relative to 21°C. The number of female flies paired with wild-type males is displayed in parentheses. For the copulation rate, chi-square test is applied. For the latency to copulation, Mann-Whitney U test is applied. Error bars indicate SEM. *p < 0.05, **p < 0.01, ***p < 0.001, NS indicates no significant difference.
-
Figure 5—figure supplement 1—source data 1
Source data for Figure 5—figure supplement 1.
- https://cdn.elifesciences.org/articles/76025/elife-76025-fig5-figsupp1-data1-v1.xlsx
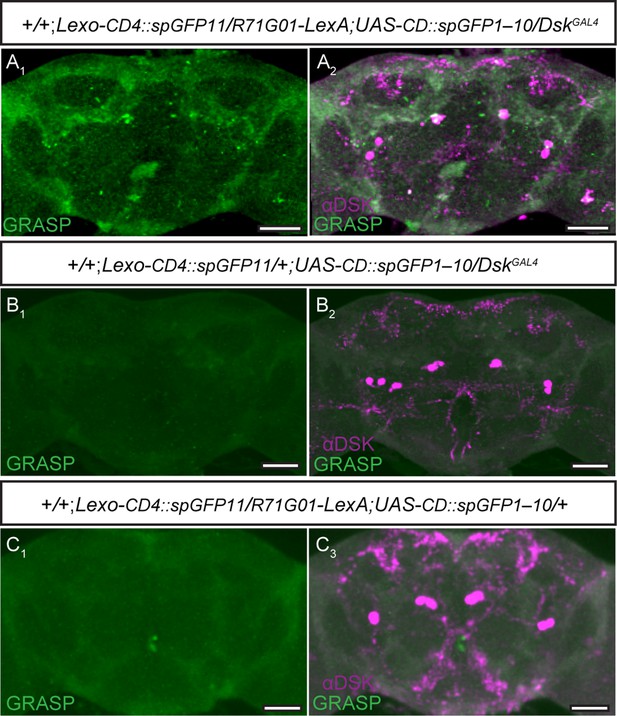
Potential connection between R71G01 neurons and Drosulfakinin (DSK) neurons was detected by GFP reconstitution across synaptic partners (GRASP) method.
(A) The reconstituted GFP signals (GRASP) (A1) revealed potential synaptic contacts between R71G01 neurons and DSK neurons. DskGAL4 expresses CD4::spGFP11, R71G01-LexA expresses CD4::spGFP1-10. GRASP (green), anti-DSK antibody staining (magenta) (A2). (B–C) No reconstituted GFP signals (GRASP) were observed in flies with either DskGAL4 alone (B1) or R71G01-LexA alone (C1). Scale bars, 50 μm.
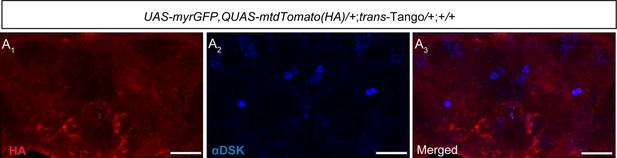
Control signals for the trans-Tango experiment.
(A) No trans-Tango signals (anti-HA, red) (A1) were detected in the central brain without a Gal4 driver. Cell bodies of Drosulfakinin (Dsk) were stained with anti-DSK (blue) (A2). Merge (A3). Scale bars, 50 μm.
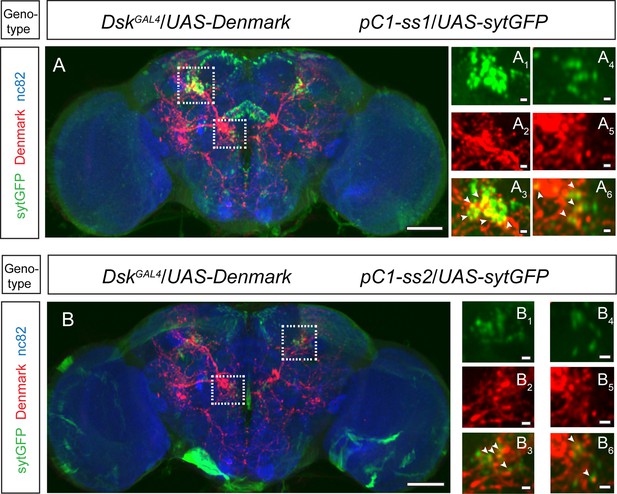
Axons of pC1 neurons overlapped with dendrites of Drosulfakinin (DSK) neurons.
(A–B) pC1 neuron axons overlapped with DSK neuron dendrites by anatomical registration. White boxed regions in (A–B) are shown in (A1–A6) and (B1–B6). White arrowheads indicated the region of overlaps between pC1 neuron axons with DSK neuron dendrites. pC1-ss1 or pC1-ss2-driven UAS-sytGFP expression (green), DskGAL4-driven UAS-Denmark expression (red). Scale bars are 50 μm in (A–B), and 5 μm in (A1–A6) and (B1–B6).
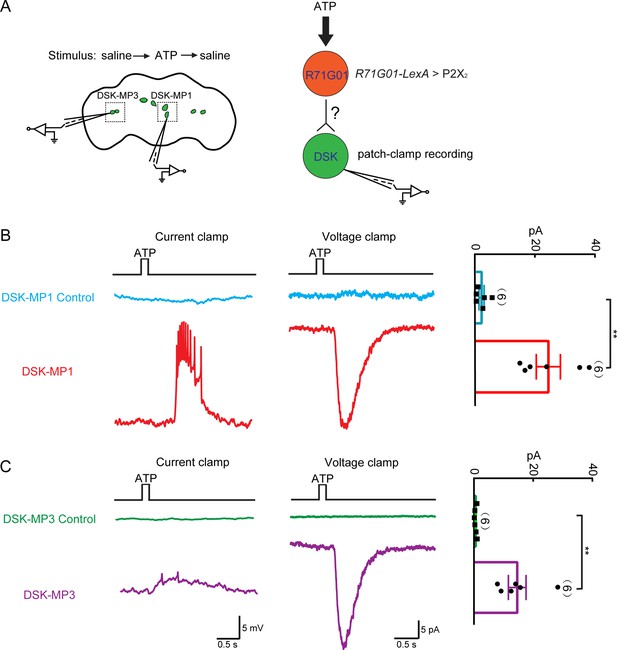
Functional connectivity between R71G01-GAL4 neurons and Drosulfakinin (DSK) neurons.
(A) Left: ATP stimulation and recording arrangement. The chemical stimulation is implemented using a three-barrel tube (with the tip positioned ~50 μm away from the brain), controlled by a stepper for rapid solution change. Right: schematic illustrating the activation of R71G01-GAL4 neurons by ATP and patch-camp recording of DSK neurons. R71G01-GAL4 neurons were activated by ATP in +/+;R71G01-LexA/+;DskGAL4/LexAop-P2X2,UAS-GCaMP6m files. (B–C) The electrical responses of medial DSK neurons (DSK-MP1) and lateral DSK neurons (DSK-MP3) to the ATP activation of 2X2-expressing R71G01-GAL4 neurons. ATP: 2.5 mM. Left: ATP-induced spiking firing (current clamp). Middle: current responses (voltage clamp). Right: quantification of absolute current responses. n = 6 for DSK-MP1, DSK-MP1 control, DSK-MP3, DSK-MP3 control. Genotype: +/+;+/+;DskGAL4/LexAop-P2X2,UAS-GCaMP6m for DSK-MP1 control and DSK-MP3 control. **p < 0.01 (Mann-Whitney U tests).
-
Figure 6—source data 1
Source for Figure 6.
- https://cdn.elifesciences.org/articles/76025/elife-76025-fig6-data1-v1.xlsx
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Antibody | Mouse anti- a-bruchpilot monoclonal (nc82) | Developmental Studies Hybridoma Bank | Cat# nc82, RRID:AB_2314866 | IHC (1:50) |
| Antibody | Rat monoclonal anti-HA | Roche | Cat# 11867431001, RRID:AB_390919 | IHC (1:100) |
| Antibody | Mouse monoclonal anti-GFP-20 | Sigma-Aldrich | Cat# G6539, RRID:AB_259941 | IHC (1:100) |
| Antibody | Chicken polyclonal anti-GFP | Thermo Fisher Scientific | Cat# A10262, RRID:AB_2534023 | IHC (1:1000) |
| Antibody | Goat anti-chicken polyclonal, Alexa Fluor 488 | Thermo Fisher Scientific | Cat# A-11039; RRID: AB_2534096 | IHC (1:500) |
| Antibody | Goat anti-rat polyclonal, Alexa Fluor 546 | Thermo Fisher Scientific | Cat# A-11081, RRID:AB_2534125 | IHC (1:500) |
| Antibody | Goat anti-rabbit polyclonal, Alexa Fluor 546 | Thermo Fisher Scientific | Cat# A-11010, RRID:AB_2534077 | IHC (1:500) |
| Antibody | Goat anti-mouse polyclonal, Alexa Fluor 633 | Thermo Fisher Scientific | Cat# A-21094, RRID:AB_2535749 | IHC (1:500) |
| Antibody | Goat anti-rabbit polyclonal, Alexa Fluor 647 | Thermo Fisher Scientific | Cat# A-21247, RRID:AB_141778 | IHC (1:500) |
| Antibody | Rabbit polyclonal anti-DSK | N/A | IHC(1:1000) | |
| Chemical compound, drug | Paraformaldehyde (PFA) | Electron Microscopy Sciences | Cat# 15713 | 8% PFA diluted in 1× PBS at 1:4 or 1:2 |
| Chemical compound, drug | DPX Mountant | Sigma-Aldrich | Cat# 44581 | |
| Chemical compound, drug | Normal goat serum | Sigma-Aldrich | Cat# G9023 | |
| Chemical compound, drug | Adenosine 5’-triphosphate disodium salt hydrate microbial | Sigma-Aldrich | Cat# A6419-1G | 2.5 mM |
| Chemical compound, drug | Mifepristone (RU486) | Sigma-Aldrich | Cat# M8046-1G | |
| Genetic reagent(Drosophila melanogaster) | UAS-myrGFP,QUAS-mtdTomato(3*HA);trans-Tango | Zhong Lab, Tsinghua University | N/A | |
| Genetic reagent(Drosophila melanogaster) | +; sp/cyo; LexAop-P2X2, UAS-GCamP/Tm2 | Luo Lab, Peking University | N/A | |
| Genetic reagent(Drosophila melanogaster) | UAS-mCD8::GFP | Bloomington Stock Center | # 5137 | |
| Genetic reagent(Drosophila melanogaster) | 10XUAS-IVS-mCD8::RFP,13XLexAop2-mCD8::GFP; nSyb-MKII::nlsLexADBD/CyO; UAS-p65AD::CaM | Bloomington Stock Center | # 61679 | |
| Genetic reagent(Drosophila melanogaster) | TβH-GAL4 | Bloomington Stock Center | # 45904 | |
| Genetic reagent(Drosophila melanogaster) | UAS > stop > dTrpAmyrc | Bloomington Stock Center | # 66871 | |
| Genetic reagent(Drosophila melanogaster) | R71G01-GAL4 | Bloomington Stock Center | # 39599 | |
| Genetic reagent(Drosophila melanogaster) | R71G01-LexA | Bloomington Stock Center | # 54733 | |
| Genetic reagent(Drosophila melanogaster) | +; UAS-syteGFP, UAS-Denmark; Sb/+ | Rao Lab, Peking University | N/A | |
| Genetic reagent(Drosophila melanogaster) | UAS > stop > Kir2.1eGFP | Rao Lab, Peking University | N/A | |
| Genetic reagent(Drosophila melanogaster) | DskFlp | Pan Lab, Southeast University | N/A | |
| Genetic reagent(Drosophila melanogaster) | elav-GS | Zhong Lab, Tsinghua University | N/A | |
| Genetic reagent(Drosophila melanogaster) | UAS-dTrpA1/cyo | Garrity Lab, Brandeis University | N/A | |
| Genetic reagent(Drosophila melanogaster) | UAS-TNT | O'Kane Lab, University of Cambridge | N/A | |
| Genetic reagent(Drosophila melanogaster) | UAS-impTNT | O'Kane Lab, University of Cambridge | N/A | |
| Genetic reagent(Drosophila melanogaster) | UAS-Kir2.1 | Bloomington Stock Center | #6595, #6596 | |
| Genetic reagent(Drosophila melanogaster) | DskGAL4 | Rao Lab, Peking University | N/A | |
| Genetic reagent(Drosophila melanogaster) | elav-GAL4 | Rao Lab, Peking University | N/A | |
| Genetic reagent(Drosophila melanogaster) | GluRIAGAL4 | Rao Lab, Peking University | N/A | |
| Genetic reagent(Drosophila melanogaster) | CCKLR-17D3GAL4 | Rao Lab, Peking University | N/A | |
| Genetic reagent(Drosophila melanogaster) | ΔDsk | Rao Lab, Peking University | N/A | |
| Genetic reagent(Drosophila melanogaster) | UAS > stop > myr::GFP | Gerald Rubin, Janelia Farm Research Campus | N/A | |
| Genetic reagent(Drosophila melanogaster) | ΔCCKLR-17D3 | Rao Lab, Peking University | N/A | |
| Genetic reagent(Drosophila melanogaster) | ΔCCKLR-17D1 | Rao Lab, Peking University | N/A | |
| Genetic reagent(Drosophila melanogaster) | UAS-Dsk | Zhou Lab, Chinese Academy of Sciences, this paper | N/A | |
| Genetic reagent(Drosophila melanogaster) | UAS-DskRNAi | Pan Lab, Southeast University | N/A | |
| Genetic reagent(Drosophila melanogaster) | elav-GAL4;UAS-dcr2 | Rao Lab, Peking University | N/A | |
| Genetic reagent(Drosophila melanogaster) | Lexo-CD4-spGFP11/CyO; UAS-CD4-spGFP1-10/Tb | Gordon and Scott, 2009 | N/A | |
| Genetic reagent(Drosophila melanogaster) | pC1-ss1 | Kaiyu Wang’s lab, Institute of Neuroscience | N/A | |
| Genetic reagent(Drosophila melanogaster) | pC1-ss2 | Kaiyu Wang’s lab, Institute of Neuroscience | N/A | |
| Genetic reagent(Drosophila melanogaster) | Dilp2-GAL4 | Zhong Lab, Tsinghua University | N/A | |
| Genetic reagent(Drosophila melanogaster) | UAS-CCKLR-17D3 | Zhou Lab, Chinese Academy of Sciences, this paper | N/A | |
| Genetic reagent(Drosophila melanogaster) | UAS-CCKLR-17D1RNAi | Bloomington Stock Center | # 27494 | |
| Genetic reagent(Drosophila melanogaster) | UAS-CCKLR-17D3RNAi | Bloomington Stock Center | # 28333 | |
| Software, algorithm | MATLAB | MathWorks, Natick, MA | https://www.mathworks.com/products/matlab.html | |
| Software, algorithm | ImageJ | National Institutes of Health | https://imagej.nih.gov/ij/ | |
| Software, algorithm | Prism 7 | GraphPad | https://www.graphpad.com/ |
Additional files
-
Supplementary file 1
Activation of Drosulfakinin (DSK) neurons did not change receptivity in mated females and very young females.
The numbers were shown in the table are the number of pairs that copulated within the 30 min divided by the number of total tested pairs. None of females successfully copulated in mated females and very young females, except one mated female successfully copulated with the genotype of UAS-dTrpA1/+ at 29°C.
- https://cdn.elifesciences.org/articles/76025/elife-76025-supp1-v1.docx
-
Supplementary file 2
Synaptic connections identified by electron microscopic (EM) reconstruction.
- https://cdn.elifesciences.org/articles/76025/elife-76025-supp2-v1.docx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/76025/elife-76025-transrepform1-v1.docx





