Spatially bivariate EEG-neurofeedback can manipulate interhemispheric inhibition
Figures
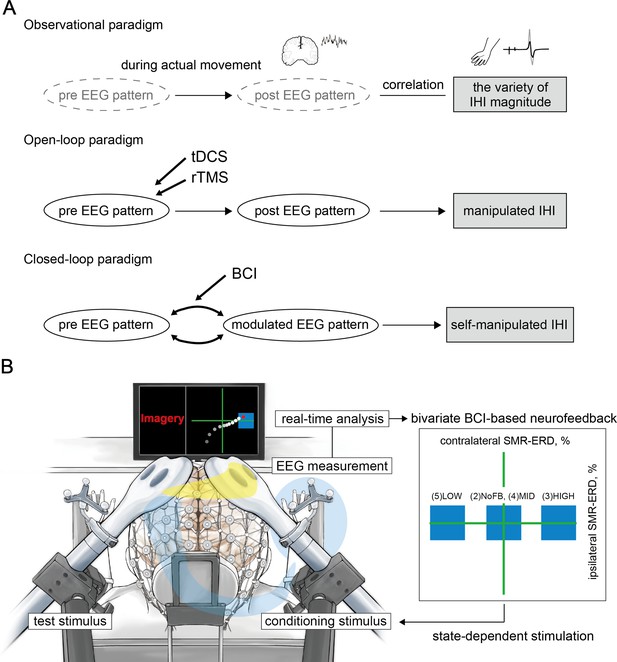
Conceptual illustration of the current study and experimental overview.
(A) When a certain stimulus was input into the system, the brain was considered to vary with the state, resulting in interhemispheric inhibition (IHI) changes. The upper panel highlights the experimental limitations in the observational paradigm due to a variety of IHI magnitudes observed during actual movement. In this case, it is unclear whether the changes in electroencephalography (EEG) patterns in both the hemispheres would affect IHI. The middle panel indicates that it is unclear whether it is possible to manipulate inhibitory interhemispheric sensorimotor activity in the open-loop neuromodulation paradigm using transcranial direct current stimulation (tDCS) or repetitive transcranial magnetic stimulation (rTMS). The lower panel shows that specific EEG patterns are associated with IHI magnitude, and brain-computer interface (BCI)-based neurofeedback modulates the EEG activities. Therefore, if bilateral EEG patterns that underlie IHI are identified, we should be able to volitionally regulate the IHI magnitude via BCI-based neurofeedback, suggesting the possibility of plastic interhemispheric balancing. (B) The current bi-EEG-triggered dual-TMS experimental system involved spatially bivariate BCI-based neurofeedback that allows volitional modulation of EEG patterns only in a targeted hemisphere to enable us to verify our hypothesis. Different states of the targeted bidirectional up- and down-regulated ipsilateral hemisphere to the imagined hand while maintaining constant contralateral excitability were tested in the following states: (1) resting-state, (2) during motor imagery without visual feedback, (3) high, (4) middle, and (5) low excitability states. A blue target box, based on the predetermined SMR-ERDs, was displayed corresponding to each session. A cue signal was generated to trigger the conditioning stimulus when the signal reached the target box. The yellow line on the head represents the signal flow from the conditioning hemisphere that modifies the contralateral side through the corpus callosum, and the blue line represents the test stimulus toward the right hand.
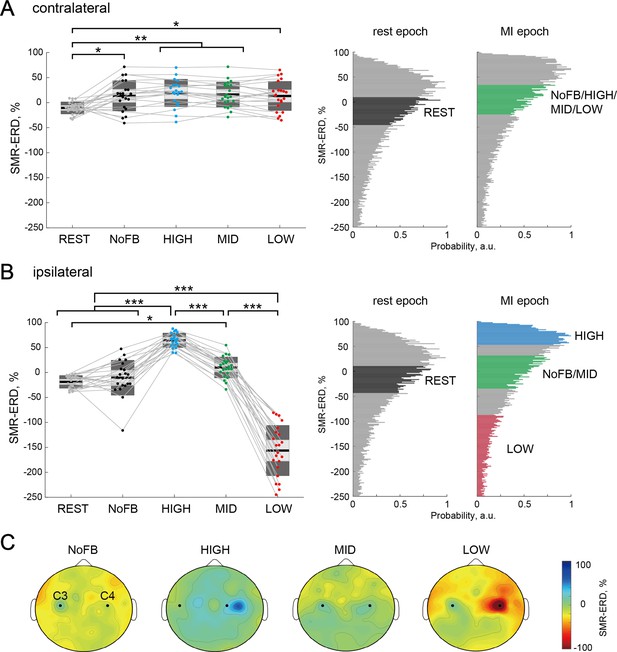
Target-hemisphere-specific modulation at the electroencephalography (EEG) level induced by spatially bivariate brain-computer interface (BCI)-based neurofeedback.
(A and B) Modulation effect of the contralateral (left) and ipsilateral (right) SMR-ERDs to the imagined right hand, respectively. Individual participants are represented by colored plots and thin gray lines. The light gray box represents 1.96 SEM (95% CI) and dark gray box represents 1 SD. The black line indicates the group mean of the studied sample and colored plots indicate a single session. Positive values indicate desynchronization as compared to rest. Complete desynchronization to zero power in the frequency of interest translates to a 100% increase in SMR-ERD, whereas synchronization in the same band could theoretically be unlimited and allows for decreases in SMR-ERD>100%. The two right-sided panels represent the SMR-ERD distributions during rest and motor imagery (MI) epoch in the calibration session. Based on the SMR-ERD distributions in the contralateral and ipsilateral hemispheres, the target ranges of SMR-ERD during bi-EEG-triggered dual-transcranial magnetic stimulation (dual-TMS) system (each color) were set for each participant. (C) Spatial patterns of SMR-ERD during the MI epoch in each session (group mean). Large positive values (blue color) represent larger SMR-ERD. The black dots represent the C3 and C4 channels.
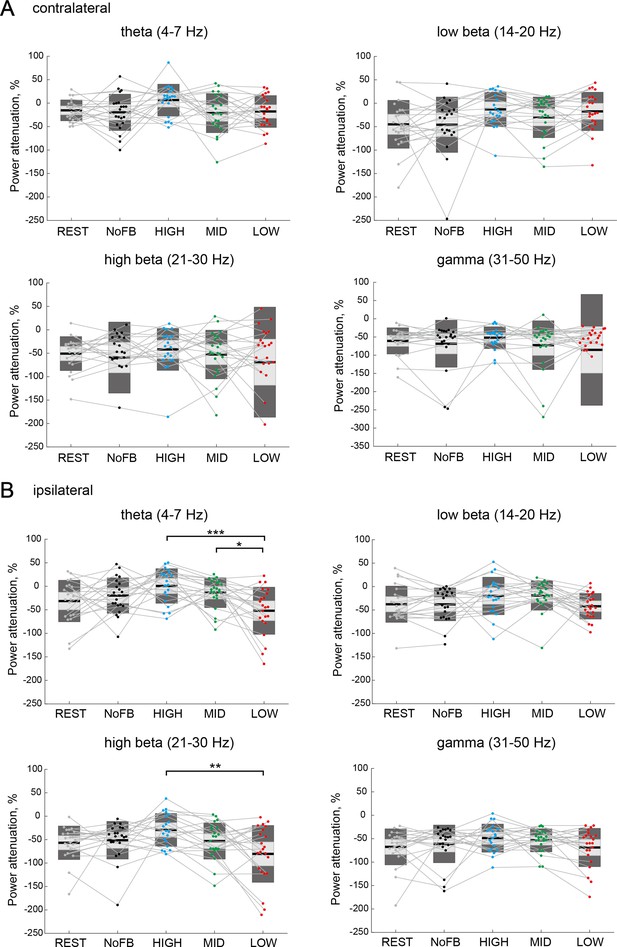
Target-hemisphere-specific modulation at the electroencephalography (EEG) level in frequency bands outside of a target alpha band.
(A and B) Modulation effect of the contralateral and ipsilateral excitabilities (power attenuation) in frequency bands outside of a target alpha band (theta [4–7 Hz], low-beta [14–20 Hz], high-beta [21–30 Hz], and gamma [31–50 Hz]), respectively. Individual participants are represented by colored plots and thin gray lines. The light gray box represents 1.96 SEM (95% CI) and dark gray box represents 1 SD. The black line indicates the group mean of the studied sample and colored plots indicate a single session.
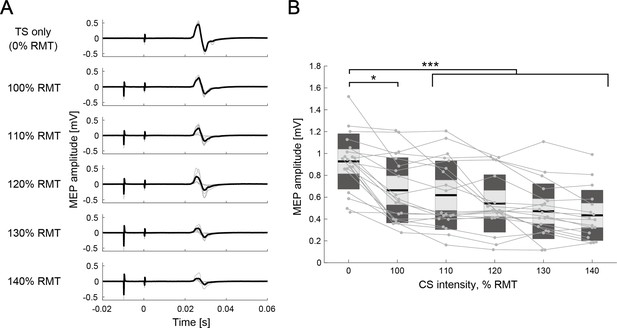
Interhemispheric inhibition (IHI) curves at rest.
(A) Motor-evoked potential (MEP) amplitudes in the different intensity of conditioning stimulus (CS) of a representative participant. The thin gray lines represent each trial and black lines indicate the trial mean. (B) The IHI curves of the individual participants at rest are represented by thin gray plots and lines. The figure presents the individual data as an alternative to a box plot. The light gray box represents 1.96 SEM (95% CI) and dark gray box indicates 1 SD. The black line indicates the group mean. The y-axis indicates raw MEP amplitude against CS intensity (x-axis, in % resting motor threshold [RMT]). Dendrograms above the bars represent the results of the post-hoc analyses. *p<0.05 and ***p<0.001; all comparisons were Bonferroni corrected.
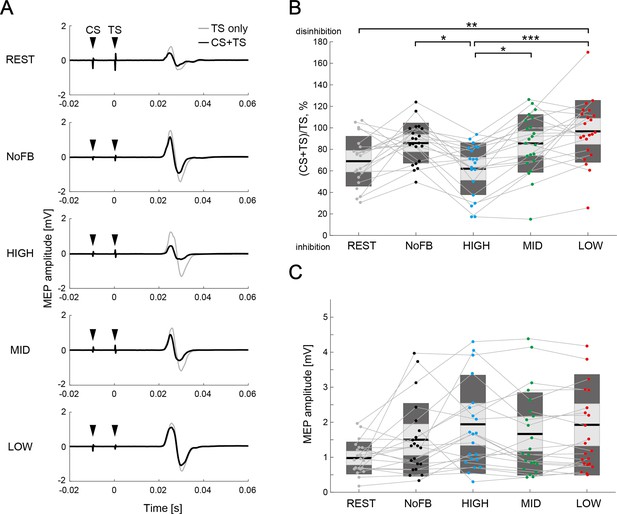
Comparison of interhemispheric inhibition (IHI) magnitude.
(A) Typical examples of mean motor-evoked potential (MEP) amplitudes elicited by single TS (TS-only; light gray color) and paired-pulse stimulation (conditioning stimulus [CS] + TS; black color) in a representative participant. The black arrows represent the stimulus timings of CS and TS. (B) The IHI magnitudes of the individual participants are represented by colored plots and thin gray lines. The light gray box represents 1.96 SEM (95% CI) and dark gray box represents 1 SD. The black line indicates the group mean of the studied sample and colored plots represent a single session. Lower values represent greater inhibitory effect from the ipsilateral hemisphere to the imagined right hand. Dendrograms above the bars represent the results of the post-hoc analyses. *p<0.05, **p<0.01, and ***p<0.001; all comparisons were Bonferroni corrected. (C) The figure shows MEP amplitude elicited by a single TS (TS-only). No significant difference in MEP amplitude was observed between sessions (all p>0.05).
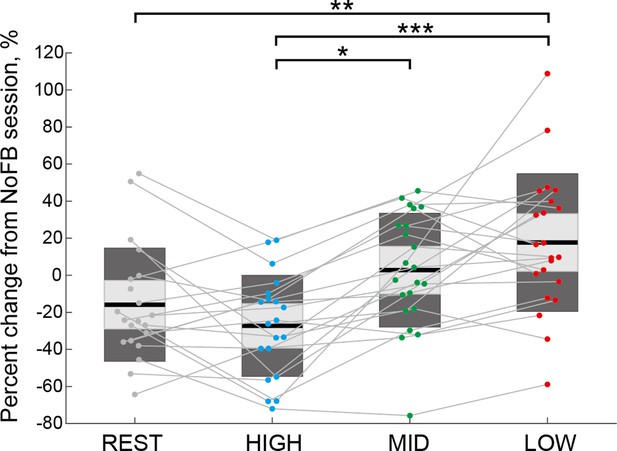
Comparison of the percentage change in interhemispheric inhibition (IHI) magnitude based on values in NoFB session.
The percentage changes in IHI magnitude based on values in the NoFB session of individual participants are represented by colored plots and thin gray lines. Dendrograms above the bars represent the results of the post-hoc analyses. *p<0.05, **p<0.01, and ***p<0.001; all comparisons were Bonferroni corrected.

Comparison of interhemispheric inhibition (IHI) magnitudes in triggered and non-triggered transcranial magnetic stimulation (TMS) trials.
The IHI magnitudes in triggered and non-triggered TMS trials of the individual participants are represented by colored plots. Bars with hatched lines represent the non-triggered TMS trials, and those without shading represent the triggered TMS trials. Dendrograms above the bar represent the results of the post-hoc analyses. *p<0.05 and ***p<0.001; all comparisons were Bonferroni corrected.

Relationship between the increase in interhemispheric inhibition (IHI) and left motor-evoked potential (MEP) amplitude elicited by conditioning stimulus (CS).
(A) Experimental overview of the MEP measurements. The yellow line on the head represents the signal flow from the conditioning hemisphere that modifies the contralateral side through the corpus callosum. The blue line represents the test stimulus toward the right hand, and the orange line indicates the CS toward the left hand. (B) The figure shows the MEP amplitude in the left first dorsal interosseous (FDI) elicited by CS. (C) A statistical comparison with the percentage changes of left MEP amplitude as a covariate of no interest during the triggered trials is shown. To compare the IHI magnitude, the percentage changes based on the values in the NoFB session were calculated. A significant difference in IHI magnitude between the sessions was observed (p<0.001), suggesting that IHI changes greater than the variance explained by the CS effect. Dendrograms above the bars represent the results of post-hoc analyses. ***p<0.001; all comparisons were Bonferroni corrected. (D) A statistical comparison with the percentage changes of left MEP amplitude as a covariate in the non-triggered trials. During the non-triggered trials, no significant difference was observed in IHI magnitude (p=0.319).
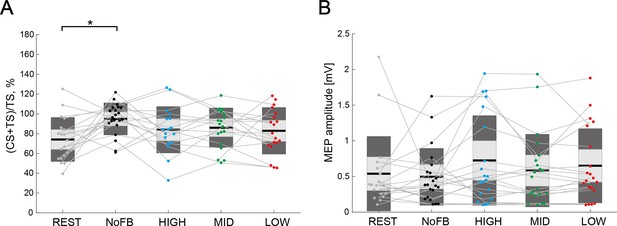
Comparison of interhemispheric inhibition (IHI) magnitude for control muscle.
(A) The IHI magnitudes of the individual participants are represented with colored plots and thin gray lines. The light gray box represents 1.96 SEM (95% CI) and the dark gray box indicates 1 SD. The black line indicates the group mean and colored plots indicate each session. Lower values indicate greater inhibitory effect from the ipsilateral hemisphere to the imagined hand. Dendrograms above the bars represent the results of the post-hoc analyses. *p<0.05; all comparisons were Bonferroni corrected. (B) The figure shows motor-evoked potential (MEP) amplitude elicited by a single TS (TS-only). No significant difference in MEP amplitude in the abductor digiti minimi (ADM) muscle was observed across the sessions (all p>0.05).
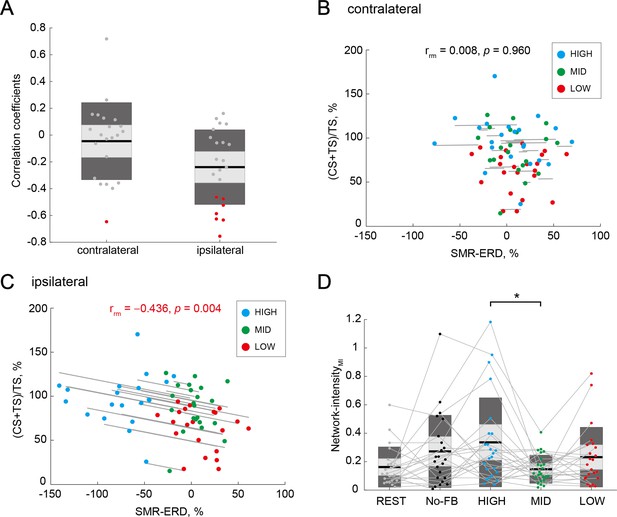
Associations of interhemispheric inhibition (IHI) magnitude and bilateral electroencephalography (EEG) patterns.
(A) Distributions of within-participant correlation coefficients in all participants. Red dots represent individuals with a significant correlation between IHI magnitude and contralateral or ipsilateral SMR-ERDs, respectively. (B and C) Repeated measures correlations between IHI magnitude and contralateral or ipsilateral SMR-ERDs, respectively. Dots represent the mean value of a single session of each individual. Only the ipsilateral SMR-ERD and IHI magnitude were significantly correlated; high sensorimotor excitability state in the ipsilateral hemisphere would induce stronger inhibition from the ipsilateral hemisphere to the imagined hand. (D) Comparison of Network-intensity across sessions. Dendrograms above the bars represent the results of the post-hoc analyses. *p<0.05; all comparisons were Bonferroni corrected. There was a significant difference in interhemispheric functional connectivity between HIGH and MID sessions, but not between HIGH and LOW sessions.
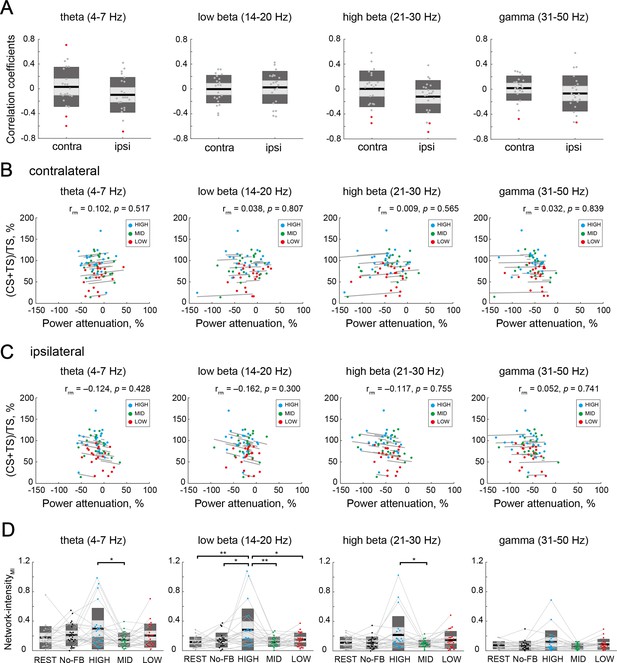
Associations of interhemispheric inhibition (IHI) magnitude and bilateral electroencephalography (EEG) patterns in frequency bands outside of a target alpha band.
(A) Distributions of within-participant correlation coefficients in frequency bands outside of a target alpha band in all participants. Red dots represent individuals with a significant correlation between IHI magnitude and contralateral or ipsilateral excitabilities (power attenuation), respectively. (B and C) Repeated measures correlations between IHI magnitude and contralateral or ipsilateral excitabilities, respectively. Dots represent the mean value of a single session of each individual. (D) Comparison of Network-intensity during motor imagery (MI) across sessions. Dendrograms above the bars represent the results of the post-hoc analyses. *p<0.05; all comparisons were Bonferroni corrected.
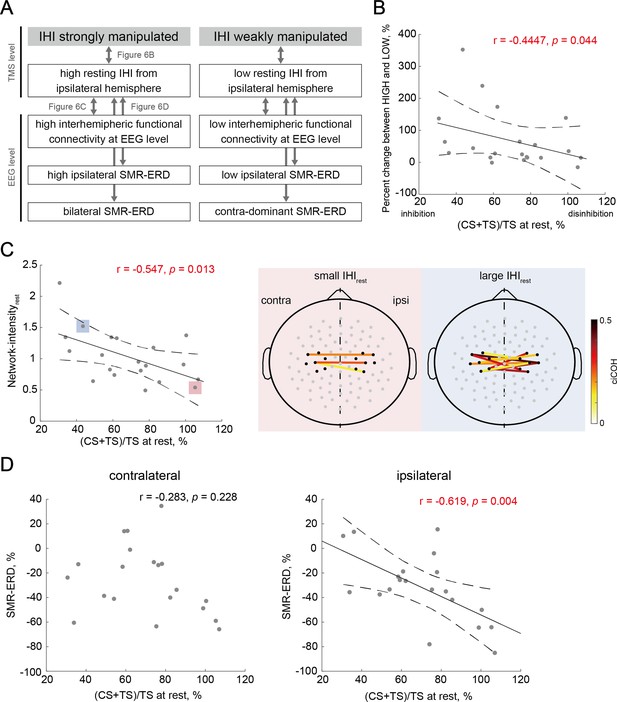
Individual characteristics associated with the manipulation capability of interhemispheric inhibition (IHI).
(A) Overview of the relationships between biomarkers from electroencephalography (EEG) and transcranial magnetic stimulation (TMS) levels to probe the individual signatures for strong versus weak manipulation of IHI. Arrows correspond to a single panel. (B) Across-participant correlations between the manipulation capability of IHI and intrinsic IHI magnitude at rest. Dots represent a single participant. Solid and dotted lines represent the estimated linear regression and 95% CI, respectively. Participants with greater IHI at rest were able to strongly manipulate IHI. (C) The left-sided panel shows across-participant correlations between IHI at rest and resting-state Network-intensity between bilateral sensorimotor cortex (SM1). The two right-sided panels indicate the significant interhemispheric connections (‘Connectivity analysis’ in Materials and methods) of the two representative participants with small and large IHIrest, respectively. The solid lines indicate a significant connection, and large positive values (dark red color) represent strong connections. The black dots around bilateral SM1 denote the seed channels, C3 or C4, and six neighboring channels. The gray dots represent other EEG channels. (D) Across-participant correlations between IHI at rest and EEG profiles showed significant correlation between IHIrest and ipsilateral SMR-ERD, but not between IHIrest and contralateral SMR-ERD.
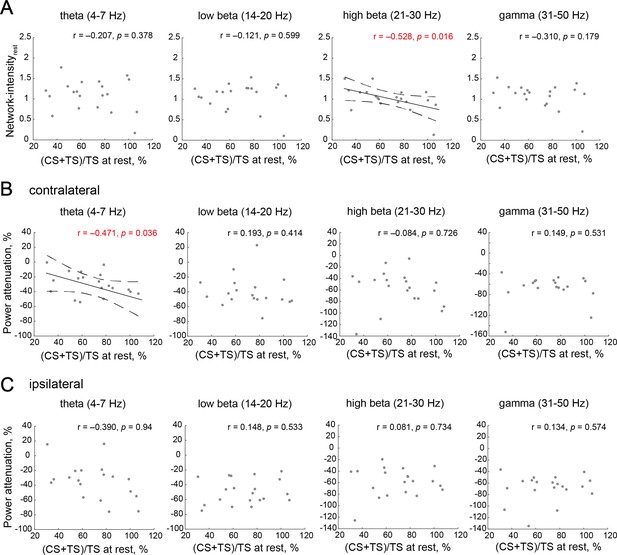
Neural characteristics depending on the manipulation capability of interhemispheric inhibition (IHI) in frequency bands outside of a target alpha band.
(A) Across-participant correlations between IHI at rest and resting-state Network-intensity between bilateral sensorimotor cortex (SM1) in frequency bands outside of a target alpha band. (B and C) Across-participant correlations between IHI at rest and contralateral or ipsilateral excitabilities (power attenuation), respectively. Dots represent a single participant. Solid and dotted lines represent the estimated linear regression and 95% CI, respectively.
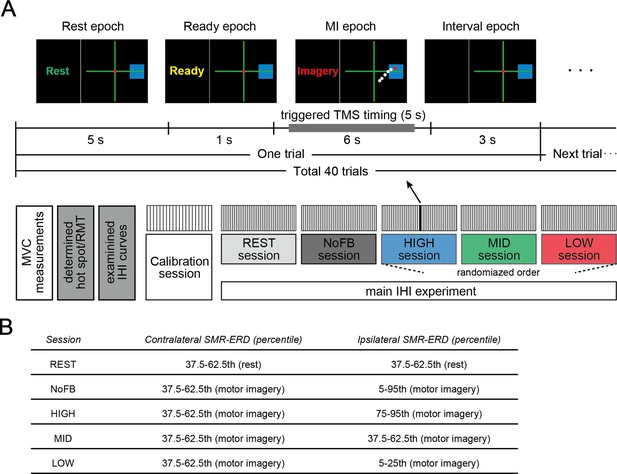
Experimental paradigm.
(A) Task instructions and visual SMR-ERD feedback in the contralateral and ipsilateral sensorimotor cortex (SM1) were provided in the form of computer cursors in a two-dimensional coordinate on a computer screen (upper panel). The electroencephalography (EEG)-triggered transcranial magnetic stimulation (TMS) timing was determined based on the intrinsic sensorimotor cortical activity of each participant in the calibration session and ranged from 0.5 to 5.5 s during the motor imagery (MI) epoch. We also applied non-triggered TMS if SMR-ERD was not achieved with the target ranges (referred to as the failed trial). In the non-triggered TMS trials, paired pulses or unconditioned test pulses were delivered in random timing ranging from 5.5 to 6 s irrespective of instantaneous SMR-ERD during the MI epoch to see the influence of spontaneous SMR fluctuations on interhemispheric inhibition (IHI). Lower panel indicates the experimental overview. The last three HIGH, MID, and LOW sessions were arranged in a random order, and in these sessions, participants received visual feedback based on bilateral SMR-ERDs. (B) The predetermined target ranges of SMR-ERD were expressed by a blue rectangle on the computer screen in each session. We aimed for participants to volitionally increase or decrease (bidirectional) the ipsilateral sensorimotor excitability while maintaining constant contralateral sensorimotor excitability. The REST and NoFB sessions were served in order to estimate the individual baseline during rest and MI for the offline analysis.
Videos
Sample video of closed-loop bivariate (bi-hemispheric brain state-dependent) electroencephalography (EEG)-triggered dual-coil transcranial magnetic stimulation (TMS) system (bi-EEG-triggered dual-TMS).
Additional files
-
Supplementary file 1
Values corresponding to each statistical figure.
- https://cdn.elifesciences.org/articles/76411/elife-76411-supp1-v2.xlsx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/76411/elife-76411-transrepform1-v2.pdf





