Enriched dietary saturated fatty acids induce trained immunity via ceramide production that enhances severity of endotoxemia and clearance of infection
Figures
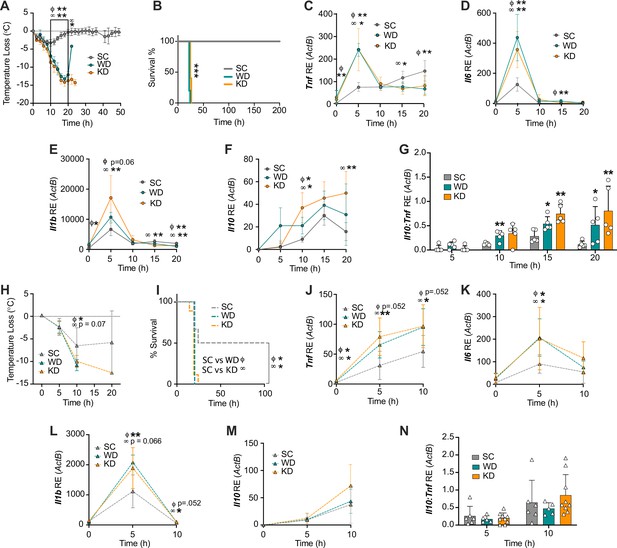
Diets enriched in saturated fatty acids lead to enhanced endotoxemia severity and altered systemic inflammatory profiles, independent of diet-associated microbiome.
(A–G) Age-matched (6–8 weeks) female BALB/c mice were fed standard chow (SC), Western diet (WD), or ketogenic diet (KD) for 2 weeks and injected intraperitoneal (i.p.) with 6 mg/kg of lipopolysaccharide (LPS). (A) Temperature loss and (B) survival were monitored every 2 hr. At indicated times, 10–20 μL of blood was drawn via the tail vein, RNA was collected, and samples were assessed for expression of (C) Tnf, (D) Il6, (E) Il1b, and (F) Il10 via qRT-PCR. (G) Il10:Tnf ratio was calculated for 5, 10, 15, and 20 hr post-injection (p.i.) with LPS. (H–N) Next, 19–23-week-old female and 14–23-week-old male and female germ-free C57BL/6 mice were fed SC, WD, or KD for 2 weeks and injected i.p. with 50 mg/kg of LPS. (H) Temperature loss and (I) survival were monitored every 5 hr p.i. (J–N) At indicated times, 10–20 μL of blood was drawn via the tail vein, RNA was collected, and samples were assessed for expression of (J) Tnf, (K) Il6, (L) Il1b, and (M) Il10 via qRT-PCR. (N) Il10:Tnf ratio was calculated for 5 and 10 hr p.i. with LPS. For (A–G), all experiments were run three times, and data are representative of one experiment, n=5 per diet group. For (H–N) SC, n=6; WD, n=5; and KD, n=9; and data are representative of one experiment. For (A, C–G, H, and J–N) a Mann Whitney test was used for pairwise comparisons. For (B) and (I) a log-rank Mantel-Cox test was used for survival curve comparison. For all panels, *p<0.05; **p<0.01; ***p<0.001. For (C–E), Φ symbols indicate WD significance, and ∞ symbols indicate KD significance. Error bars shown mean ± SD.
-
Figure 1—source data 1
Data and statistics for graphs depicted in Figure 1A–N.
- https://cdn.elifesciences.org/articles/76744/elife-76744-fig1-data1-v2.xlsx
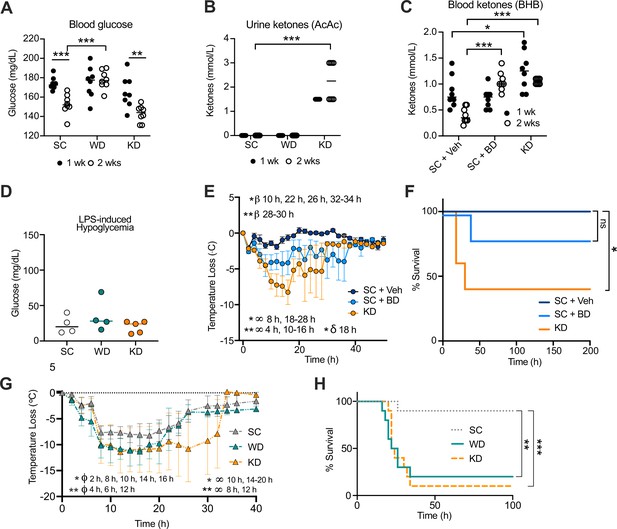
Increase in disease severity in ketogenic diet (KD) mice is independent of ketosis.
Age-matched (6–8 weeks) female BALB/c mice were fed standard chow (SC), Western diet (WD), or KD for 2 weeks. At 1 week and 2 weeks, (A) blood was collected via the tail vein to measure blood glucose levels using a glucose testing meter (Keto-Mojo), and (B) urine was collected on ketone indicator strips to measure levels of systemic acetoacetate (AcAc). Age-matched (6–8 weeks) female BALB/c mice were fed SC supplemented with 1,3-butanediol (SC + BD) or with a saccharine vehicle solution as a control (SC + Veh), or KD for 2 weeks. At 1 week and 2 weeks, (C) blood was collected via the tail vein to measure levels of systemic β-hydroxybutyrate (BHB) using a ketone testing meter (Keto-Mojo). At 2 weeks, SC-, WD-, and KD-fed mice were injected intraperitoneal (i.p.) with lipopolysaccharide (LPS; 6 mg/kg) and (D) 25 hr post-injection (p.i.), blood glucose levels were measured as stated in (A). (E) Temperature loss and (F) survival were monitored every 2 hr for mice treated as in (C) followed by i.p. injection with LPS (10 mg/kg). Age-matched (20–21 weeks) female C57BL/6 mice were fed SC, WD, or KD for 2 weeks followed by i.p. injection with LPS (4.5 mg/kg). (G) Temperature loss and (H) survival were monitored every 2 hr. For (A, B, D), all experiments were run three times, and data are representative of one experiment, n=5–8 mice/group. For (C, E, F), all experiments were run three times, and data are representative of one experiment, n=5–8 mice/group. For (G, H), data are representative of one experiment, n=10 mice/group. For (A–C, E, G), a Mann-Whitney U test was used for pairwise comparisons. For (F, H), a log-rank Mantel-Cox test was used for survival curve comparison. For (E), β symbols indicate SC +Veh vs. SC + BD significance, ∞ symbols indicate SC + Veh vs. KD significance, and δ symbols indicate SC + BD vs. KD significance. For (G), ϕ symbols indicate SC vs. WD significance, and ∞ symbols indicate SC vs. KD significance. For all panels, * p<0.05; ** p<0.01; *** p<0.001; **** p<0.0001. Error bars show mean ± SD.
-
Figure 1—figure supplement 1—source data 1
Data for graphs depicted in Figure 1—figure supplement 1A-H.
- https://cdn.elifesciences.org/articles/76744/elife-76744-fig1-figsupp1-data1-v2.xlsx

Ketogenic diet (KD) feeding alters HSC populations and bone marrow-derived macrophages (BMDMs) from KD-fed mice show a hyper-inflammatory response to lipopolysaccharide (LPS) ex vivo.
Bone marrow was extracted from the femurs and tibias of age-matched (6–8 weeks) female BALB/c mice fed standard chow (SC), Western diet (WD), or KD for 2 weeks. (A) Fluorescence-Activated Cell Sorting (FACS) plots of total HSCs (CD201+CD27+) and (B) LT-HSCs, ST-HSCs, and multipotent progenitors (MPPs) from mice fed SC, WD, or KD for 2 weeks. Quantification of (C) the total numbers of LT- and ST-HSCs, and MPPs in bone marrow from mice fed SC, WD, or KD for 2 weeks. Next, BMDMs were plated at 5×10^6 cells/mL and differentiated for 7 days in media supplemented with macrophage colony-stimulating factor. Cells were split and plated in 24-well plates to adhere for 12 hr and treated with media (Ctrl) or LPS (24 hr; 10 ng/mL). Supernatants were assessed via ELISA for (D) TNF and (E) IL-6 secretion at 24 hr post-LPS treatment. IL-6 Ctrl supernatants were below the limit of detection; ND = no data. For (A-E), all experiments were run three times, and data are representative of one experiment, n=5 per diet group. (C) A Mann Whitney test was used for pairwise comparisons. (D, E) For all plates, all treatments were performed in triplicate, and a student’s t-test was used for statistical significance. *, p<0.05; **, p<0.01; ***, p<0.001; ****, p<0.0001. Error bars show the mean ± SD.
-
Figure 2—source data 1
Data and statistics for graphs depicted in Figure 2A–E.
- https://cdn.elifesciences.org/articles/76744/elife-76744-fig2-data1-v2.xlsx
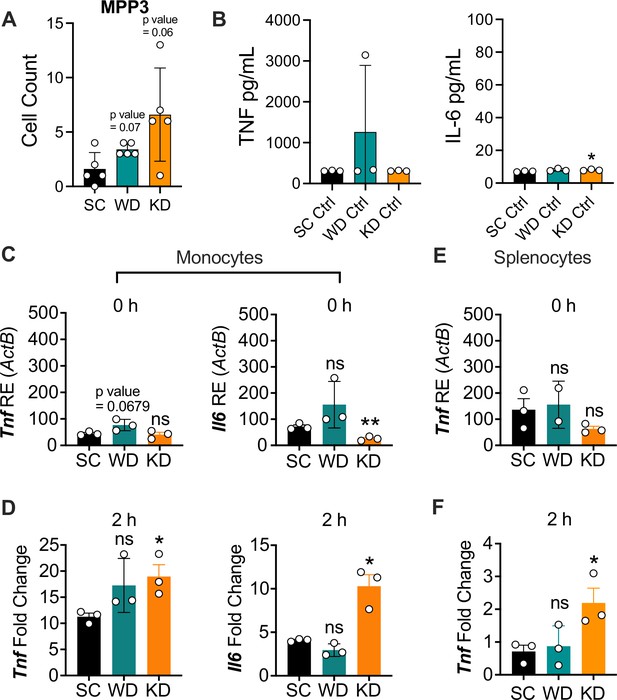
Ketogenic diet (KD) does not alter multipotent progenitor (MPP) differentiation or basal inflammation in bone marrow-derived macrophages (BMDMs), and monocytes and splenocytes show a hyper-inflammatory response to lipopolysaccharide (LPS) ex vivo.
Age-matched (6–8 weeks) conventional, wild-type, female BALB/c mice were fed standard chow (SC), Western diet (WD), or KD for 2 weeks. Bone marrow was extracted from the femurs and tibias of mice, HSCs were isolated via FACS, and (A) MPPs were quantified. BMDMs were plated at 5×106 cells/mL and differentiated for 7 days in media supplemented with macrophage colony-stimulating factor. Cells were split and plated in 24-well plates to adhere for 12 hr and treated with media (Ctrl) for 24 hr. Supernatants were assessed via ELISA for (B) TNF and IL-6 secretion. Monocytes were isolated from the femurs and tibias of mice and plated at 2×106 cells/mL. RNA was extracted from (C) untreated monocytes (0 hr) or (D) monocytes with LPS (10 ng/mL) for 2 hr. Expression of Tnf and Il6 was analyzed via qRT-PCR. Splenocytes were isolated and plated at 1×106 cells/mL. RNA was isolated from (E) untreated splenocytes (0 hr) or (F) splenocytes treated with LPS (10 ng/mL) for 2 hr. Expression of Tnf and Il6 was analyzed via qRT-PCR. For (A, B), all experiments were run three times, and data are representative of one experiment, n=5 per diet group. For (C-F), all experiments were run twice, and data are representative of one experiment, n=3 per diet group.(A) A Mann Whitney test was used for pairwise comparisons. (B-F) For all plates, all treatments were performed in triplicate, and a student’s t-test was used for statistical significance. For all panels, * p<0.05; ** p<0.01; *** p<0.001; **** p<0.0001. Error bars show mean ± SD.
-
Figure 2—figure supplement 1—source data 1
Data for graphs depicted in Figure 2—figure supplement 1.
- https://cdn.elifesciences.org/articles/76744/elife-76744-fig2-figsupp1-data1-v2.xlsx
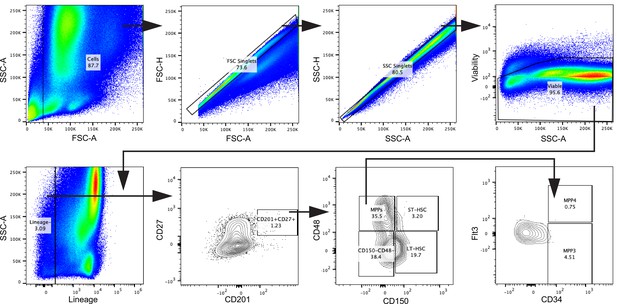
Gating strategy for HSCs, related to Figure 2.
Cells were gated in FSC-A against SSC-A. Doublets were excluded using FSC-A against FSC-H and subsequently SSC-A against SSC-H. Viable cells were gated, and lineage-committed cells were excluded. Within the lineage-negative cells, the CD201+CD27+ population was gated. In a CD150 against CD48 plot, the CD201+CD27+ cells were divided into LT-HSC, ST-HSC, multipotent progenitor (MPP), and the remaining CD150−CD48− population. MPPs were characterized as MPP3 and MPP4 by their surface expression of CD34 and Flt3.
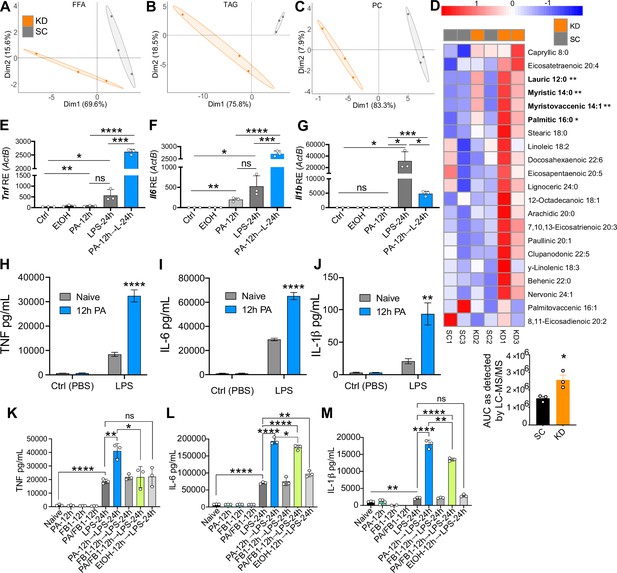
Ketogenic diet (KD) alters lipid profiles, and palmitic acid (PA) is mediating a hyper-inflammatory response to secondary challenge with lipopolysaccharide (LPS).
Data points represent single animal samples, and colors represent groups fed standard chow (SC; gray) or KD (orange) diets for 2 weeks. A 95% confidence ellipse was constructed around the mean point of each group for (A) free fatty acids (FFA), (B) triglycerides (TAG), and (C) phosphatidylcholines (PC). (D) Heatmap analysis of FFA in SC and KD mice. Components that are significantly different between the two groups are in bold. Below the heatmap is a comparison of PA 16:0 peak area detected by Liquid Chromatography Quadruple Time of Flight Mass Spectrometry (LC-QToF MS/MS) between SC and KD groups; AUC = area under the curve. Statistical significance is determined by unpaired two-tailed t-test between SC and KD groups with n=3 per group. Primary bone marrow-derived macrophages (BMDMs) were isolated from age-matched (6–8 weeks) C57BL/6 female and male mice. BMDMs were plated at 1×106 cells/mL and treated with either ethanol (ethanol (EtOH); media with 0.83% ethanol), media (Ctrl for LPS), or LPS (10 ng/mL) for 12 hr, or PA (PA stock diluted in 0.83% EtOH; 1 mM PA conjugated to 2% bovine serum albumin [BSA]) for 12 hr, with and without a secondary challenge with LPS. After indicated time points, RNA was isolated, and expression of (E) Tnf, (F) Il6, and (G) Il1b was measured via qRT-PCR. BMDMs were plated at 1×106 cells/mL and treated with either ethanol (EtOH; media with 0.83% ethanol), media (Naïve), or 1 mM PA for 12 hr followed by PBS (control) or LPS (10 ng/mL). Supernatants were assessed via ELISA for (H) TNF, (I) IL-6, and (J) IL-1β secretion. Next, BMDMs were plated at 1×106 cells/mL and treated with either media (Ctrl), LPS (10 ng/mL) for 24 hr, PA (PA stock diluted in 0.83% EtOH; 0.5 mM PA conjugated to 2% BSA) for 12 hr, Fumonisin B1 (FB1; 10 μM; diluted in 0.14% EtOH) or EtOH (0.97% to mimic simultaneous PA/FB1 treatment). Controls for all treatments are shown next to experimental groups treated additionally with LPS (10 ng/mL) for 24 hr. Supernatants were assessed via ELISA for (K) TNF, (L) IL-6, and (M) IL-1β secretion. For all plates, all treatments were performed in triplicate. For all panels, a student’s t-test was used for statistical significance. *, p<0.05; **, p<0.01; ***, p<0.001; ****, p<0.0001. Error bars show the mean ± SD.
-
Figure 3—source code 1
- https://cdn.elifesciences.org/articles/76744/elife-76744-fig3-code1-v2.zip
-
Figure 3—source data 1
Data and statistics for graphs depicted in Figure 3A–M.
- https://cdn.elifesciences.org/articles/76744/elife-76744-fig3-data1-v2.xlsx
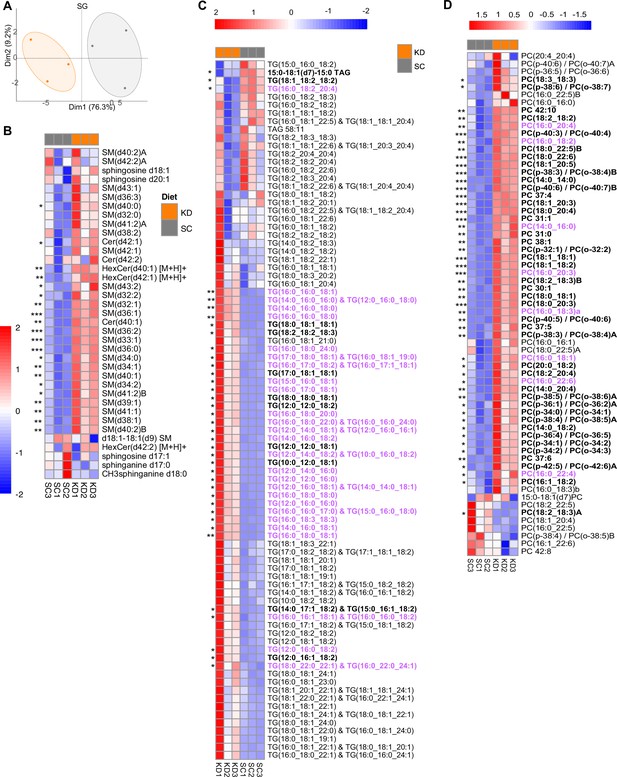
Principal component analysis and heatmap analysis of sphingolipid lipidomic data in mouse serum samples.
(A) Data points represent single animal samples, and colors represent groups fed standard chow (SC; gray) or Ketonic diet (KD; orange) diets for 2 weeks, and a 95% confidence ellipse was constructed around the mean point of each group. Heatmap analysis of (B) sphingolipids (SM), (C) triglycerides (TG), and (D) phosphatidylcholines (PC) in SC and KD groups. Lipid components containing 16:0 palmitic chains are highlighted in purple, and components that are significantly different between the two groups are in bold. Statistical significance determined by unpaired two-tailed t-test between SC and KD groups. * p<0.05; ** p<0.01; *** p<0.001; **** p<0.0001. n=3 per group.
-
Figure 3—figure supplement 1—source data 1
- https://cdn.elifesciences.org/articles/76744/elife-76744-fig3-figsupp1-data1-v2.xlsx
-
Figure 3—figure supplement 1—source code 1
- https://cdn.elifesciences.org/articles/76744/elife-76744-fig3-figsupp1-code1-v2.zip
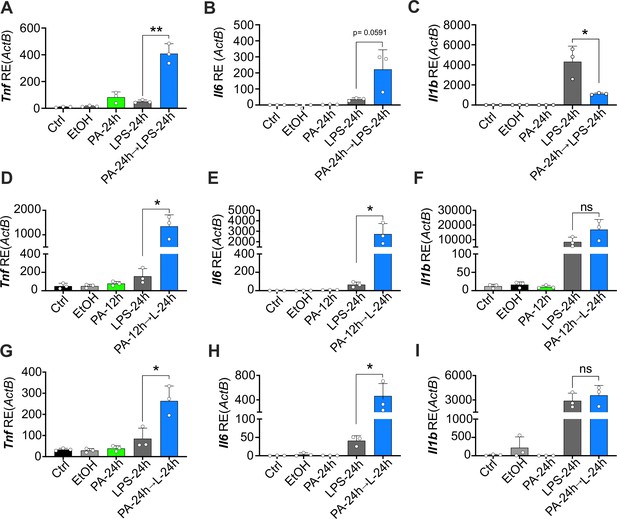
Physiological levels of palmitic acid (PA) induce a hyper-inflammatory response to secondary challenge with lipopolysaccharide (LPS) in macrophages.
Primary bone marrow-derived macrophages (BMDMs) were isolated from age-matched (6–8 weeks) female and male mice. (A–C) BMDMs were plated at 1×106 cells/mL and treated with ethanol (EtOH; media with 1.69% ethanol), media (Ctrl for LPS), or PA (1 mM; diluted in 1.69% EtOH) for 12 hr. Next, PA-treated cells were treated with LPS (10 ng/mL) for 24 hr, and all other wells were given fresh media. (D–I) BMDMs were plated at 1×106 cells/mL and treated with PA (0.5 mM; diluted in 1.69% EtOH) for 12 or 24 hr. Next, PA-treated cells were treated with LPS (10 ng/mL) for 24 hr, and all other wells were given fresh media. After indicated time points, RNA was isolated and expression of (A, D, G) Tnf, (B, E, H) Il6, and (C, F, I) Il1b was measured via qRT-PCR. For all plates, treatments were performed in triplicate. For all panels, a student’s t-test was used for statistical significance. * p<0.05; ** p<0.01; *** p<0.001; **** p<0.0001. Error bars show mean ± SD.
-
Figure 3—figure supplement 2—source data 1
Data for graphs depicted in Figure 3—figure supplement 2A-D.
- https://cdn.elifesciences.org/articles/76744/elife-76744-fig3-figsupp2-data1-v2.xlsx
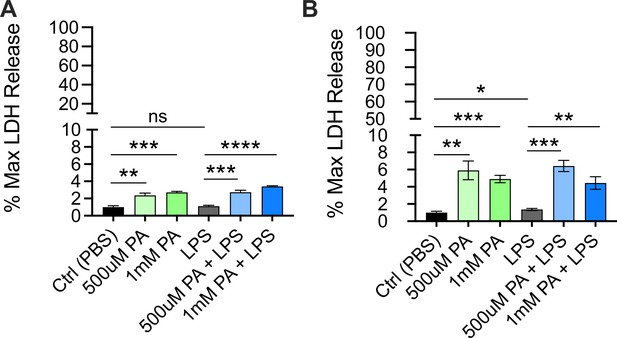
Cytotoxicity as determined by lactate dehydrogenase (LDH) release from bone marrow-derived macrophages (BMDMs) pre-treated with palmitic acid (PA) followed by lipopolysaccharide (LPS) stimulation.
BMDMs from age-matched (6–8 weeks) male and female C57BL/6 mice were plated in 96-well plates at 5×104 cells/well and incubated for 12 hr with PA (0.5 mM or 1 mM). Next, media was removed, and cells were treated with PBS for 10 ng/mL LPS in phenol-red-free Opti-MEM media and incubated for an additional 24 hr. Supernatants were collected, and LDH release was quantified using CytoTox96 non-radioactive cytotoxicity assay. (A, B) Cytotoxicity is shown as percentage of max LDH release. For all plates, all treatments were performed in triplicate, and a student’s t-test was used for statistical significance. * p<0.05; ** p<0.01; *** p<0.001; **** p<0.0001. Error bars show mean ± SD.
-
Figure 3—figure supplement 3—source data 1
Data for graphs depicted in Figure 3—figure supplement 3A, B.
- https://cdn.elifesciences.org/articles/76744/elife-76744-fig3-figsupp3-data1-v2.xlsx
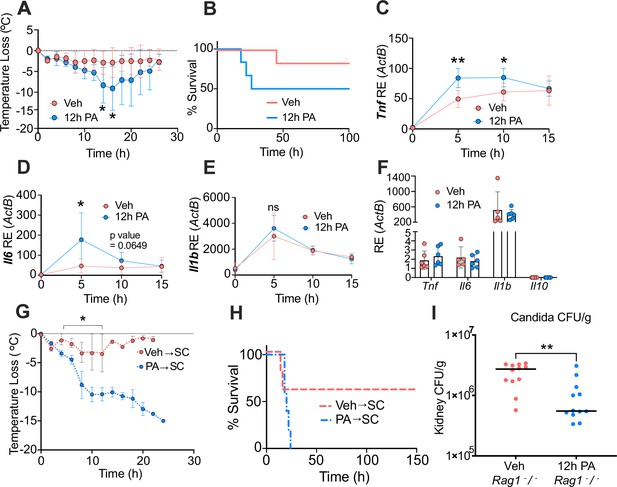
Palmitic acid (PA) acts as a novel mediator of trained immunity by inducing a hyper-inflammatory response lipopolysaccharide (LPS)-induced endotoxemia and enhancing clearance of Candida albicans infection.
Age-matched (6–8 weeks) female BALB/c mice were fed standard chow (SC) for 2 weeks and injected intraperitoneal (i.p.) with ethyl palmitate (PA, 750 mM) or vehicle (Veh) solutions 12 hr before i.p. LPS injections (10 mg/kg). (A) Temperature loss was monitored every 2 hr as a measure of disease severity or (B) survival. At indicated times blood was collected via the tail vein, RNA was extracted, and samples were assessed for expression of (C) Tnf, (D) Il6, and (E) Il1b via qRT-PCR. (F) Blood was collected via the tail vein from Veh and PA pre-treated (12-hr PA) mice immediately prior to LPS injection, and samples were assessed for expression of Tnf, Il6, Il1b, and Il10 via qRT-PCR. Additionally, age-matched (6–8 weeks) female BALB/c mice fed SC, injected i.p. with ethyl palmitate (PA, 750 mM) or Veh solutions every day for 9 days, and then rested for 7 days before i.p. LPS injections (10 mg/kg) (G) Temperature loss and (H) survival were monitored during endotoxemia. (I) Age-matched (8–9 weeks) female Rag1−/− mice were injected i.p. with ethyl palmitate (PA, 750 mM) or Veh solutions 12 hr before intravenous C. albicans infection. Fungal burden of kidneys from Veh and PA pre-treated (12-hr PA) mice 24 hr after C. albicans infection. For (A–F), experiments were run three times, and data are representative of one experiment, n=3 mice/group. For (G, H), experiments were run twice, and data are representative of one experiment, n=5 mice/group. For (I), experiments were run three times, and data are representative of one experiment, n=6 mice/group. For (A), (C–E), (G), and (I), a Mann Whitney test was used for pairwise comparisons. For (B) and (H), a log-rank Mantel-Cox test was used for survival curve comparison. For all panels, *, p<0.05; **, p<0.01; ***, p<0.001; ****, p<0.0001. Error bars shown mean ± SD.
-
Figure 4—source data 1
Data and statistics for graphs depicted in Figure 4A–I.
- https://cdn.elifesciences.org/articles/76744/elife-76744-fig4-data1-v2.xlsx
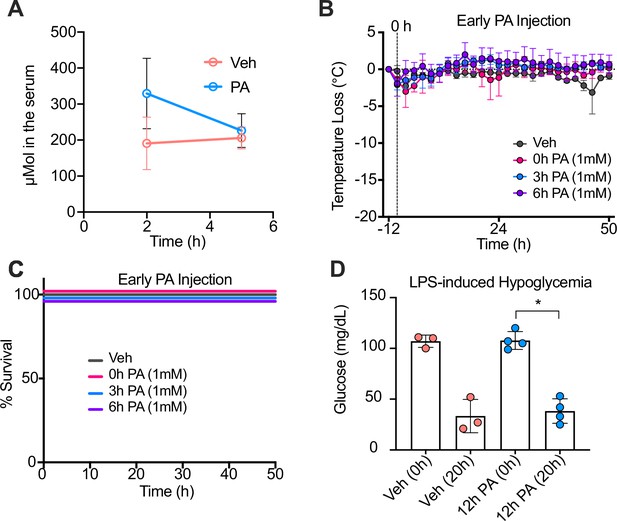
Palmitic acid (PA) intraperitoneal (i.p.) injections enhance serum PA concentrations, and PA-induced trained immunity is time-dependent.
Conventional wild-type, age-matched (6–8 weeks), female BALB/c mice were fed standard chow (SC) for 2 weeks and injected i.p. with ethyl palmitate (PA 750 mM in 1.6% lecithin and 3.3% glycerol in endotoxin-free limulus amebocyte lysate [LAL] reagent water) or a vehicle solution (Veh, 1.6% lecithin and 3.3% glycerol in endotoxin-free LAL reagent water). (A) Serum was collected via cardiac punctures from mice 2 hr and 5 hr post-injection (p.i.). Serum samples were analyzed for absolute PA concentrations using qualitative tandem liquid chromatography quadrupole time of flight mass spectrometry. At 0, 3, and 6 hr after PA injection, endotoxemia was induced via a single i.p. injection of lipopolysaccharide (LPS; 10 mg/kg). (B) Temperature loss and (C) survival were monitored every 2 hr. (D) Blood was collected via the tail vein to measure blood glucose levels at 0 and 20 hr p.i. with LPS using a glucose testing meter (Keto-Mojo). For (D), a Mann Whitney test was used for pairwise comparisons. Data is representative of one experiment, n=3–4 mice/group. * p<0.05; ** p<0.01; *** p<0.001; **** p<0.0001. Error bars show mean ± SD.
-
Figure 4—figure supplement 1—source data 1
Data for graphs depicted in Figure 4—figure supplement 1A-D.
- https://cdn.elifesciences.org/articles/76744/elife-76744-fig4-figsupp1-data1-v2.xlsx
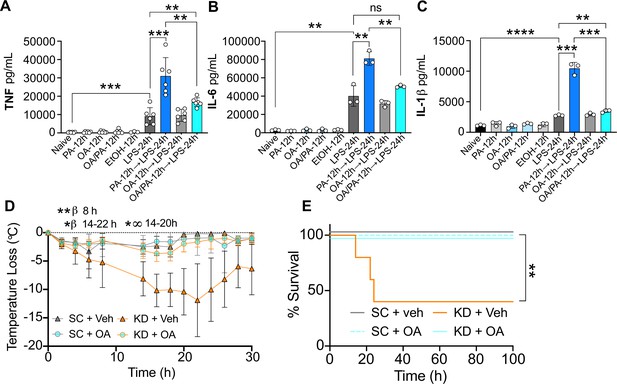
Oleic acid (OA) reverses palmitic acid (PA)-dependent hyper-inflammation in response to lipopolysaccharide (LPS) in vitro, and PA-dependent enhanced endotoxemia disease severity in vivo.
Primary bone marrow-derived macrophages (BMDMs) were isolated from age-matched (6–8 weeks) C57BL/6 female and male mice. BMDMs were plated at 1×106 cells/mL and treated with either media (Ctrl), LPS (10 ng/mL) for 24 hr, PA (PA stock diluted in 0.83% EtOH; 0.5 mM PA conjugated to 2% bovine serum albumin) for 12 hr, or OA (200 μM; diluted in endotoxin-free water). Controls for all treatments are shown next to experimental groups treated additionally with LPS (10 ng/mL) for 24 hr. Supernatants were assessed via ELISA for (A) TNF, (B) IL-6, and (C) IL-1β secretion. Age-matched (6–8 weeks) female BALB/c mice were fed standard chow (SC) or ketonic diet (KD) for 2 weeks and injected intraperitoneal with 7 mg/kg LPS. (D) Temperature loss and (E) survival were monitored every 2 hr. For (A–C), experiments were run three times and data are representative of (A) two experiments and (B, C) one experiment. For all plates, all treatments were performed in triplicate, and a student’s t-test was used for statistical significance. For (D), a Mann Whitney test was used for pairwise comparisons. For (E), a log-rank Mantel-Cox test was used for survival curve comparison. For (D, E), experiments were run three times, and data are representative of one experiment, n=5 mice/group. β symbols indicate KD + Veh vs KD + OA significance, and ∞ symbols indicate KD + Veh vs. SC + Veh. For all panels, *, p<0.05; **, p<0.01; ***, p<0.001; ****, p<0.0001. Error bars shown mean ± SD.
-
Figure 5—source data 1
Data and statistics for graphs depicted in Figure 5A–E.
- https://cdn.elifesciences.org/articles/76744/elife-76744-fig5-data1-v2.xlsx
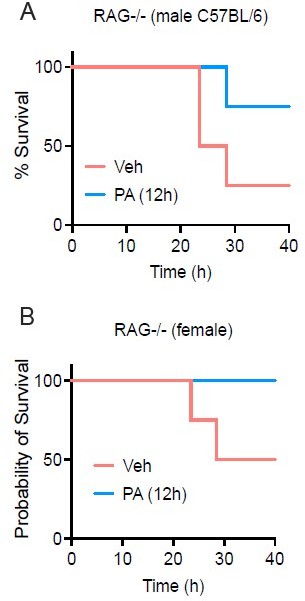
PA treatmentenhances survival in both female and male RAG-/- mice.
Age-matched (8-9 wk) RAG-/- mice were injected i.v. with ethyl palmitate (PA, 750mM) or vehicle (Veh) solutions 12 h before C.albicans infection. Survival was monitored for 40h post-infection fed SC (Figure S1G-H).
Additional files
-
Supplementary file 1
Diet compositions (values represent percentage of total kcal).
- https://cdn.elifesciences.org/articles/76744/elife-76744-supp1-v2.docx
-
Supplementary file 2
List of primers used in this study.
- https://cdn.elifesciences.org/articles/76744/elife-76744-supp2-v2.docx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/76744/elife-76744-transrepform1-v2.pdf





