Chloride-dependent mechanisms of multimodal sensory discrimination and nociceptive sensitization in Drosophila
Figures
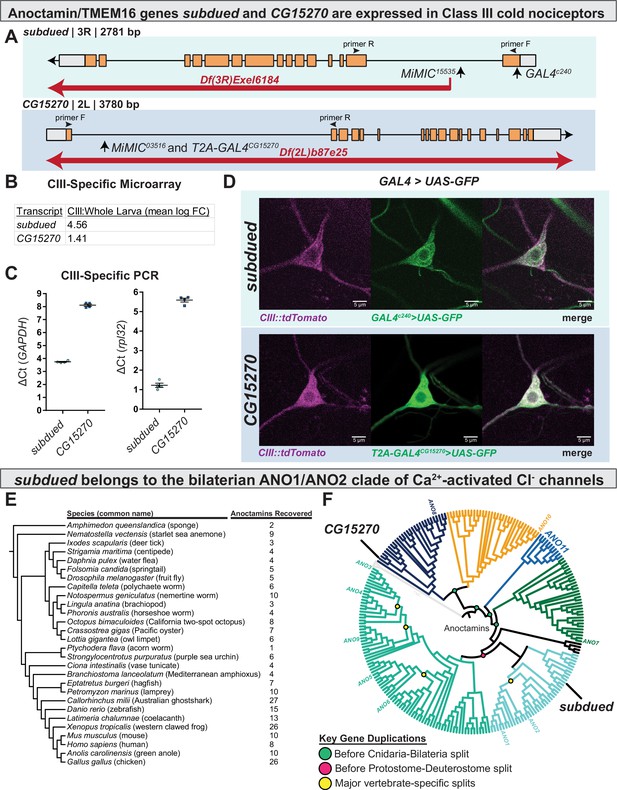
subdued and white walker (CG15270) are anoctamin/TMEM16 channels expressed in class III (CIII) neurons.
(A) Alleles, chromosomal deficiencies, and enhancer trap or T2A GAL4s used in this study, as well as approximate location for primers (F and R) used to validate mutants. (B) Class III expression of subdued and CG15270 from cell-type-specific microarray (GSE69353), expressed as mean log fold-change difference between isolated CIII and whole-larval samples. Positive values indicate enrichment/upregulation and negative values downregulation. (C) qRT-PCR validating CIII expression of subdued and CG15270 (n=4, each condition). (D) UAS-GFP driven under the control of a subdued enhancer trap, GAL4c240 or T2A-GAL4wwk, evidences that subdued and white walker are expressed in CIII neurons. (E) Species, cladogram, and number of sequences used in phylogenetic analysis. (F) Maximum likelihood phylogeny of animal anoctamins, with weak branches rearranged in a species-aware manner against cladogram in E. subdued is a member of the ANO1/2 clade of nephrozoan calcium-activated chloride channels and of the clade of metazoan anoctamins that includes mammalian ANO1/2/3/4/5/6/9. Mammalian ANO8 is homologous to CG15270. Additionally, this phylogeny evidences a separate clade of ANOs of unknown function we have deemed ANO11 (cyan).
Overlapping expression of GAL4nompC and the newly described class III (CIII)::tdTomato fusion.
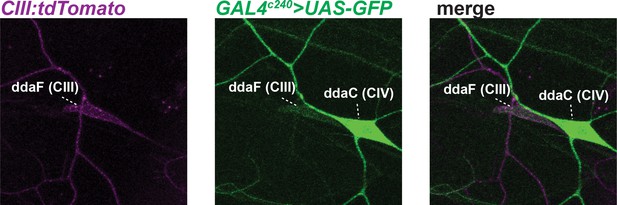
GAL4c240 driven UAS-GFP expression.
Consistent with previous reports, GAL4c240 also shows expression in class IV (CIV) neurons. CIII, class III.
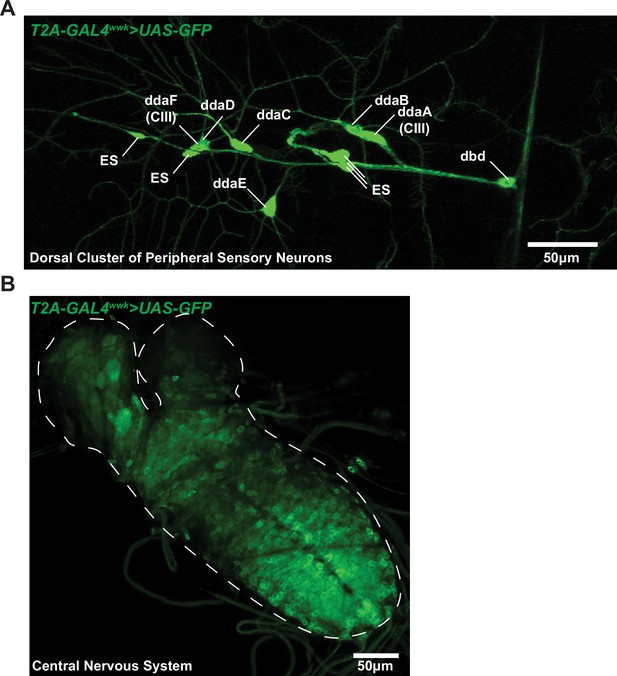
T2A-GAL4wwk driven UAS-GFP expression.
T2A-GAL4wwk does not singularly drive expression in class III (CIII) neurons but rather appears to broadly mark neural tissues. Examples: (A) T2A-GAL4wwk>GFP expression patterns in the dorsal sensory neuron cluster. "ES" indicates external sensory neurons. (B) T2A-GAL4wwk>GFP in the larval central nervous system; expression appears through the central nerve cord and in restricted regions of the brain lobes.
Maximum likelihood phylogeny of anoctamins corresponding to Figure 1.
NOTUNG-generated phylogeny of anoctamins corresponding to Figure 1.
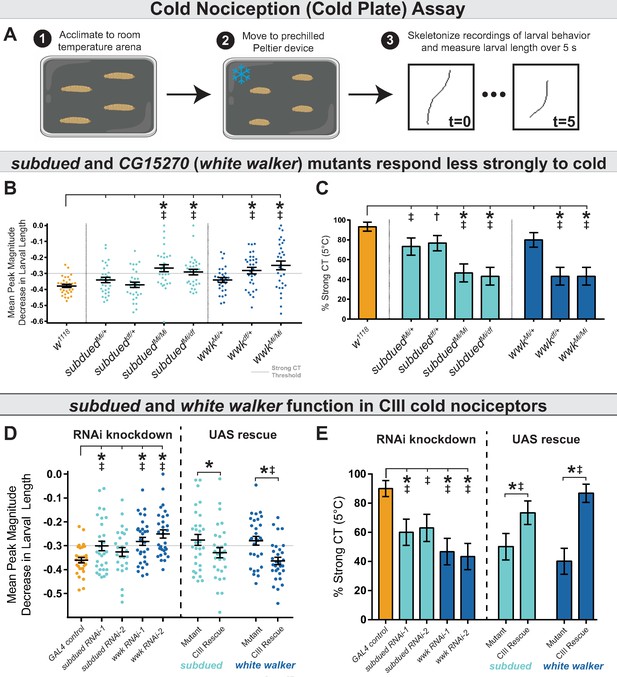
Anoctamins function in class III (CIII) neurons.
(A) For cold plate assay, larvae were acclimated to a room temperature arena before being transferred to a pre-chilled cold plate. Contraction (CT) was identified by measuring the length of skeletonized larvae over the course of chilling. (B) Mutant analysis; mean peak magnitude in larval CT. w1118 (n=30); subduedMi/+ (n=30; p=0.58; BF10=1.18); subdueddf/+ (n=30; p=1; BF10=0.28); subduedMi/Mi (n=30; p<0.001; BF10=228.63); subduedMi/df (n=30; p=0.008; BF10=737.03); wwkMi/+ (n=30; p=0.58; BF10=1.60); wwkdf/+ (n=30; p=0.003; BF10=324.11); wwkMi/Mi (n=30; p<0.001; BF10=433.18). Also see Figures 1—3. (C) Mutant analysis; % of animals which strongly CT in response to noxious cold (≥30% reduction in body length). Mutations in subdued and white walker result in a reduced percent of larvae which strongly CT in response to noxious cold (5°C). w1118 (n=30); subduedMi/+ (n=30; p=0.13; BF10=4.417); subdueddf/+ (n=30; p=0.25; BF10=2.826); subduedMi/Mi (n=30; p<0.001; BF10=461.34); subduedMi/df (n=30; p<0.001; BF10=997.24); wwkMi/+ (n=30; p=0.45; BF10=2.00); wwkdf/+ (n=30; p<0.001; BF10=997.24); wwkMi/Mi (n=30; p<0.001; BF10=997.24). (E) CIII-specific knockdown and rescue analyses; mean peak magnitude in larval CT. Knockdown: GAL4 control (n=30); subdued RNAi-1 (n=30; p=0.049; BF10=3.98); subdued RNAi-2 (n=27; p=0.437; BF10=0.78); wwk RNAi-1 (n=30; p=0.005; BF10=89.25); wwk RNAi-2 (n=30; p<0.001; BF10=6932.18). Rescue: GAL4nompC;subduedMi/Mi (mutant, n=30); GAL4nompC/UAS-subdued;subduedMi/Mi (CIII rescue, n=30; p=0.049; BF10=1.61). wwkMi/Mi;GAL419-12 (mutant, n=30); wwkMi/Mi;GAL419-12/UAS-wwk (CIII rescue, n=30; p<0.001; BF10=83.59). (D) CIII-specific knockdown and rescue analyses; % of animals which strongly CT in response to noxious cold (≥30% reduction in body length). CIII-specific knockdown (GAL419-12) of subdued and white walker results in a reduced percent of larvae which strongly CT in response to noxious cold. GAL4 control (n=30); subdued RNAi-1 (n=30; p=0.039; BF10=7.64); subdued RNAi-2 (n=27; p=0.075; BF10=4.93); wwk RNAi-1 (n=30; p=0.002; BF10=71.17); and wwk RNAi-2 (n=30; p<0.001; BF10=138.15). GAL4-UAS-mediated CIII rescue of subdued and white walker in mutant backgrounds increased cold sensitivity. GAL4nompC;subduedMi/Mi (n=30); GAL4nompC/UAS-subdued;subduedMi/Mi (n=30; p=0.031; BF10=3.57). wwkMi/Mi;GAL419-12 (n=30); wwkMi/Mi;GAL419-12/UAS-wwk (n=30; p<0.001; BF10=281.95).
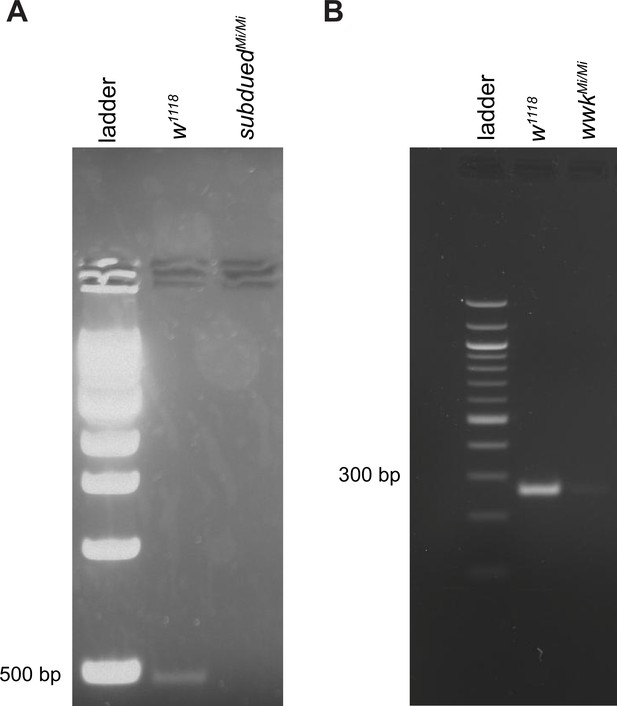
Validation of mutagenic effects of subdued and white walker MiMIC mutations.
(A) RT-PCR validating the subdued mutant. (B) RT-PCR validating the white walker (wwk) mutant. Relative to controls, which produced amplicons of the predicted sizes (see Materials and methods), homozygous Minos-mediated integration cassette (MiMIC) insertions for subdued or wwk led to the absence or severe reduction in amplicon products suggesting that these mutations are severely hypomorphic for subdued or wwk expression.
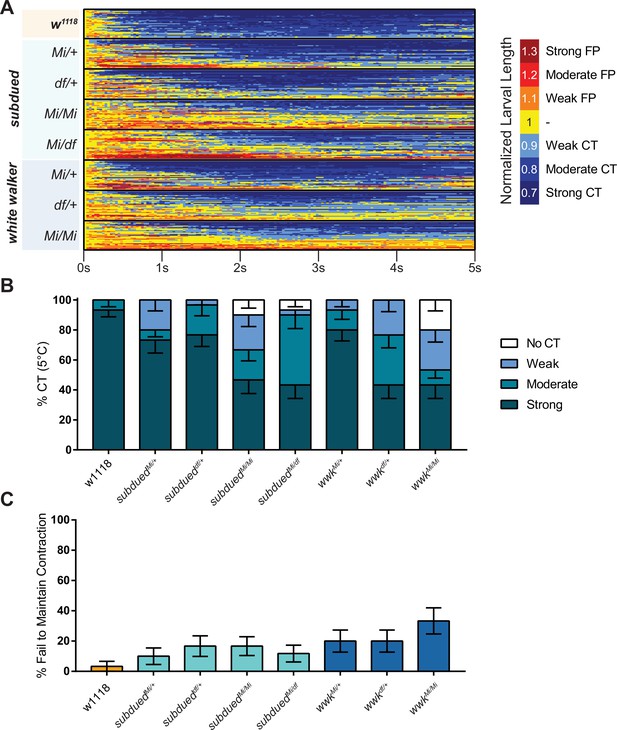
Cold nociception defects of subdued and white walker mutations.
(A) Heatmap representation of cold-evoked contraction (CT) in genetic control (w1118) and mutant conditions, where FP is flaccid paralysis (full body relaxation with an inability to locomote). N=240, n=30 for each condition. (B) % of animals from anoctamin allele/deficiency experiments performing a particular CT response, where NR is no response (<10% reduction in length), weak is ≥10% reduction in length, moderate is ≥20% reduction in length, and strong is ≥30% reduction in length; bars show proportions in % ± standard error of the proportion (SEP). (C) % ± SEP of animals which ceased any contractile behavior whatsoever (returned to normal length) within the experimental time frame.
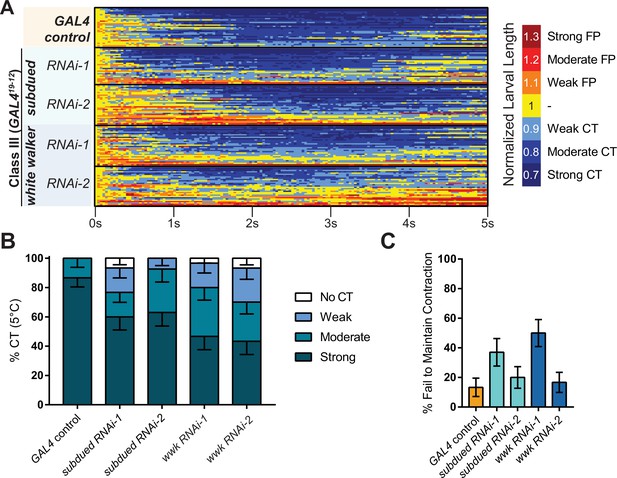
Cold nociception defects of subdued and white walker RNAi in CIII cold nociceptive neurons.
(A) Heatmap representation of cold-evoked contraction (CT) in genetic control (GAL4) and knockdown conditions, where FP is flaccid paralysis. N=147, n=30 for each condition except subdued RNAi-2, where n=27. (B) % of animals from anoctamin knockdown experiments performing a particular CT response, where NR is no response (<10% reduction in length), weak is ≥10% reduction in length, moderate is ≥20% reduction in length, and strong is ≥30% reduction in length; bars show proportions in % ± standard error of the proportion (SEP). (C) % ± SEP of animals which ceased any contractile behavior whatsoever (returned to normal length) within the experimental time frame.
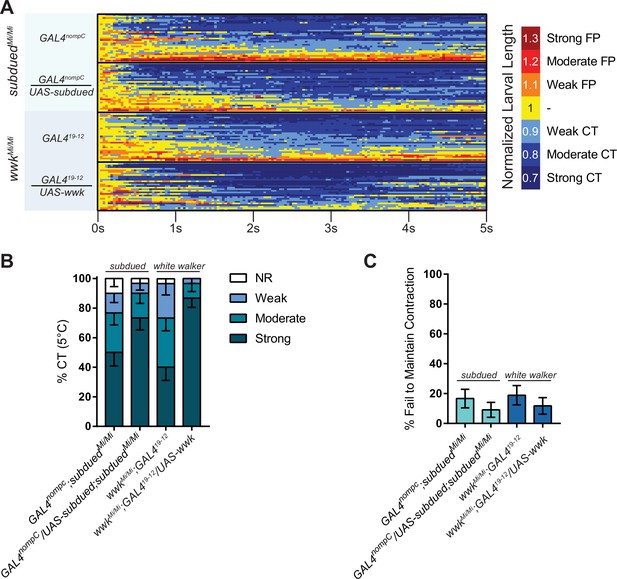
Rescue analyses of cold nociceptive defects for subdued and white walker.
(A) Heatmap representation of cold-evoked contraction (CT) in mutant GAL4 control and GAL4-UAS-mediated rescue conditions, where FP is flaccid paralysis. N=120, n=30 for each condition. (B) % of animals from rescue experiments performing a particular CT response, where NR is no response (<10% reduction in length), weak is ≥10% reduction in length, moderate is ≥20% reduction in length, and strong is ≥30% reduction in length; bars show proportions in % ± standard error of the proportion (SEP). (C) % ± SEP of animals which ceased any contractile behavior whatsoever (returned to normal length) within the experimental time frame.
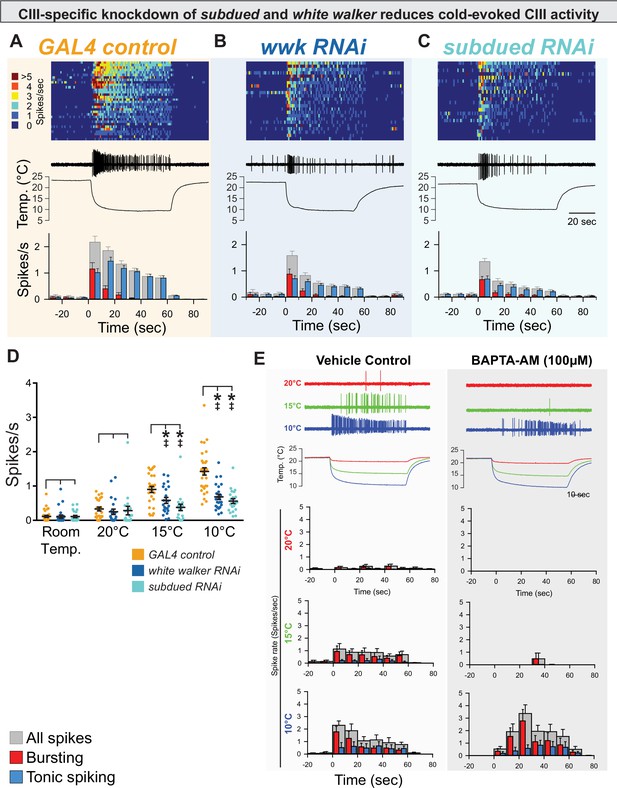
CIII-specific knockdown of subdued and white walker reduces cold-evoked neural activity.
(A, B, C) Top: Heatmap representation of cold-evoked class III (CIII) activity (10°C), with each line representing an individual sample prep. Middle: Representative traces of cold-evoked neural activity over graph of temperature ramp. Knockdown of subdued and white walker results in decreased cold-evoked firing, particularly in the steady-state tonic firing at steady-state temperature. Bottom: Representation of average frequency from population binned by 10 s. Red and blue bars show the proportion of bursting vs tonic spiking activity. Knockdown of subdued and white walker results in decreased tonic firing. (D) CIII-specific knockdown of subdued and white walker results in decreased cold-evoked firing frequency at temperature ramps to 15 and 10°C. Room temp (N=78): GAL4 control (n=32); white walker RNAi (n=24; p>0.99; BF10=0.274); subdued RNAi (n=22; p≥0.99; BF10=0.279). 20°C (N=65): GAL4 control (n=25); white walker RNAi (n=22; p=0.69; BF10=0.39); subdued RNAi (n=18; p=0.90; BF10=0.32). 15°C (N=68): GAL4 control (n=27); white walker RNAi (n=22; p=0.0082; BF10=3.64); subdued RNAi (n=19; p<0.001; BF10=92.73). 10°C (N=78): GAL4 control (n=32); white walker RNAi (n=24; p<0.001; BF10=3310.09); subdued RNAi (n=22; p<0.001; BF10=44346.95). (E) Cold-evoked CIII activity in the presence of vehicle (left; n=6) or BAPTA-AM (right; n=6).
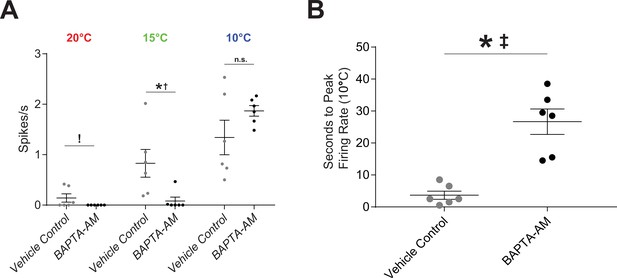
Application of BAPTA results in reduced cold sensitivity; n=6 for each condition.
(A) Cold-evoked neural activity was completely ablated at 20°C (exclamation point indicating inability to compare by t-test due to 0 variance), severely reduced at 15°C (p=0.026; BF10=2.77), and not affected at 10°C (p=0.17; BF10=0.90). (B) At 10°C, it took more time to reach peak firing rate (p<0.001; BF10=83.28).
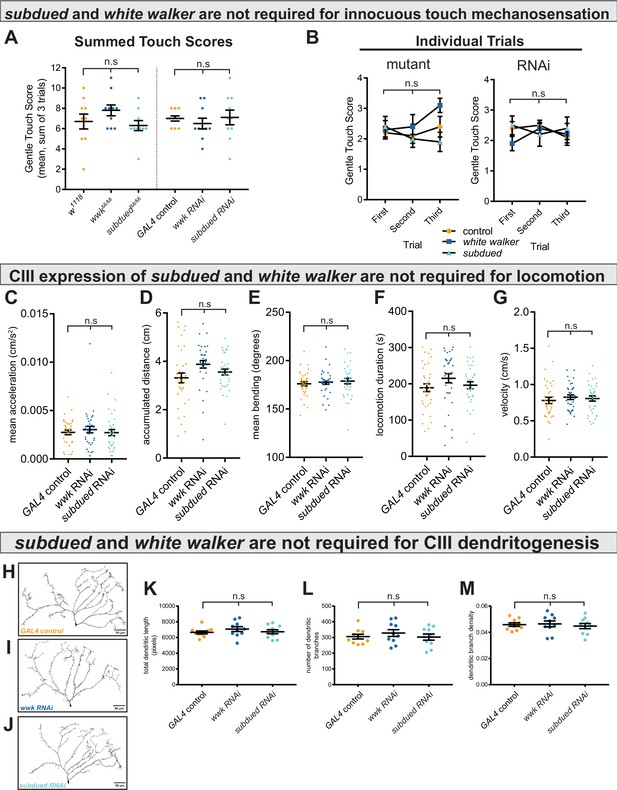
subdued and white walker are not required for innocuous mechanosensation, locomotion, or class III (CIII) dendritogenesis.
(A) There is no difference in innocuous touch mechanosensation (sum Kernan touch scores, three trials for each sample) in either subdued or white walker mutants (n=10 for each condition; p=0.20; BF10=0.62) or GAL4-UAS-mediated CIII-knockdown (n=10 for each condition; p=0.70; BF10=0.27). (B) For each genotype, there were no within-subjects effects in innocuous touch sensation across trials. w1118 (n=10; p=0.69; BF10=0.27); wwkMi/Mi (n=10; p=0.19; BF10=0.85); subduedMi/Mi (n=10; p=0.42; BF10=0.42); GAL4 control (n=10; p=0.39; BF10=0.50); wwk RNAi (n=10; p=0.37; BF10=0.47); subdued RNAi (n=10; p=0.80; BF10=0.25). (C–G) There are no differences in acceleration (p=0.59; BF10=0.13), accumulated distance (p=0.056; BF10=0.96), bending behavior (p=0.71; BF10=0.025), locomotion duration (p=0.22; BF10=0.30), or average velocity (p=0.69; BF10=0.12), between control and knockdown (ncontrol = 36, nwwk = 35, and nsubdued = 36). (H–J) Representative neural images of CIII ddaF neuron dendritic arbors under control and knockdown conditions. (K–M) There is no difference in total dendritic length (p=0.48; BF10=0.34), number of branches (p=0.70; BF10=0.27), or dendritic branch density (p=0.81; BF10=0.24) between control and knockdown (n=10 for each condition).
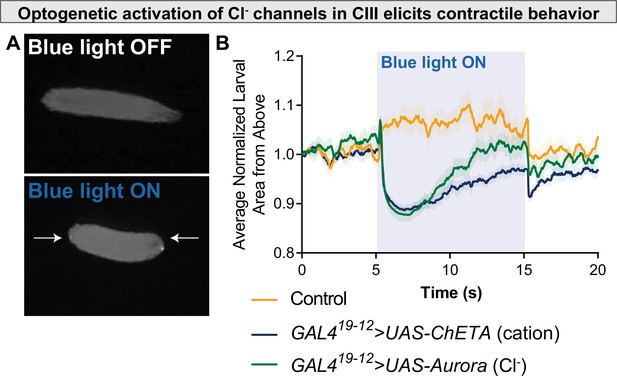
Optogenetic activation of chloride channels in CIII neurons elicits contractile behavior.
(A) Optogenetic activation of class III (CIII) >Aurora activates CIII neurons, resulting in contraction (CT) behavior. (B) CT behavior represented as larval area from above. Blue light activation of the Ca2+ channel ChETA and the Cl− channel Aurora causes a rapid reduction in normalized larval area from above, an indication of CT behavior.
Behavior elicited by blue light in a control larva.
Behavior elicited by blue light in a larva expressing ChETA in class III neurons.
Behavior elicited by blue light in a larvae expression Aurora in class III neurons.
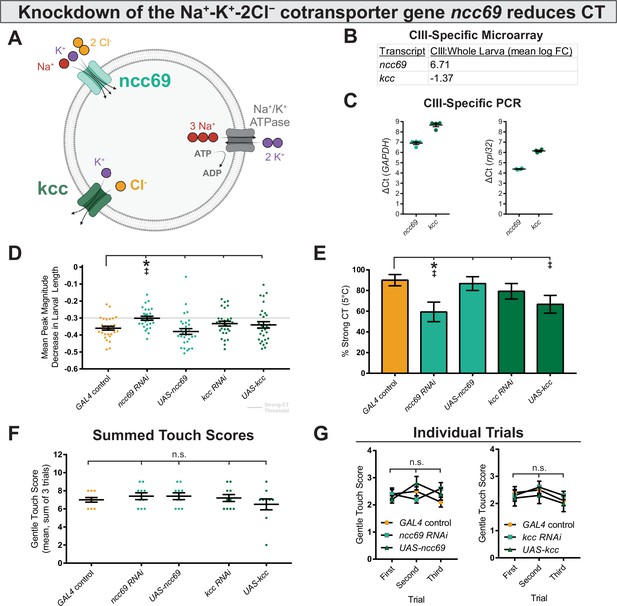
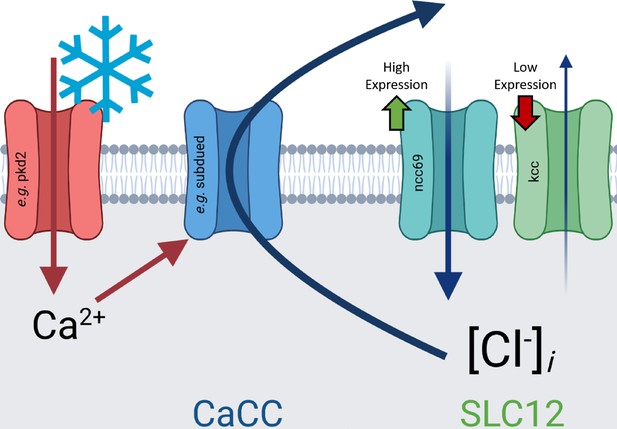
Genetically perturbing Cl− homeostasis affects cold sensitivity, but not touch sensitivity.
(A) Schematic of neural Cl− homeostasis as modulated by the secondary active cotransporters ncc69 and kcc. (B) Class III (CIII) expression of ncc69 and kcc from cell-type specific microarray (GSE69353), expressed as mean log fold-change difference between isolated CIII and whole-larval samples. Positive values indicate enrichment/upregulation and negative values downregulation. (C) qRT-PCR validating CIII expression of ncc69 and kcc (n=4, each condition). (D) Mean peak magnitude in larval contraction. GAL4 control (n=30); ncc69 RNAi (n=29; p=0.025; BF10=46.38); ncc69 OE (n=30; p=0.77; BF10=0.38); kcc RNAi (n=29; p=0.51; BF10=0.68); kcc OE (n=30; p=0.76; BF10=0.37). (E) % of animals which strongly contract (CT) in response to noxious cold (≥30% reduction in body length). CIII-specific knockdown (GAL419-12) of ncc69 results in a reduced percent of larvae which strongly CT in response to noxious cold. GAL4 control (n=30); ncc69 RNAi (n=29; p=0.038; BF10=7.96); ncc69 OE (n=30; p=1; BF10=0.58); kcc RNAi (n=29; p=0.91; BF10=0.99); kcc OE (n=30; p=0.13; BF10=3.29). (F) Average summed touch scores. (G) Average touch scores across trials.
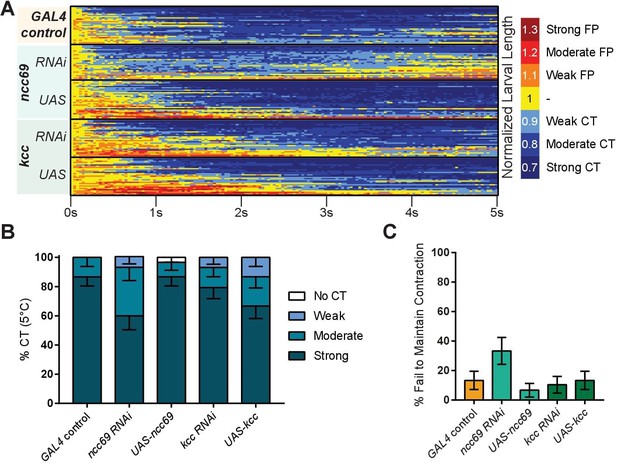
Cold nociception defects of ncc69 and kcc RNAi in CIII cold nociceptive neurons.
(A) Heatmap representation of cold-evoked ontraction (CT) in genetic control (GAL4), knockdown, and overexpression conditions, where FP is flaccid paralysis. N=148; control n=30, ncc69 RNAi n=29, ncc69 OE n=30, kcc RNAi n=29, kcc OE n=30. (B) % of animals from SLC12 manipulation experiments performing a particular CT response, where NR is no response (<10% reduction in length), weak is ≥10% reduction in length, moderate is ≥20% reduction in length, and strong is ≥30% reduction in length; bars show proportions in % ± standard error of the proportion (SEP). (C) % ± SEP of animals which ceased any contractile behavior whatsoever (returned to normal length) within the experimental time frame.
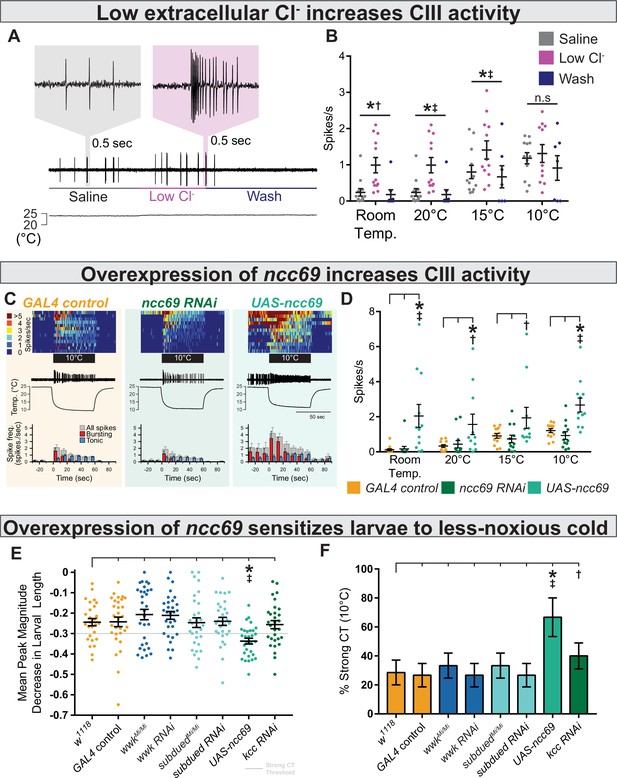
Decreasing extracellular Cl− and overexpression of ncc69 causes nociceptor and behavioral sensitization.
(A) Extracellular application of low Cl− saline to fileted electrophysiology preps causes spontaneous bursting activity. At room temperature, neurons are largely silent but may show occasional, low-frequency spiking (likely associated with mechanosensation from saline flow). (B) The presence of low Cl− saline causes spontaneous firing and sensitizes class III (CIII) neurons to cooling. Room temp: saline (n=12); low Cl− (n=12); wash (n=8); p=0.003, BF10=1.89. 20°C: saline (n=12); low Cl− (n=12); wash (n=8); p=0.003, BF10=66.38. 15°C: saline (n=12); low Cl− (n=12); wash (n=7); p=0.011, BF10=14.51. 10°C: saline (n=12); low Cl− (n=11); wash (n=7); p=0.25, BF10=0.75. (C) Top: Heatmap representation of cold-evoked CIII activity, with each line representing an individual sample prep. Middle: Representative traces of cold-evoked neural activity over graph of temperature ramp. Overexpression of ncc69 results in spontaneous nociceptor activity and increased cold sensitivity. Bottom: Representation of average spike frequency from population binned by 10 s. Red and blue bars show the proportion of bursting vs tonic spiking activity. (D) Overexpression of ncc69 causes spontaneous neural activity and sensitizes neurons to cooling. Room temp: GAL4 control (n=13); ncc69 RNAi (n=12; p=0.99; BF10=0.22); UAS-ncc69 (n=12; p<0.001; BF10=7.86). 20°C: GAL4 control (n=13); ncc69 RNAi (n=12; p=0.96; BF10=0.40); UAS-ncc69 (n=11; p=0.020; BF10=2.23). 15°C: GAL4 control (n=13); ncc69 RNAi (n=12; p=0.90; BF10=0.26); UAS-ncc69 (n=11; p=0.062; BF10=1.17). 10°C: GAL4 control (n=13); ncc69 RNAi (n=12; p=0.77; BF10=0.60); UAS-ncc69 (n=11; p=0.0042; BF10=25.18). (E) Mean peak magnitude in larval contraction in response to noxious cold (10°C). w1118 (n=28); GAL4 control (n=30; p=1; BF10=0.27); wwkMi/Mi (n=30; p=1; BF10=0.48); wwk RNAi (n=30; p=1; BF10=0.52); subduedMi/Mi (n=30; p=1; BF10=0.27); subdued RNAi (n=30; p=1; BF10=0.27); UAS-ncc69 (n=30; p=0.037; BF10=199.79); kcc RNAi (n=30; p=1; BF10=0.29). (F) % of animals which strongly contract (CT) in response to noxious cold (10°C). CIII-specific (GAL419-12) overexpression of ncc69 results in an increased percentage of strong CT in response to less-noxious cold. w1118 (n=28); GAL4 control (n=30; p=1; BF10=0.46); wwkMi/Mi (n=30; p=1; BF10=0.69); wwk RNAi (n=30; p=1; BF10=0.46); subduedMi/Mi (n=30; p=1; BF10=0.69); subdued RNAi (n=30; p=1; BF10=0.46); UAS-ncc69 (n=30; p=0.026; BF10=28.79); kcc RNAi (n=30; p=1; BF10=1.07).
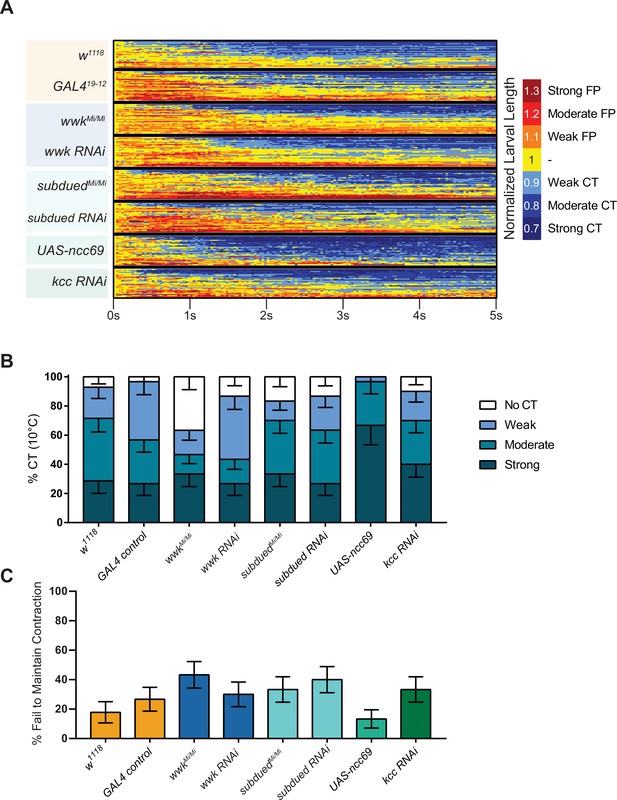
Cold nociception defects of subdued, white walker, ncc69, and kcc at less-noxious cold temperature.
(A) Heatmap representation of cold-evoked contraction (CT) in genetic control (w1118 or GAL419-12), knockdown, and overexpression conditions, where FP is flaccid paralysis. N=239, n=30 for each condition except w1118, where n=28. (B) % of animals from SLC12 manipulation experiments performing a particular CT response, where NR is no response (<10% reduction in length), weak is ≥10% reduction in length, moderate is ≥20% reduction in length, and strong is ≥30% reduction in length; bars show proportions in % ± standard error of the proportion (SEP). (C) % ± SEP of animals which ceased any contractile behavior whatsoever (returned to normal length) within the experimental time frame.