Botulinum neurotoxin accurately separates tonic vs. phasic transmission and reveals heterosynaptic plasticity rules in Drosophila
Figures
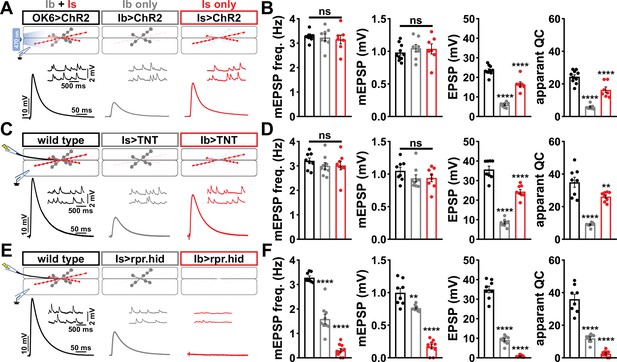
Suboptimal approaches for selectively isolating tonic and phasic neurotransmission at the Drosophila neuromuscular junction (NMJ).
(A) Schematic of recording configuration and representative miniature excitatory postsynaptic potential (mEPSP) and excitatory postsynaptic potential (EPSP) traces illustrating that overexpression of ChR2T159C in MN-Ib or -Is enables optically evoked EPSP events from either MN-Ib or -Is NMJs. Genotypes: OK6>ChR2 (w;OK6-GAL4/UAS-ChR2T159C;+); Ib>ChR2 (w;UAS- ChR2T159C/+;dHB9-GAL4/+); Is>ChR2 (w;UAS- ChR2T159C/+;R27E09-GAL4/+). (B) Quantification of mEPSP frequency, amplitude, EPSP, and apparent quantal content values in the indicated genotypes in (A). Note that because input-specific mEPSP values cannot be determined using this optogenetic approach, inaccurate quantal content values are shown by simply dividing the EPSP values by the same averaged mEPSP values. (C) Schematic and representative traces following selective expression of tetanus toxin (TNT) in MN-Ib or -Is. Genotypes: wild-type (w1118); Is>TNT (w;+;R27E09-GAL4/UAS-TNT); Ib>TNT (w;+;dHB9-GAL4/UAS-TNT). (D) Quantification of the indicated values of the genotypes shown in (C), with the same inaccuracies in determining quantal content. (E) Schematic and representative traces following input-specific expression of the pro-apoptotic genes reaper (rpr) and head involution defective (hid). Note that MN-Is NMJs are completely absent following MN-Ib ablation. Genotypes: wild-type (w); Is>rpr.hid (UAS-rpr.hid/+;+; R27E09-GAL4/+); Ib>rpr.hid (UAS-rpr.hid/+;+; dHB9-GAL4/+). (F) Quantification of the indicated values of the genotypes shown in (E). Error bars indicate ± SEM. ****p<0.0001; ***p<0.001; **p<0.01; ns, not significant. Additional statistical details are shown in Supplementary file 2.
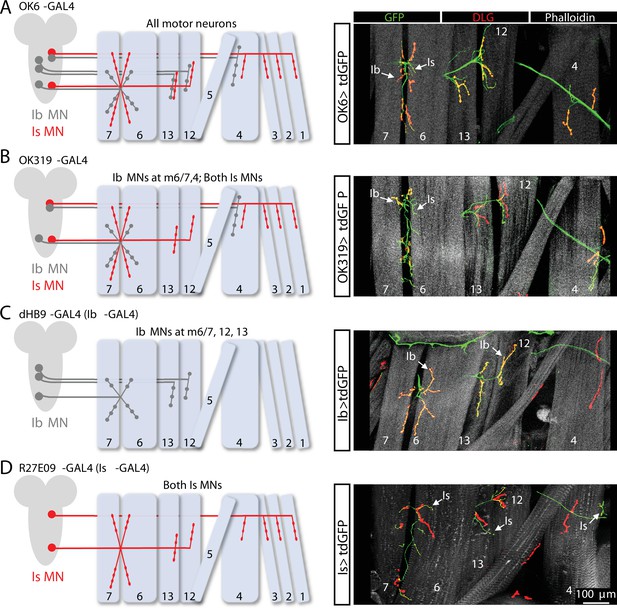
Motor neuron (MN)-specific GAL4 expression at the Drosophila neuromuscular junction (NMJ).
(A) Schematic of the Ib and Is MNs that innervate the indicated muscles. The pan-MN driver OK6-GAL4 is expressed in all larval MNs. Right: images of larval body wall muscles (labeled by Phalloidin) innervated by Ib and Is MNs (labeled with GFP; OK6>tdGFP: w;OK6-GAL4/UAS-CD4::tdGFP;+) and the postsynaptic density marker DLG to distinguish Ib vs. Is NMJs. (B) Schematic and images of the Ib and Is MNs targeted by OK319-GAL4, which is expressed in two Is MNs and the two Ib MNs that innervate muscles 6/7 and 4 (OK319>tdGFP: w;OK319-GAL4/UAS-CD4::tdGFP;+). (C) Schematic and images of the two Ib MNs targeted by the MN-Ib-specific driver dHB9-GAL4, which is expressed in the three MN-Ibs that innervate muscles 6/7, 12, and 13 (Ib>tdGFP: w;UAS-CD4::tdGFP/+;dHb9-GAL4/+). (D) Schematic and images of the two Is MNs targeted by the MN-Is-specific driver R27E09-GAL4, which is expressed in both Is MNs (Is>tdGFP: w;UAS-CD4::tdGFP/+;R29E07-GAL4/+).
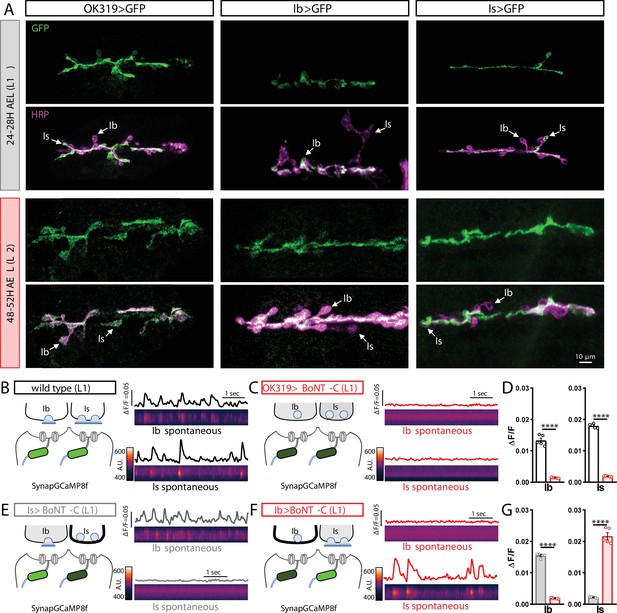
MN-Is and MN-Ib drivers are expressed by early first-instar larval stages and block neurotransmission when crossed to botulinum neurotoxin (BoNT-C).
(A) Representative images of muscle 6/7 neuromuscular junctions (NMJs) immunostained with anti-GFP and anti-HRP in OK319>GFP (w;OK319-GAL4/+;UAS-CD4::tdGFP/+), Ib>GFP (w;UAS-CD4::tdGFP/+;dHb9-GAL4/+), and Is>GFP (w;UAS-CD4::tdGFP/+;R29E07-GAL4/+) at early L1 (24–28H AEL) and early L2 (48–52H AEL) stages. Note that in all three genotypes, GFP immunostaining is observed in MN-Is and/or MN-Ib by early first-instar stages. (B, C) Postsynaptic Ca2+ imaging of spontaneous neurotransmission with SynapGCaMP8f at early first-instar (24–28H AEL) NMJs of wild-type (w;MHC>GCaMP8 f/+;+) (B) or BoNT-C (w;MHC>GCaMP8f/OK319-GAL4;+/UAS-BoNT-C) (C). (D) Quantification of GCaMP8f signals at MN-Ib or MN-Is NMJs in the indicated genotypes. Note that synaptic activity is silenced at both Is and Ib NMJs after BoNT-C silencing. (E, F) SynapGCaMP8f imaging following selective silencing of MN-Is (E) or MN-Ib (F) at early first-instar NMJs. Note that while robust spontaneous activity is apparent at the unsilenced input, all activity is blocked at the input expressing BoNT-C. (G) Quantification of GCaMP signals in (E, F). Error bars indicate ± SEM. ****p<0.0001.
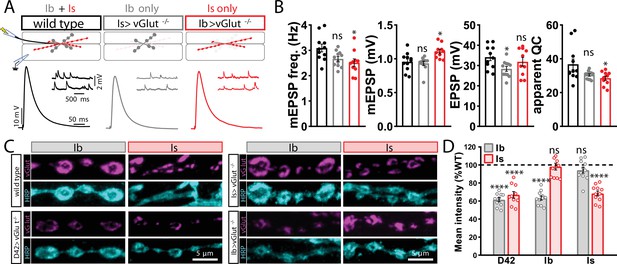
vGlut expression persists despite conditional knockout in motor neurons.
(A) Schematic and representative miniature excitatory postsynaptic potential (mEPSP) and excitatory postsynaptic potential (EPSP) traces illustrating transmission persists in conditional knockout of vGlut in MN-Ib or -Is. Genotypes: wild-type (w1118); Is>vGlut-/- (w;vGlutSS1/B3RT-vGlut-B3RT;UAS-B3/R7E09-GAL4); Ib>vGlut-/- (w;vGlutSS1/B3RT-vGlut-B3RT;UAS-B3/dHB9-GAL4). (B) Quantification of mEPSP frequency, amplitude, EPSP, and quantal content in the indicated genotypes. (C) Representative images of MN-Ib and -Is NMJs immunostained with anti-vGlut, and the neuronal membrane marker HRP in the indicated genotypes. D42>vGlut-/- (pan-motor neuron; w;vGlutSS1/B3RT-vGlut-B3RT;UAS-B3/D42-GAL4)>vGlut-/-. (D) Quantification of mean fluorescence intensity of vGlut in the indicated genotypes normalized to wild-type values. ****p<0.0001; *p<0.05; ns, not significant. Absolute values for normalized data and additional statistical details are summarized in Supplementary file 2.
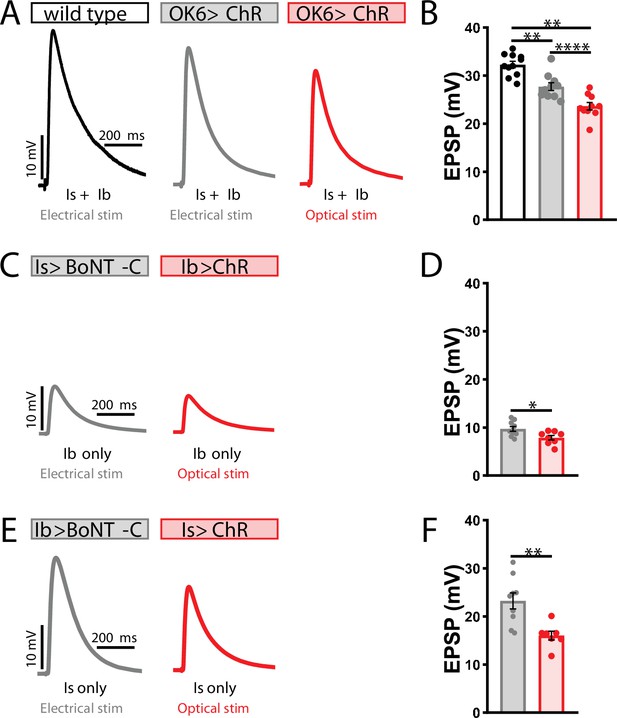
Electrophysiological differences between optogenetic and electrical stimulation at the Drosophila neuromuscular junction (NMJ).
(A) Representative excitatory postsynaptic potential (EPSP) traces demonstrating that ChR expression in motor neurons reduces evoked transmission using either electrical or optical stimulation. Genotype: OK6>ChR2T159C (w;OK6-GAL4/UAS-ChR2T159C;+). (B) Quantification of EPSP amplitude in the indicated genotypes and stimulation. (C–F) Representative EPSP traces and quantification showing that selective ChR expression in MN-Is (C, D) or -Ib (E, F) similarly reduces transmission compared to input-specific silencing by botulinum neurotoxin (BoNT-C) expression. Genotypes: Is>BoNT-C (w;+;R29E07-GAL4/UAS-BoNT-C); Ib>ChR2T159C (w;dHB9-GAL4/UAS-ChR2T159C;+); Ib>BoNT-C (w;+;dHB9-GAL4/UAS-BoNT-C); Is>ChR2T159C (w;R29E07-GAL4/UAS-ChR2T159C;+). ****p<0.0001; **p<0.01; *p<0.05. Additional statistical details are summarized in Supplementary file 2.
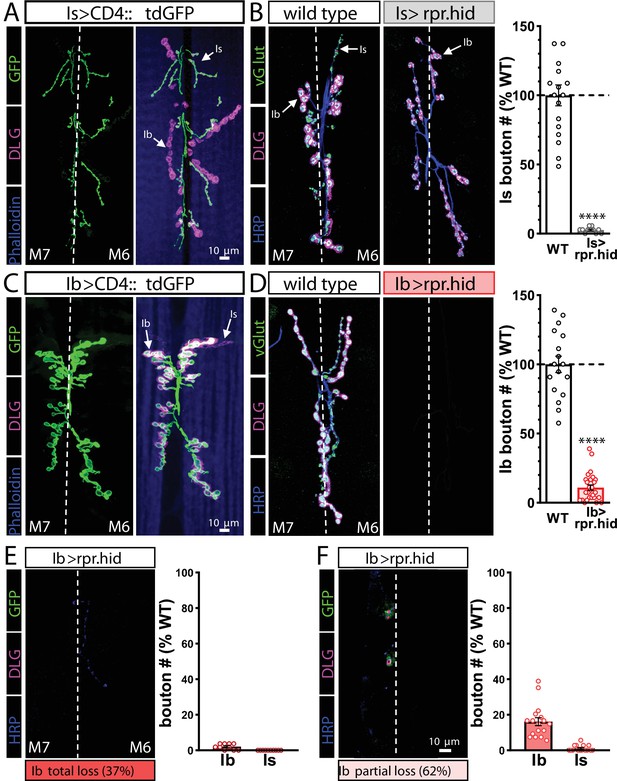
Genetic ablation of MN-Ib abolishes innervation by MN-Is on the same target.
(A) Representative muscle 6/7 neuromuscular junction (NMJ) images showing MN-Is labeled by CD4::tdGFP (Is>CD4::tdGFP: w;UAS-CD4::tdGFP/+;R29E07-GAL4/+), co-stained with anti-DLG and -Phalloidin. (B) Representative NMJ images of MN-Is ablation by rpr.hid expression (Is>rpr.hid; rpr.hid/+;+;R29E07-GAL4/+) co-stained with anti-vGlut, -DLG, and -HRP. Right: quantification of MN-Is bouton number normalized to wild-type values in the indicated genotype, confirming full MN-Is ablation. (C) Representative NMJ images of MN-Ib labeled with GFP expression (Ib>CD4::tdGFP: w;UAS-CD4::tdGFP/+;dHb9-GAL4/+), co-stained with anti-DLG and -Phalloidin. (D) Representative NMJ images of wild-type and MN-Ib ablation (Ib>rpr.hid; rpr.hid/+;+;dHB9-GAL4/+) immunostained with anti-vGlut, -DLG, and -HRP, demonstrating that ablation of MN-Ib leads to loss of MN-Is innervation. Right: quantification of MN-Ib bouton number normalized to wild-type values in the indicated genotypes. (E, F) Representative NMJ images of MN-Ib ablation illustrating two outcomes across 27 samples. Although MN-Is innervation was completely lost in all samples, 37% of NMJ samples exhibited a total loss of MN-Ib innervation as well (consistent with a failure to evoke an excitatory postsynaptic potential [EPSP] response before staining) (E). In contrast, 62% of cases were found to retain partial MN-Ib innervation (F). Quantification of bouton numbers of MN-Ib and -Is boutons in each condition normalized to wild-type values. ****p<0.0001. Absolute values for normalized data and additional statistical details are summarized in Supplementary file 2.
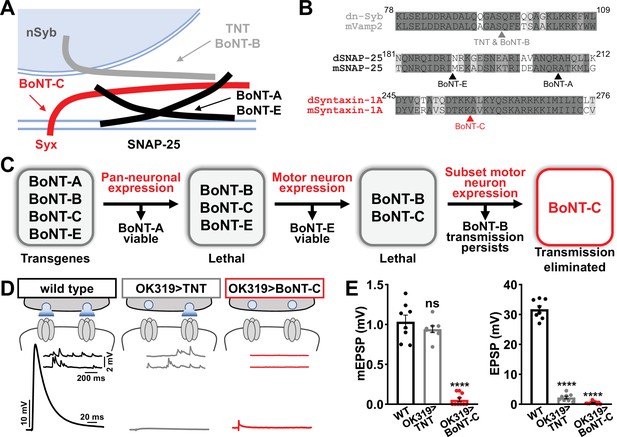
Botulinum neurotoxin (BoNT-C) eliminates both spontaneous and evoked transmission.
(A) Schematic of synaptic SNARE proteins that are targets for enzymatic cleavage by the tetanus (TNT) and botulinum (BoNT) neurotoxins. (B) Amino acid sequence alignments of the cleavage sites for the indicated TNT and BoNT toxins in the mouse and Drosophila SNARE components synaptobrevin (Vamp2 and neuronal synaptobrevin [dN-Syb]), SNAP25, and Syntaxin (Syntaxin-1A). Arrows illustrate the conserved cleavage sites of TNT, BoNT-A, BoNT-B, BoNT-C, and BoNT-E. (C) BoNT screening flowchart: four BoNT lines were first tested for lethality when expressed using the pan-neuronal c155-GAL4 driver, then similarly tested when crossed to the motor neuron-specific OK6-GAL4 driver, resulting in only BoNT-B and BoNT-C causing lethality. These transgenes were then crossed to the OK319-GAL4 driver, which expressed in only a subset of motor neurons, and electrophysiological recordings revealed that while transmission persisted in BoNT-B, transmission was completely blocked in BoNT-C. (D) Schematic and representative electrophysiological traces illustrating that while miniature transmission persists at neuromuscular junctions (NMJs) poisoned by TNT expression, transmission was completely blocked at NMJs expressing BoNT-C. (E) Quantification of miniature excitatory postsynaptic potential (mEPSP) and excitatory postsynaptic potential (EPSP) amplitudes in the indicated genotypes: wild-type (w); OK319>TNT (w;OK319-GAL4/UAS-TNT;+); OK319>BoNT-C (w;OK319-GAL4/+;UAS- BoNT-C/+). Error bars indicate ± SEM. ****p<0.0001; ns, not significant. Additional BoNT screening results and statistical details are shown in Supplementary file 1 and Supplementary file 2.
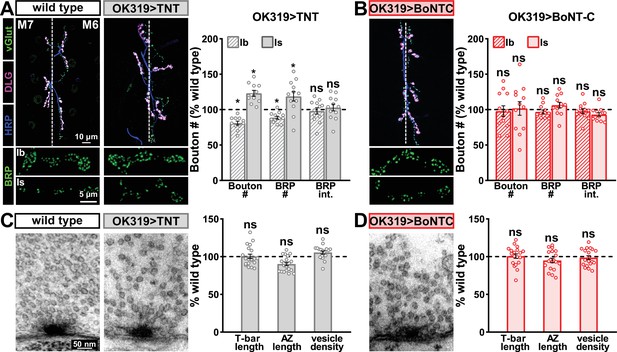
Botulinum neurotoxin (BoNT-C) expression does not perturb presynaptic growth or structure.
(A) Representative muscle six neuromuscular junction (NMJ) images immunostained with anti-vGlut, -DLG, and -HRP at wild-type and NMJs expressing tetanus toxin (TNT); BRP immunostaining in individual boutons is shown below. Right: quantification of bouton number, BRP number/NMJ, and mean BRP fluorescence intensity at TNT NMJs of MN-Ib or -Is expressing normalized to wild-type values. (B) Representative images and quantification of NMJs silenced by BoNT-C expression as described in (A). Note that in contrast to TNT, BoNT-C expression does not change bouton or BRP numbers at NMJs of either MN-Ib or -Is. (C) Representative electron micrographs of wild-type and TNT NMJs showing synaptic vesicles and active zone structures. Right: quantification of T-bar length (µm), active zone length (µm), and synaptic vesicle density (#/µm2) normalized to wild-type values in the indicated genotypes. (D) Representative electron micrographs and analysis of BoNT-C NMJs as presented in (C). Note that no significant differences are observed compared to wild-type values. Error bars indicate ± SEM. *p<0.05; ns, not significant. Absolute values for normalized data and additional statistical details are summarized in Supplementary file 2.
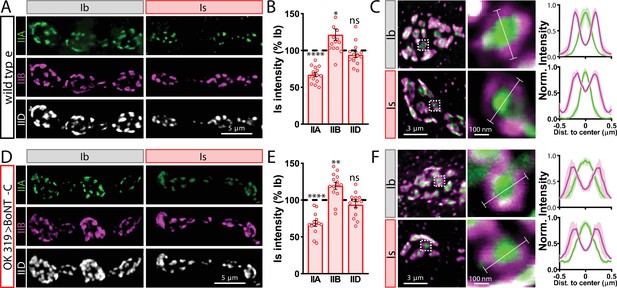
Tonic vs. phasic activity patterns do not specialize postsynaptic GluR fields.
(A) Representative images of boutons from MN-Ib and -Is neuromuscular junctions (NMJs) immunostained with anti-GluRIIA, -GluRIIB, and -GluRIID in wild-type. (B) Quantification of the mean fluorescence intensity of each GluR subunit at MN-Is NMJs normalized as a percentage of those at MN-Ib. MN-Is NMJs exhibit a significant decrease in GluRIIA-containing GluRs, an increase in GluRIIB-containing GluRs, and no significant change in total GluR levels (indicated by the common subunit GluRIID) compared to GluR fields at MN-Ib NMJs. (C) High-magnification image of individual NMJs imaged as in (A). Averaged fluorescence line profiles show GluRIIA and GluRIIB normalized to peak fluorescence values across 10 receptor fields in MN-Ib or -Is NMJs. Note that peak fluorescence of GluRIIA is at the center of the GluR field, while peak fluorescence of GluRIIB is located more peripherally. The white line indicates the line profile region of interest (ROI). (D–F) Similar analysis of MN-Ib and -Is NMJs silenced by botulinum neurotoxin (BoNT-C) expression (OK319>BoNT-C). Similar GluR levels and localizations are observed, indicating that tonic vs. phasic patterns of transmission, and indeed glutamate release itself, are not required to establish the input-specific specialization of GluR fields. Error bars indicate ± SEM. ****p<0.0001; **p<0.01; *p<0.05; ns, not significant. Absolute values for normalized data and additional statistical details are summarized in Supplementary file 2.
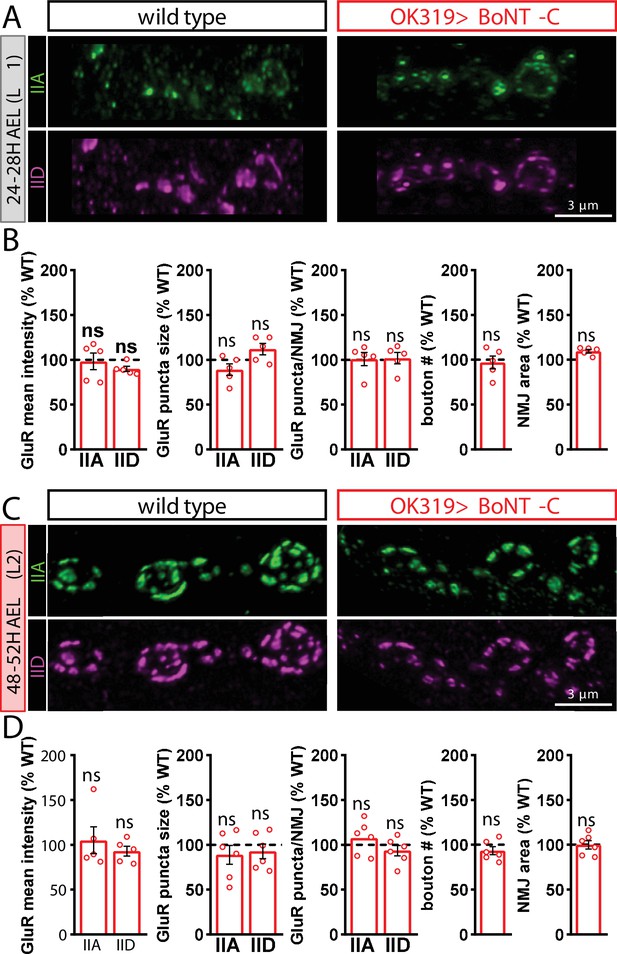
Botulinum neurotoxin (BoNT-C) silencing does not delay postsynaptic maturation.
(A) Representative images of wild-type and OK319>BoNT-C early first-instar neuromuscular junctions (NMJs) (24–28H AEL) immunostained with anti-GluRIIA and anti-GluRIID. (B) Quantification of GluR mean fluorescence intensity, puncta size, puncta number, bouton number, and NMJ area in OK319>BoNT-C normalized as a percentage of wild-type values. No significant differences are observed. (C, D) Same immunostaining and quantification as (A, B) but in second-instar stages (48–52H AEL). ns, not significant. Absolute values for normalized data and additional statistical details are summarized in Supplementary file 2.
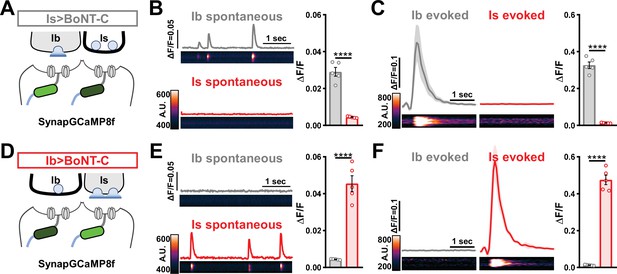
Postsynaptic Ca2+ signals are selectively eliminated by input-specific botulinum neurotoxin (BoNT-C) expression.
(A) Schematic depicting silencing of MN-Is transmission by selective expression of BoNT-C in MN-Is. The postsynaptic Ca2+ indicator SynapGCaMP8f is indicated in the postsynaptic compartment, which should receive transmission only at MN-Ib neuromuscular junctions (NMJs). (B) Representative images and traces of spontaneous postsynaptic Ca2+ transients at MN-Ib and -Is NMJs using the new postsynaptic GCaMP8f indicator in Is>BoNT-C (w;MHC>GCaMP8 f /+;R29E07-GAL4/UAS-BoNT-C). Right: quantification of averaged transients, confirming that synaptic Ca2+ events are selectively eliminated at MN-Is NMJs but intact at MN-Ib. (C) Averaged traces and images of evoked postsynaptic Ca2+ transients at MN-Ib and -Is NMJs. Right: quantification of averaged evoked transients, confirming that evoked synaptic Ca2+ events are selectively eliminated at MN-Is NMJs but intact at MN-Ib. (D) Schematic depicting silencing of MN-Ib transmission by selective expression of BoNT-C in MN-Ib. (E, F) Similar analysis as shown in (B, C) at MN-Ib-silenced NMJs (Ib>BoNT-C; w;MHC>GCaMP8 f /+;dHB9-GAL4/UAS-BoNT-C). Synaptic Ca2+ events are selectively eliminated at MN-Ib NMJs but intact at MN-Is. Error bars indicate ± SEM. ****p<0.0001. Additional statistical details are summarized in Supplementary file 2.
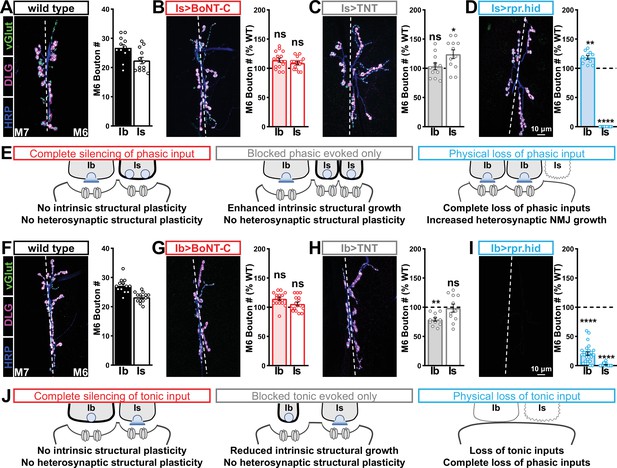
Selective silencing by botulinum neurotoxin (BoNT-C) does not induce heterosynaptic structural plasticity.
(A–D) Representative neuromuscular junction (NMJ) images immunostained with anti-vGlut, -DLG, and -HRP in wild-type (A); selective silencing of MN-Is (Is>BoNT-C; w;+;R29E07-GAL4/UAS-BoNT-C) (B); tetanus toxin (TNT) expression in MN-Is (Is>TNT; w;UAS-TNT/+;R29E07-GAL4/+) (C); and ablation of MN-Is (Is>rpr.hid; rpr.hid/+;R29E07-GAL4/+) (D). Quantification of MN-Ib and MN-Is bouton number in wild-type and each condition normalized to wild-type values is shown on the right. Note that while no heterosynaptic structural plasticity is observed in bouton number Is>BoNT-C and Is>TNT, a compensatory heterosynaptic increase is found in Is>rpr.hid. (E) Schematic summarizing the results of (A–D). (F–I) Similar NMJ images and quantification as shown in (A–D) but with MN-Ib expression of BoNT-C (Ib>BoNT-C; w;+;dHB9-GAL4/UAS-BoNT-C), -TNT (Ib>TNT; w;UAS-TNT/+;dHB9-GAL4/+), or -rpr.hid (Ib>rpr.hid; rpr.hid/+;dHB9-GAL4/+). (J) Schematic summarizing the results of (F–I). Error bars indicate ± SEM. ****p<0.0001; ***p<0.001; *p<0.05; ns, not significant. Absolute values for normalized data and additional statistical details are summarized in Supplementary file 2.
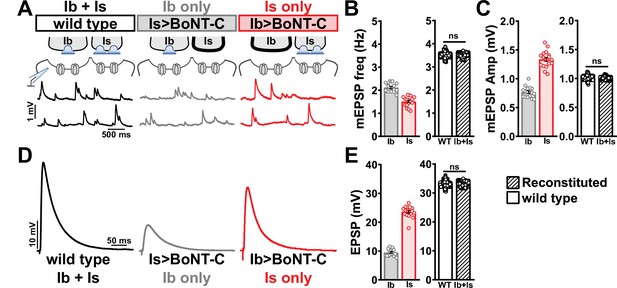
Selective silencing by botulinum neurotoxin (BoNT-C) fully reconstitutes wild-type neuromuscular junction (NMJ) physiology.
(A) Schematic and representative miniature excitatory postsynaptic potential (mEPSP) traces of recordings from wild-type (‘Ib+Is’) or input-specific silencing of MN-Is (‘Ib only’) or MN-Ib (‘Is only’) by BoNT-C expression. (B) Quantification of mEPSP frequency in Ib only and Is only. A simple addition of these values (reconstituted) recapitulates the observed blended values observed in wild-type. (C) Quantification of mEPSP amplitude in Ib only and Is only. Note the substantial input-specific difference in mEPSP amplitude. A weighted average of these values from Ib and Is (reconstituted) fully recapitulates the average mEPSP amplitude values observed from recordings of wild-type NMJs. Reconstituted data sets were acquired by bootstrapping and resampling from the seed data sets (see ‘Materials and methods’ for additional details). (D) Representative EPSP traces from wild-type, Ib-only, and Is-only NMJs. (E) Quantification of EPSP amplitude from Ib-only and Is-only NMJs. A simple addition of these values (reconstituted) fully recapitulates the blended EPSP values obtained from wild-type recordings. Error bars indicate ± SEM. ns, not significant. Additional statistical details are summarized in Supplementary file 2.
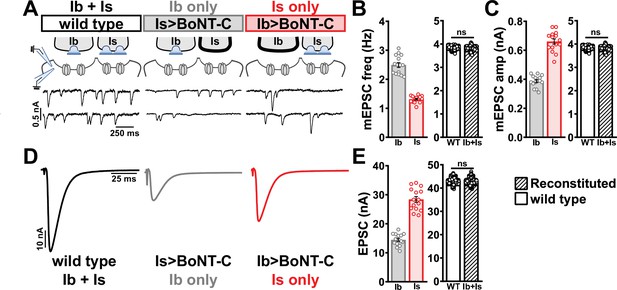
Selective silencing by botulinum neurotoxin (BoNT-C) fully reconstitutes wild-type neuromuscular junction (NMJ) physiology using two-electrode voltage clamp.
(A) Schematic and representative miniature excitatory postsynaptic current (mEPSC) traces of two-electrode voltage clamp (TEVC) recordings from wild-type (‘Ib+Is’) or input-specific silencing of MN-Is (‘Ib only’) or MN-Ib (‘Is only’) by BoNT-C expression. (B) Quantification of mEPSC frequency in Ib only and Is only. A simple addition of these values (reconstituted) recapitulates the observed blended values observed in wild-type. (C) Quantification of mEPSC amplitude in Ib only and Is only. Note the substantial input-specific difference in mEPSC amplitude. A weighted average of these values from Ib and Is (reconstituted) fully recapitulates the average mEPSP amplitude values observed from recordings of wild-type NMJs. Reconstituted data sets were acquired by bootstrapping and resampling from the seed data sets (see ‘Materials and methods’ for additional details). (D) Representative EPSC traces from wild-type, Ib-only, and Is-only NMJs. (E) Quantification of EPSC amplitude from Ib-only and Is-only NMJs. A simple addition of these values (reconstituted) fully recapitulates the blended EPSC values obtained from wild-type recordings. Error bars indicate ± SEM. ns, not significant. Additional statistical details are summarized in Supplementary file 2.
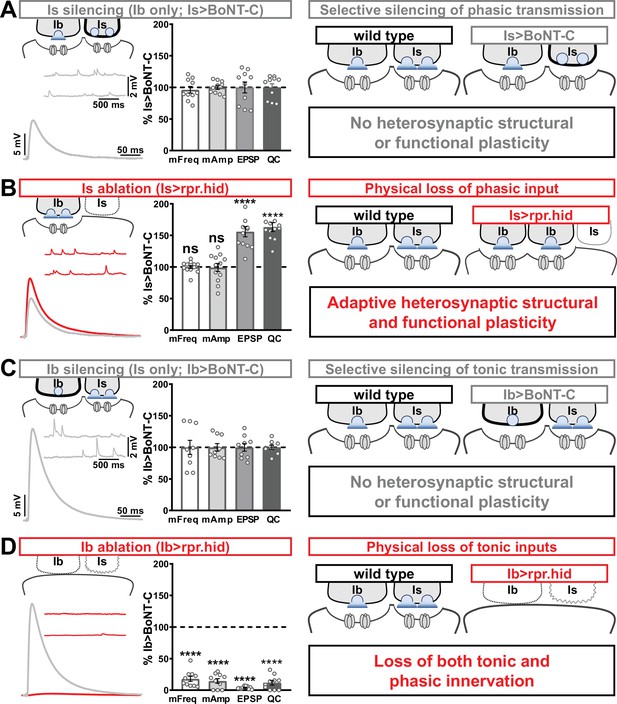
Selective silencing by botulinum neurotoxin (BoNT-C) does not induce heterosynaptic functional plasticity.
(A) Schematic and representative electrophysiological traces of Is-silenced neuromuscular junctions (NMJs) (Ib only; Is>BoNT-C) as a baseline for isolated synaptic transmission from MN-Ib. Electrophysiological data (miniature excitatory postsynaptic potential [mEPSP] frequency, mEPSP amplitude, excitatory postsynaptic potential [EPSP] amplitude, and quantal content) are normalized to this same genotype (Is>BoNT-C). Right: schematic illustrating no heterosynaptic structural or functional plasticity is induced. (B) Schematic, traces, and quantification of recordings from NMJs in which MN-Is is ablated (Is<rpr.hid), normalized to baseline (Is>BoNT-C) values. Note that no differences in mEPSP values are observed following MN-Is ablation, while an apparent adaptive heterosynaptic increase in EPSP amplitude and quantal content is observed from the remaining MN-Ib, summarized at right. (C) Schematic, traces, and quantification of recordings from Ib-silenced NMJs (Is only; Ib>BoNT-C) as a baseline for isolated synaptic transmission from MN-Is. Electrophysiological data are normalized to this same genotype (Ib>BoNT-C). Right: schematic illustrating no heterosynaptic structural or functional plasticity is induced. (D) Schematic, traces, and quantification of recordings from NMJs in which MN-Ib are ablated (Ib<rpr.hid), normalized to baseline (Ib>BoNT C) values. Note that in this case synaptic transmission is essentially eliminated due to ablation of both MN-Ib and -Is inputs, summarized at right. Thus, when transmission is silenced by BoNT-C but both motor inputs are physically present, no heterosynaptic structural or functional plasticity is elicited, while when one of the motor inputs is physically absent, either adaptive heterosynaptic structural and functional plasticity or loss of the convergent input is observed. Error bars indicate ± SEM. ****p<0.0001; ***p<0.001; ns, not significant. Absolute values for normalized data and additional statistical details are summarized in Supplementary file 2.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Antibody | Mouse monoclonal | Developmental Studies Hybridoma Bank (DSHB) | AB_528269 | 1:50 |
| Antibody | Mouse monoclonal | DSHB | AB_2314866 | 1:100 |
| Antibody | Mouse monoclonal | DSHB | AB_528203 | 1:100 |
| Antibody | Mouse monoclonal | DSHB | AB_2617422 | 1:500 |
| Antibody | Rabbit polyclonal | Pielage et al., 2005 | 1:10000 | |
| Antibody | Rabbit polyclonal | Perry et al., 2017 | 1:1000 | |
| Antibody | Guinea polyclonal | Goel and Dickman, 2018 | 1:2000 | |
| Antibody | Guinea polyclonal | Perry et al., 2017 | 1:1000 | |
| Antibody | Alexa Fluor 647 conjugated Goat anti-Horseradish Peroxidase | Jackson ImmunoResearch Laboratories (Jackson) | 123-605-021 | 1:400 |
| Antibody | Alexa Fluor 488 conjugated secondary antibodies | Jackson | 706-545-148, 715-545-150, 711-545-152 | 1:400 |
| Antibody | Cy3-conjugated secondary antibodies | Jackson | 706-165-148, 715-165-150, 711-165-152 | 1:400 |
| Antibody | DyLight 405-conjugated secondary antibodies | Jackson | 706-475-148, 715-475-150 | 1:400 |
| Antibody | Alexa Fluor 647 conjugated Goat anti-Phalloidin | ThermoFisher | A22287 | 1:1000 |
| Genetic reagent (D. melanogaster) | UAS-BoNT-C | This study | See Materials and methods, subsection Molecular biology. | |
| Genetic reagent (D. melanogaster) | UAS-BoNT-A | This study | Same as above | |
| Genetic reagent (D. melanogaster) | UAS-BoNT-B | This study | Same as above | |
| Genetic reagent (D. melanogaster) | UAS-BoNT-E | This study | Same as above | |
| Genetic reagent (D. melanogaster) | MHC-CD8-GCaMP8f-Sh (SynapGCaMP8f) | This study | Same as above | |
| Genetic reagent (D. melanogaster) | OK6-GAL4 | Aberle et al., 2002 | ||
| Genetic reagent (D. melanogaster) | UAS-rpr.hid | Zhou et al., 1997 | ||
| Genetic reagent (D. melanogaster) | OK319-GAL4 | Sweeney et al., 1995 | ||
| Genetic reagent (D. melanogaster) | vGlutSS1 | Sherer et al., 2020 | ||
| Genetic reagent (D. melanogaster) | B3RT-vGlut-B3RT | Sherer et al., 2020 | ||
| Genetic reagent (D. melanogaster) | UAS-B3 | Sherer et al., 2020 | ||
| Genetic reagent (D. melanogaster) | UAS-ChR2T159C | Bloomington Drosophila Stock Center (BDSC) | 58373 | |
| Genetic reagent (D. melanogaster) | UAS-CD4::tdGFP | BDSC | 35839 | |
| Genetic reagent (D. melanogaster) | dHb9-GAL4 | BDSC | 83004 | |
| Genetic reagent (D. melanogaster) | GMR27E09-GAL4 | BDSC | 49227 | |
| Genetic reagent (D. melanogaster) | UAS-TNT | BDSC | 28838 | |
| Genetic reagent (D. melanogaster) | w1118 | BDSC | 5905 | |
| Genetic reagent (D. melanogaster) | D42-GAL4 | BDSC | 8816 | |
| Recombinant DNA reagent | pUASt destination vector | Drosophila Genetics Resource Center (DGRC) | 1129 | |
| Chemical, compound, drug | all trans-Retinal | Sigma-Aldrich | R2500 | |
| Software | NIS Elements software | Nikon | 4.51.01 | |
| Software | Axon pCLAMP Clampfit | Molecular Devices | 10.7 | |
| Software | MiniAnalysis | Synaptosoft | 6.0.3 | |
| Software | GraphPad Prism | GraphPad | 8.0.1 | |
| Software | Jupyter Notebook | Anaconda | 6.0.1 | |
| Software | ImageJ (Fiji) | Rueden et al., 2017 |
Additional files
-
Supplementary file 1
Characterization of BoNT-A, BoNT-B, and BoNT-E.
- https://cdn.elifesciences.org/articles/77924/elife-77924-supp1-v2.docx
-
Supplementary file 2
Absolute values for normalized data and complete statistical details.
- https://cdn.elifesciences.org/articles/77924/elife-77924-supp2-v2.docx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/77924/elife-77924-transrepform1-v2.pdf
-
Source code 1
Customized Jupyter Note codes.
- https://cdn.elifesciences.org/articles/77924/elife-77924-code1-v2.zip





