Geometric control of myosin II orientation during axis elongation
Figures
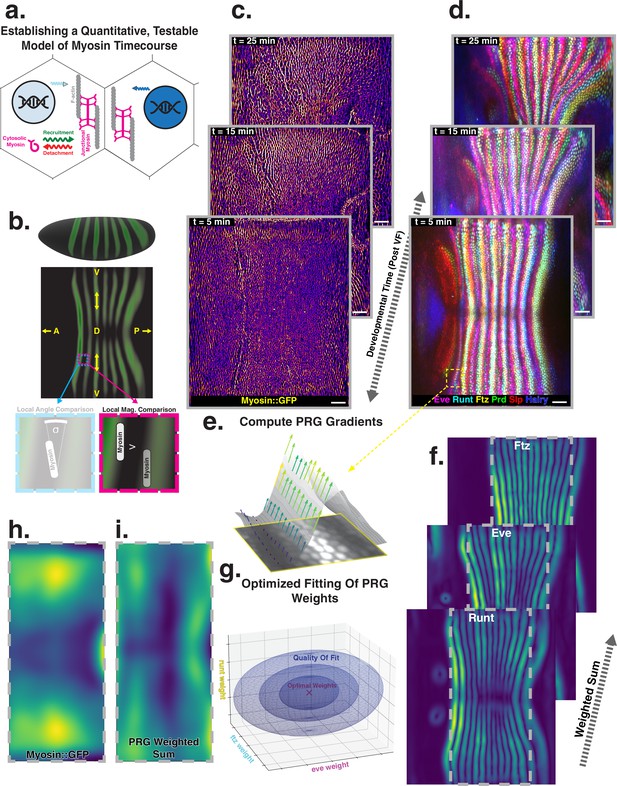
Global analysis of myosin vs. pair rule gene (PRG) expression patterns reveals no linear correlation.
(a) Junctional myosin could be regulated by gene expression patterns, by mechanical cues, or both. (b) Tissue cartography extracts embryo surfaces from volumetric in toto light sheet imaging that are projected onto a cylindrical chart. This allows measuring quantities on a tissue scale, here the intensity and orientation of junctional myosin and PRGs. All figures follow the orientation indicated here: anterior left, posterior right. For 3D-rendered embryos, dorsal is up, and for full-embryo cylindrical projections, dorsal is in the middle. (c) Time series of junctional myosin, starting at 5 min post ventral furrow initiation (d) Time series of PRGs, starting at 5 min post ventral furrow initiation. Time series created by digitally stitching together different stained and live imaged embryos. (e) The smoothed gradient of PRG expression patterns computes local cell–cell differences. The gradient vector points in the direction in which the signal increases most. (f) Gradient magnitude of the expression patterns of the PRGs Runt, Eve, and Ftz in three representative embryos shows 14 stripes with a dorsal-ventral (DV) modulation of intensity. (g) In regression analysis, the smoothed gradients of PRGs are combined into a weighted sum to approximate the observed myosin pattern. The weights are adjusted to optimize the quality of fit, exploring the entire space of possible weights, both positive and negative. (h) Smoothed junctional myosin intensity at the onset of germband extension (GBE) (ensemble average of five embryos). (i) Result of PRG gradient regression. The best possible fit using the weighted sum of PRG gradients does not resemble the large-scale myosin pattern.
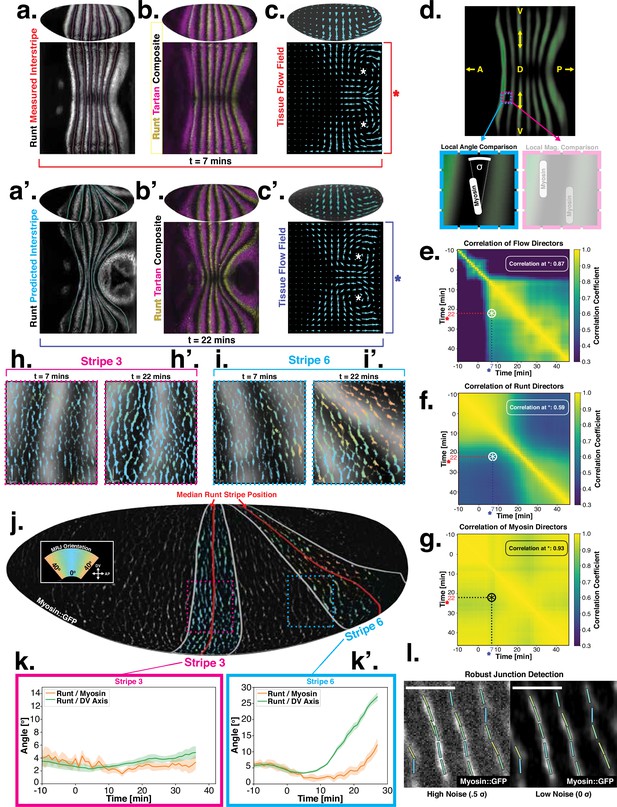
Pair rule genes (PRGs) flow with tissue while myosin pattern does not.
(a) Runt stripes with measured inter-stripe lines, 7 min post ventral furrow (VF) initiation from a representative Runt::LlamaTag-GFP embryo. All PRG stripes are initially approximately parallel to the dorsal-ventral (DV) axis. (a’) PRG stripes deform due to advection by tissue flow. Runt stripes with inter-stripe lines predicted by advection, 22 min post VF initiation. Same embryo as in (a). (b, b’) Digitally stitched Runt/Tartan composite, 7 min (b) and 22 min (b’) post VF initiation, showing that PRG and TLR stripes remain parallel. (c, c’) Tissue flow field, 7 min (c) and 22 min (c’) post VF initiation. Calculated from an average of 5 WT Myosin::GFP embryos. (d) Using tissue cartography, we compare MRJ and PRG orientation across the entire embryo. (e) Temporal autocorrelation of the tissue flow field. Each pixel in the matrix shows the correlation (similarity in direction, ranging from 0 to 1, averaged over the embryo surface) of the flow fields at two different time points. (f) Temporal autocorrelation of the Runt stripe direction shows rapid decay during tissue flow. Data from 5 WT Runt::LlamaTag-GFP embryos (see Appendix ‘Definition of Runt stripe angle’ and ‘Definition of correlation coefficient for nematic fields’ for mathematical details). (g) Temporal autocorrelation of the tissue-scale myosin direction, showing an approximately static pattern of myosin orientation. Data from 5 WT Myosin::GFP embryos. (h–h’, i–i’) Digitally stitched images showing Runt and junctional myosin (colored according to angle with DV axis) in a part of the regions surrounding runt stripes 3 (h-h’) and 6 (i-i’) at 7 min (h,i) and 22 min (h’, i’) post VF initiation. In regions where gene patterns are deformed by flow, an angle discrepancy between myosin orientation and PRG stripes develops. Orientation is dorsal up, anterior left. (j) Junctional myosin at 22 min post VF initiation in a representative WT Myosin::GFP embryo. In highlighted regions (defined by Runt stripes 3 and 6), junction color corresponds to the junction/DV axis angle and junction brightness to the myosin fluorescent intensity. Red lines show median runt stripe position. (k–k’) Angle between myosin anisotropy orientation and Runt stripe, and angle between Runt stripe and DV axis, averaged over the regions corresponding to runt stripes 3 and 6. Runt angle measured by the direction of Runt gradient, rotated by 90°. (l) The radon transform method detects MRJs and is insensitive to noise. Bars indicate junctions detected by the radon transform, color-coded according to their angle. Orientation is dorsal up, anterior left. Scale bar .
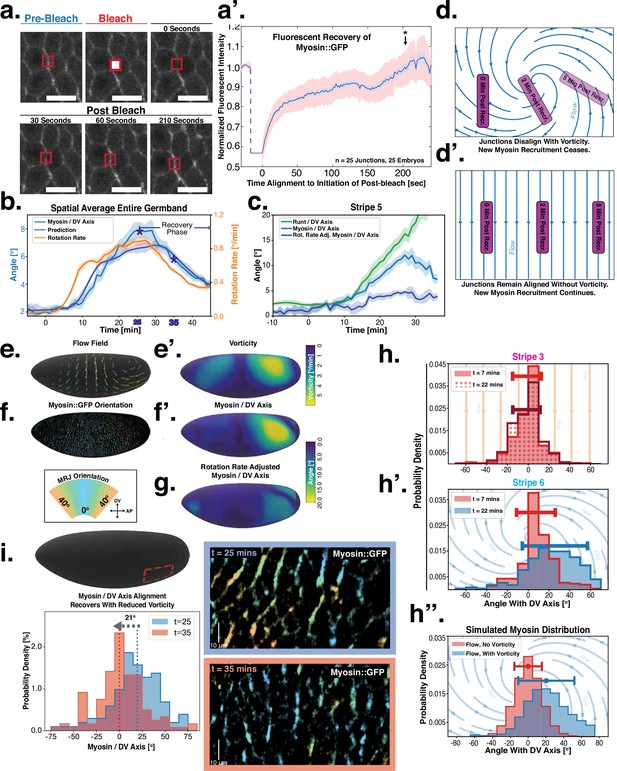
Dynamics of myosin orientation can be quantitatively captured by embryo geometry and vorticity.
(a) Time series of a representative fluorescence recovery after photo bleaching (FRAP) experiment of junctional myosin. (a’) FRAP of junctional myosin (=25 embryos) shows multiple timescales and complete recovery of myosin fluorescence, indicating transient binding to the cortex. Rose shaded area indicates standard deviation and blue shaded error the standard error of the mean. * indicates the time of full recovery. (b) Spatial average of vorticity and myosin/dorsal-ventral (DV) axis angle across germband versus time in WT Myosin::GFP embryos. Blue line shows prediction for the myosin/DV axis angle calculated from vorticity. Stars highlight times shown in panel (i), showing myosin/DV axis alignment recovery. (c) Spatial average of Runt/DV axis angle, myosin/Runt stripe, myosin/DV axis angle, and rotation-rate corrected myosin/DV axis angle over the region corresponding to Runt stripe 5 versus time in WT Myosin::GFP and WT Runt::LlamaTag-GFP embryos. (d, d’) Myosin-rich junctions (MRJs) deflect away from the axis of preferential recruitment in rotational (d) flow but remain aligned in irrotational (d’) flow. (e) Tissue flow field during germband extension (GBE), temporal average from 15 to 25 min post VF initiation. Computed from ensemble of WT Myosin:GFP embryos. (e’) Vorticity of tissue flow field, temporal average from 15 to 25 min post VF initiation. Computed from ensemble of WT Myosin::GFP embryos. (f) MRJs in a representative WT Myosin::GFP embryo 22 min post VF initiation. Junction color corresponds to the junction/DV axis angle and junction brightness to the myosin fluorescent intensity. (f’) Smoothed myosin/DV axis angle, temporal average from 15 to 25 min post VF initiation. Computed from ensemble of WT Myosin::GFP embryos. (g) Rotation rate-adjusted myosin/DV axis angle, temporal average from 15 to 25 min post VF initiation. Computed from ensemble of WT Myosin::GFP embryos. (h, h’) Histogram of myosin angular distribution in the region corresponding to Runt stripe 3 (h) and stripe 6 (h’), at two times during GBE. Data corresponds to region shown in Figure 2h’–i’ (one representative WT Myosin::GFP embryo). Histograms colored in shades of red, resp. blue, show data at times and regions where vorticity is low, resp. high. (h”) Simulated histograms of Myosin angular distribution the presence or absence of vorticity (rotation rates of and , myosin-effective lifetime of 5 min). Compare with histograms in (h’) and (i). (i) Junctional myosin in a ventro-posterior region of the embryo at 25 and 35 min post VF initiation, showing the recovery of myosin/DV axis alignment. Junctions colored according to their orientation and fluorescent intensity as in (f). Histogram shows the distribution of orientations observed at 25 and 35 min.
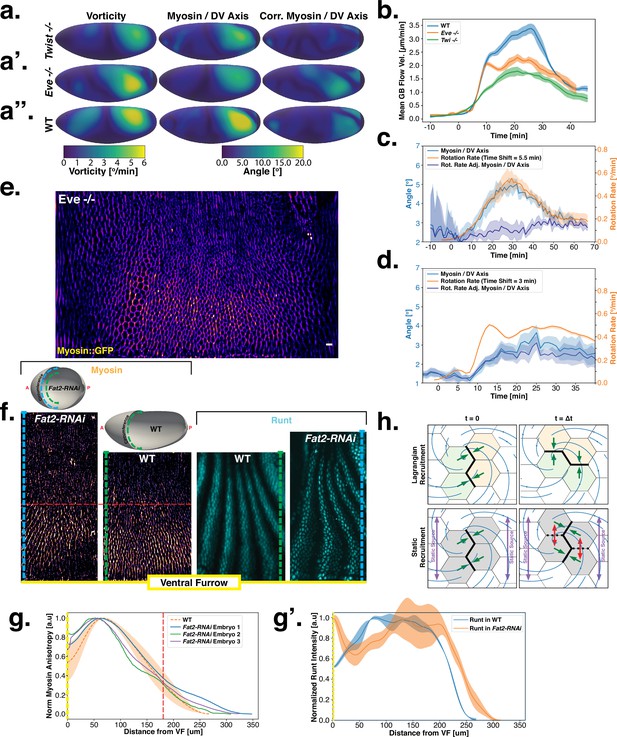
Dynamics of myosin orientation in mutants affecting vorticity or embryo geometry can be quantitatively described.
(A–A”) Vorticity, myosin/dorsal-ventral (DV) axis angle, and rotation-rate adjusted myosin/DV axis angle prediction in WT, , and embryos. Heatmaps show a temporal average from 15 to 25 min post ventral furrow (VF) initiation. The correlation of vorticity and myosin/DV axis angle persists in all mutants, and the rotation rate-corrected myosin/DV axis angle remains low. (B) Spatial average of tissue flow velocity in the germband over time in WT, , and embryos. and mutants show markedly lower flow velocity than WT, but differ in their kinetics. (C) Spatial average of vorticity, myosin/DV axis angle, and rotation rate-adjusted myosin/DV axis angle over time in , Myosin::GFP embryos. (D) Spatial average of vorticity, myosin/DV axis angle, and rotation rate-adjusted myosin/DV axis angle over time in , Myosin:mCherry embryos. (E) Junctional myosin in mutants remains anisotropic and aligned with the DV axis, although the degree of anisotropy is reduced. One lateral half of a representative , Myosin::mCherry embryo, 18 min post VF initiation. (F) Top: 3D shape of WT embryos and embryos from Fat2-RNAi mothers, extracted by tissue cartography pipeline. Compared to WT, Fat2-RNAi embryos are spherical and have a greatly increased circumference (marked in green resp. blue). Bottom: patterns of junctional myosin and Runt in the germband of WT and Fat2-RNAi embryos at equivalent phases in germband extension (GBE) (10 min post VF initiation in WT). Only one lateral half is shown, orientation is dorsal up, anterior left. Junctional myosin is visible up to the same distance from the VF in both WT and Fat2-RNAi embryos. (G–G’) Quantification of the decay of junctional myosin (G) and Runt (G’) away from the ventral furrow in WT and Fat2-RNAi. Myosin data from WT and Fat2-RNAi embryos. Runt data from both lateral halves of WT and Fat2-RNAi embryo shown in (F). (H) Myosin recruitment by a passively advected source vs by a static source leads to qualitatively and quantitatively different behavior.
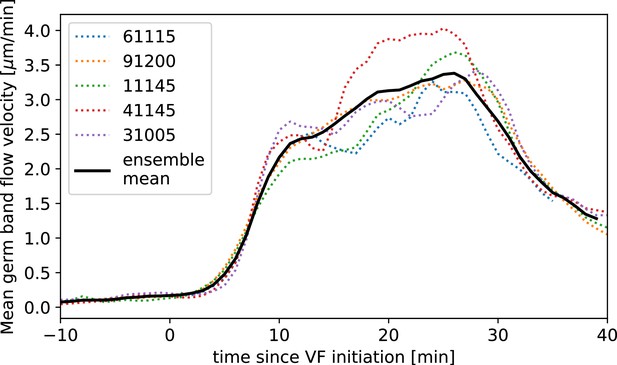
Time-alignment using particle image velocimetry (PIV) curves.
Average flow velocity in germband during germband extension (GBE) in WT Myosin::GFP, time-aligned by matching PIV fields.
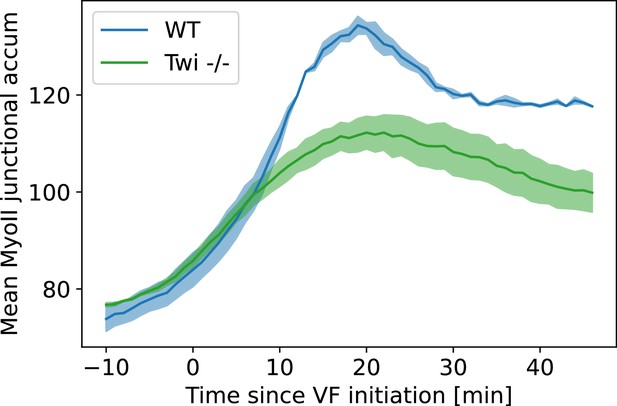
Average junctional myosin levels in WT and embryos.
Average junctional myosin levels, computed using the cytosolic normalization filter, in germband during germband extension (GBE) in WT Myosin::GFP and Myosin::GFP embryos. Included in the spatial average are image regions with a junctional accumulation level of , which we classify as ‘junction.’
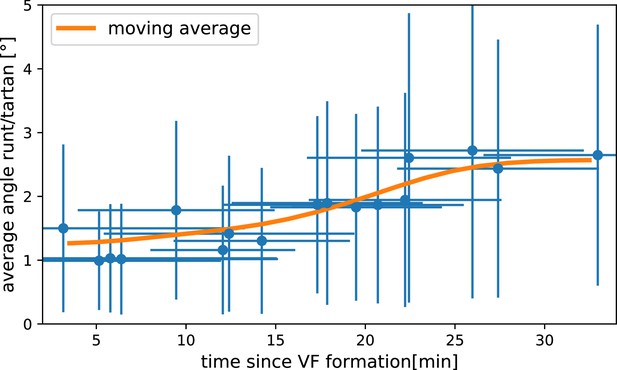
Measured angle between Runt and Tartan stripes.
Runt angle from Runt::LLamaTag-GFP embryos, Tartan data from stained embryos.
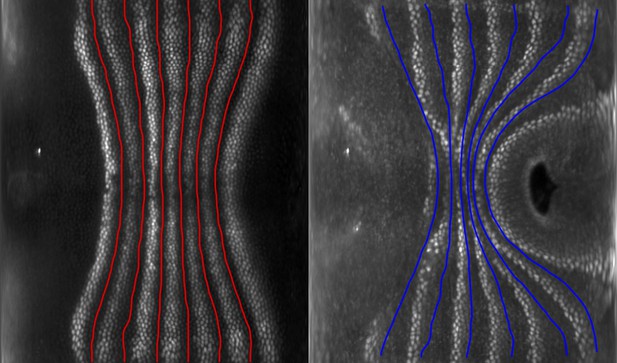
Eve stripes are advected by tissue flow.
Eve stripes 5 min before ventral furrow (VF) initiation (left), and Eve stripes 20 min post VF initiation, with predicted inter-stripe locations based on advection (right).
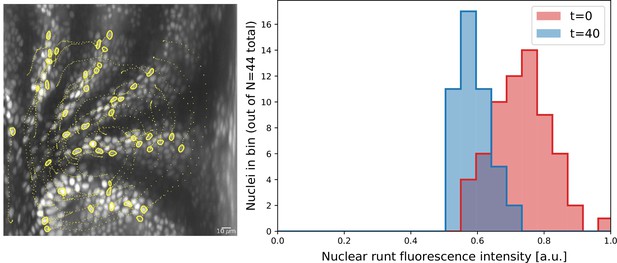
Nuclei initially expressing Runt expression after 20 min.
Cell tracks obtained by semi-automatic cell tracking in Ilastik.
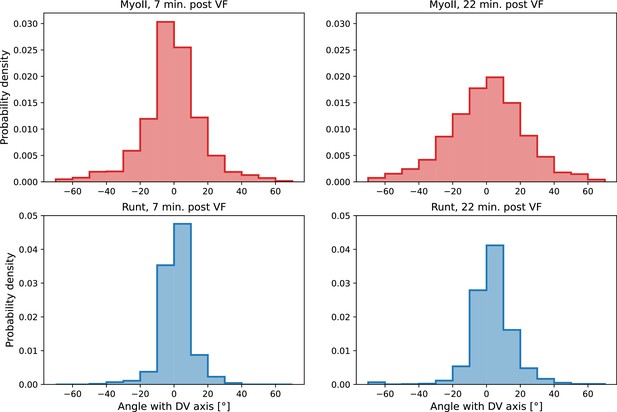
Local distribution of Runt and myosin orientations in the ventro-lateral region of two representative embryos 22 min post ventral furrow (VF) initiation.
Runt angle and myosin data taken from to the regions shown Figure 2E. Window size used for querying the local distribution is .
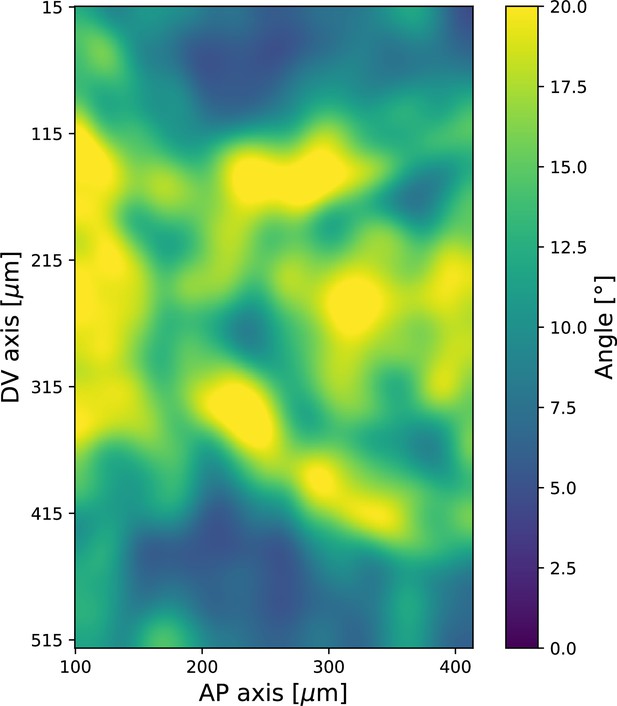
Spatial pattern of local standard deviation of Runt orientation, 20 min post ventral furrow (VF) initiation.
Data from Runt::LlamaTag-GFP embryos. Window size used for querying the local distribution is .
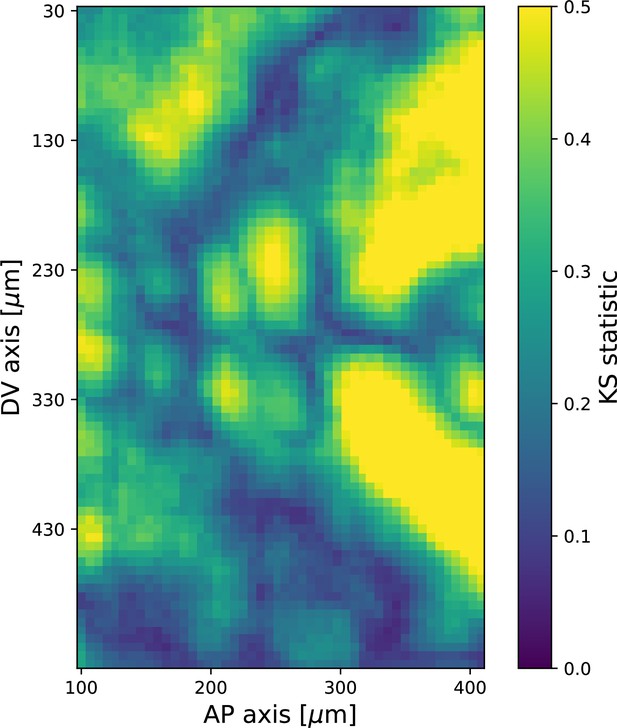
Kolmogorov–Smirnov test statistic comparing local angular distributions of Runt and myosin 20 min post ventral furrow (VF) initiation.
Observed values of the test statistic strongly indicate that the two distributions are dissimilar. Runt angle from Runt::LLamaTag-GFP embryos, myosin data from Myosin::GFP embryos. Each local window contains ∼100 junctions.
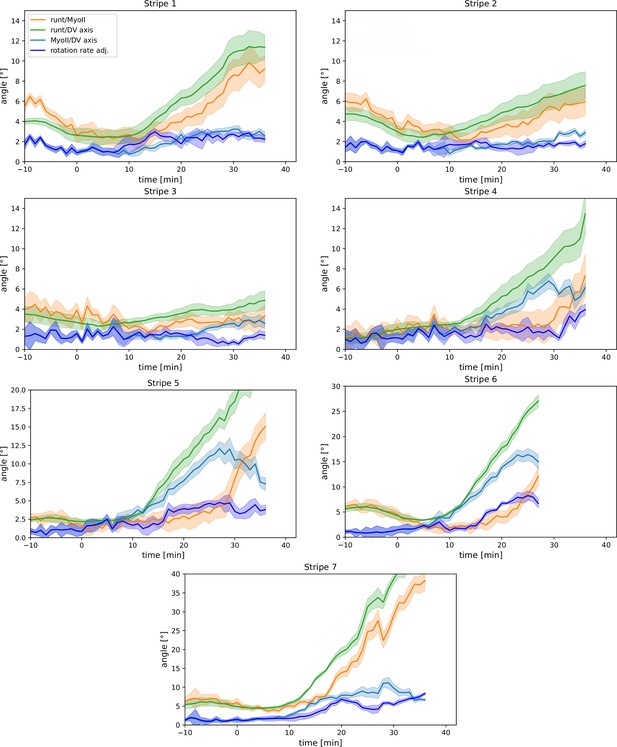
Per-Runt stripe averages of myosin and Runt orientations.
Angle between myosin anisotropy orientation and Runt stripe, angle between Runt stripe and dorsal-ventral (DV) axis, angle between myosin anisotropy and DV axis and rotation rate-adjusted myosin/DV axis angle, averaged over the regions corresponding to Runt stripes 1–7. Data from WT Myosin::GFP and Runt::LlamaTag-GFP embryos. Data ends early in stripe 6 since the region becomes difficult to separate from stripe 7.
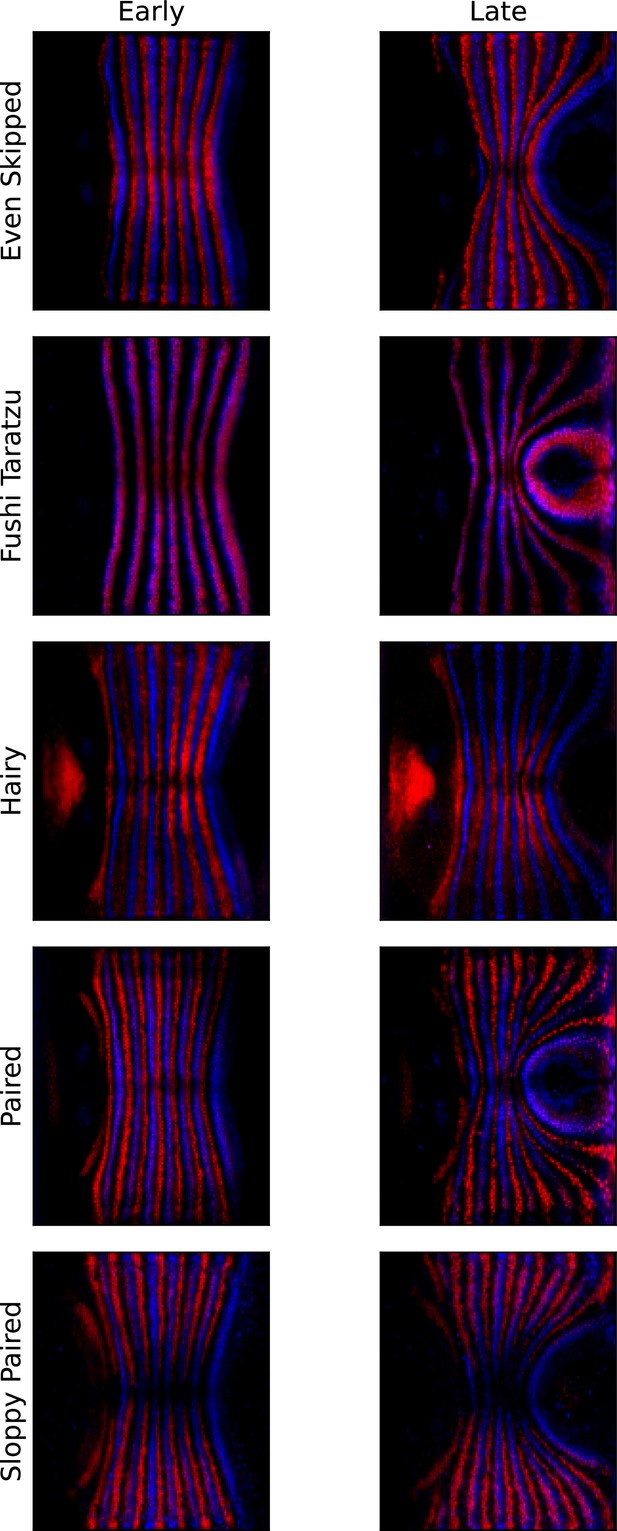
Pair rule genes (PRGs) remain parallel with respect to one another during germband extension (GBE).
Pair-rule gene co-stains, showing Runt (blue) and another PRG (red, PRG indicated on the left), before the onset of GBE flow, and after significant flow has occurred (ca. 20 min later).
The PRG stripes remain parallel to one another and do not penetrate one another, showing that Runt and the other PRGs are advected by tissue flow in the same way.
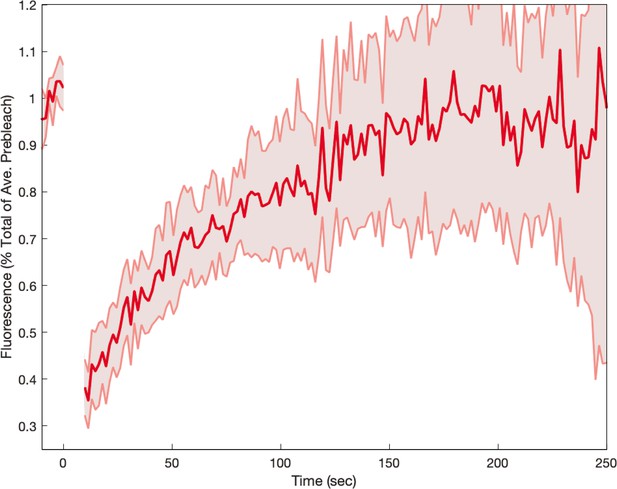
Myosin fluorescence recovery after photobleaching (FRAP) recovery in embryos.
The data is compatible with the hypothesis that myosin kinetics is more rapid in embryos than in WT. Shaded error represent the standard error on the mean. FRAPed junctions.
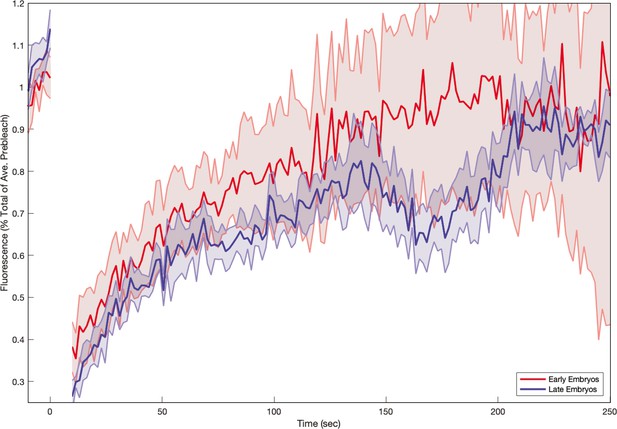
Myosin fluorescence recovery after photobleaching (FRAP) recovery in embryos as a function of embryo age.
In , moysin kinetics appears to depend sensitively on time, with less recovery in embryos during later germband extension (GBE). Shaded error represents the standard error on the mean. (red) resp. (blue) FRAPed junctions.
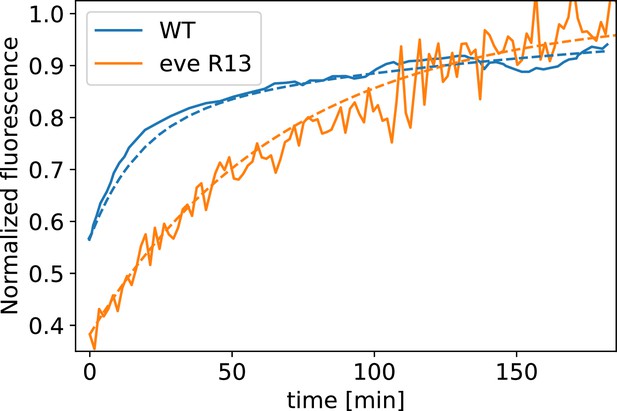
Myosin fluorescence recovery after photobleaching (FRAP) recovery in embryos, and WT, together with modeling fit.
The curve is fit by a single exponential, whereas for the WT curve, two exponential terms are required.
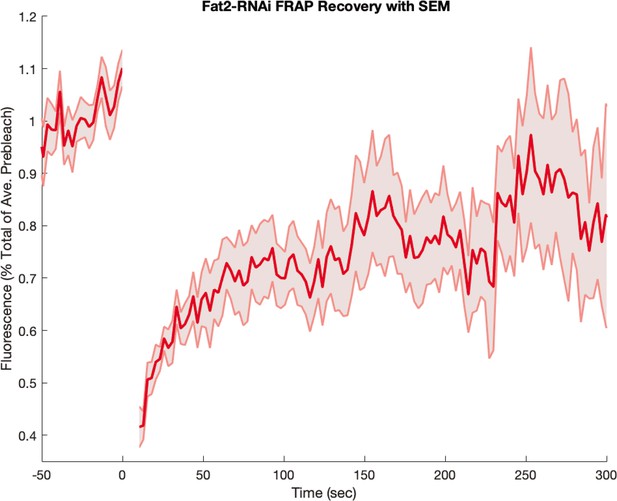
Myosin fluorescence recovery after photobleaching (FRAP) recovery in embryos from Fat2-RNAi mothers.
Embryos were visualized with a fluorescent myosin only (and not also with a fluorescent membrane marker). Shaded error represents the standard error on the mean. FRAPed junctions.
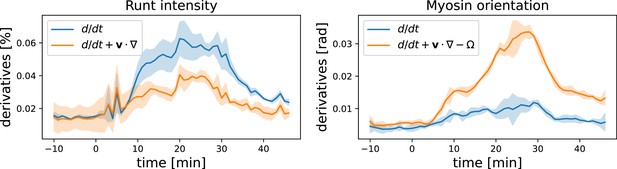
Convective vs. partial time derivatives of the Runt and myosin patterns.
For the Runt intensity, the convective derivative is significantly lower than the partial derivative, while for the myosin orientation the opposite is the case.
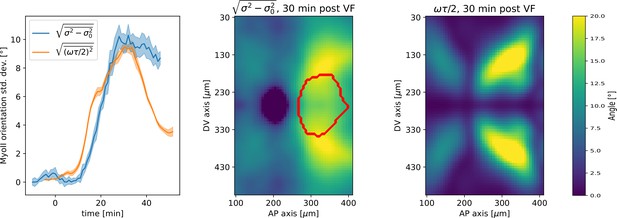
Embryo-scale prediction of myosin angular distribution width.
From left to right: spatial average over the germband of standard deviation of myosin angular distribution and vorticity contribution to standard deviation – heatmap of change in standard deviation of myosin myosin angular distribution, showing germband only. Red outline indicates region of the invaginating posterior midgut – heatmap of vorticity contribution to myosin standard deviation.
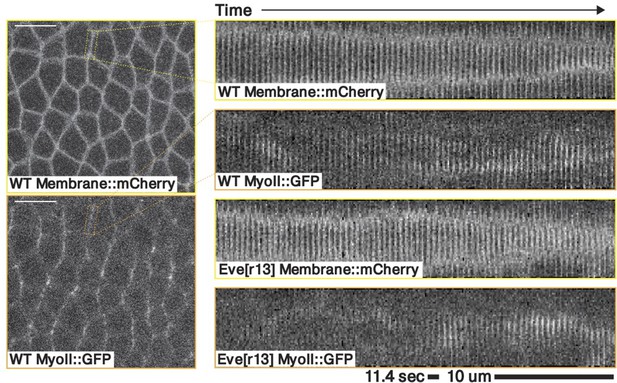
Kymograph of a contracting junction in a representative WT and a representative eve embryo.
Both kymographs shows a junction in the germband ∼10–20 minutes post VF initiations, marked with both a membrane and a myosin fluorescent tag.
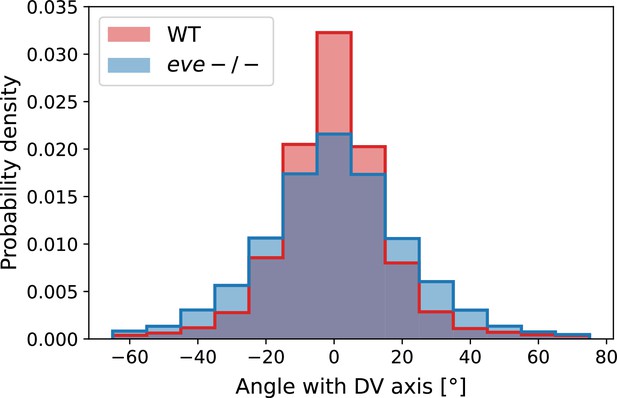
Histogram of myosin orientations in the germband of Myosin::GFP WT Myosin:mCherry eve embryos.
Data corresponds to 15 min post VF initiations. For each embryo, more than 7000 edges are detected. The two-sided KS statistic (maximal area difference between cumulative distribution functions) is 0.076.
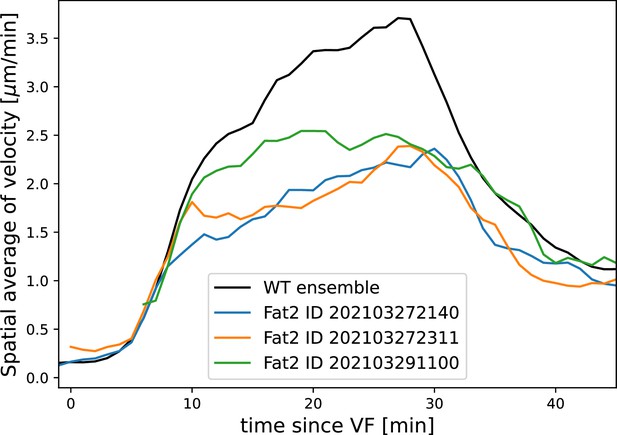
Average tissue flow velocity in WT and three Fat2-RNAi embryos.
Flow in round embryos is noticeably reduced. All measurements computed using the induced metric to correct for any distortions of the cylindrical projections.
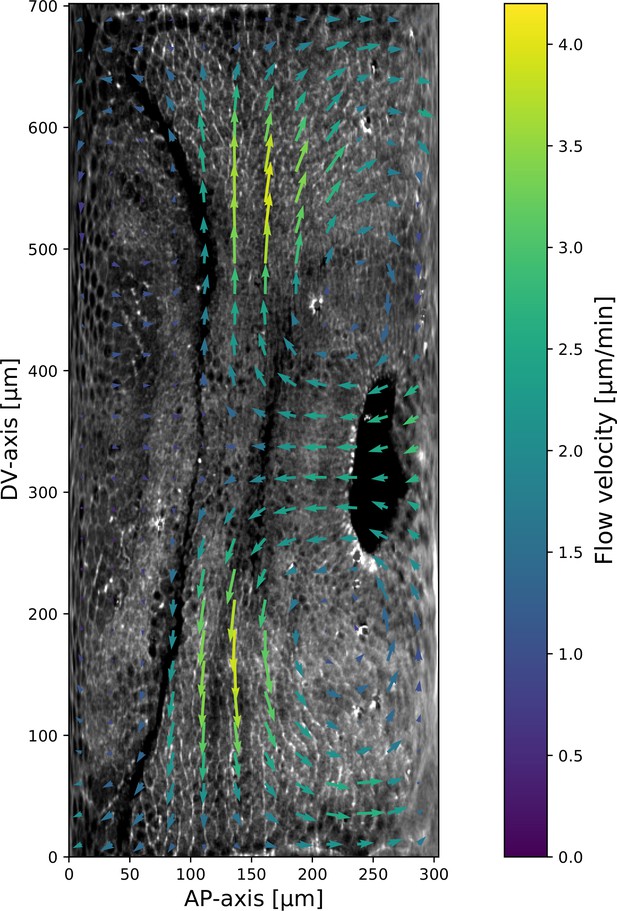
Myosin and particle image velocimetry (PIV) field on a representative Fat2-RNAi embryo.
Time 10 min post VF initiation. The signal shown is the raw myosin signal, not subjected to the cytosolic normalization procedure, to show the embryo anatomy. The PIV vortices (regions of maximal vorticity) are removed from the regions with significant junctional myosin accumulation.





