Airway basal cells show regionally distinct potential to undergo metaplastic differentiation
Figures
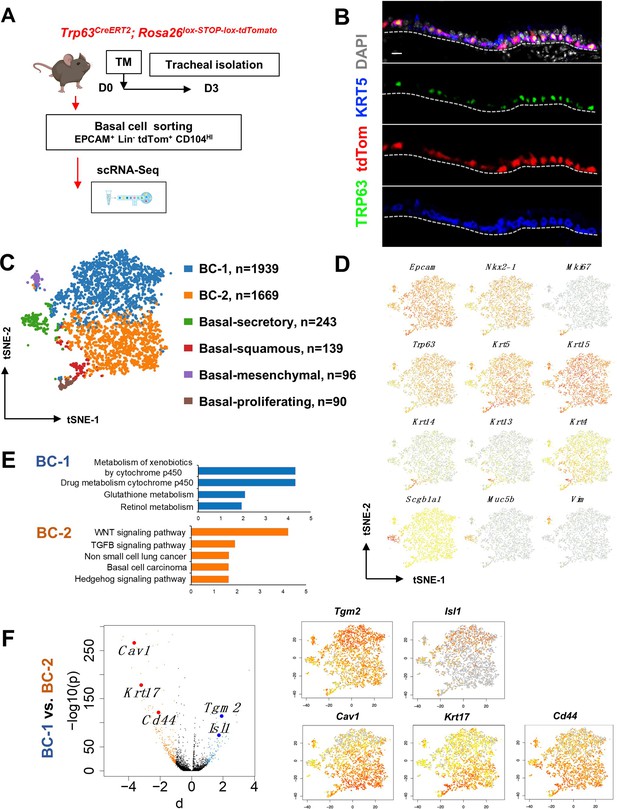
Single cell RNA sequencing (scRNA-Seq) reveals six distinct airway basal cell (BC) subpopulations in the murine trachea.
(A) Diagram: strategy for labeling, isolation and scRNA-Seq analysis of BCs from Trp63CreERT2; Rosa26lox-STOP-lox-tdTomato adult tracheas. (B) Immunofluorescence (IF) of tracheal sections showing efficient tdTom co-labeling with TRP63 +KRT5+BCs restricted to the basal layer of the airway epithelium. (C) tSNE visualization of the six airway BC subpopulations identified by scRNA-Seq, colored by cluster assignment of each population annotated using established lineage-specific or proliferation markers. (D) tSNE visualization of airway BC scRNA-Seq, colored by expression (log2(TPM +1)) of representative marker genes of the different BC subpopulations. (E) Enriched KEGG gene sets in BC-1 and BC-2 subpopulations. (F) Left panel: Volcano plot showing differentially expressed genes in BC-1 and BC-2 (right; black dots); genes significantly enriched in BC-1 were colored in blue (-log10(p)>10; d: average expression difference >1); those significantly enriched in BC-2 were colored in orange (-log10(q)>10; d < –1). Right panel: tSNE visualization of Tgm2, Isl1, Cav1, Krt17, and Cd44 distribution in the BC subpopulations. Scale bar in (B): 10 μm.
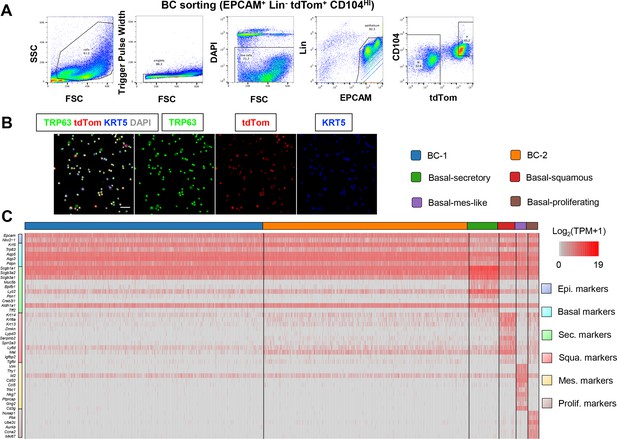
scRNA-Seq of sorted mouse tracheal BCs reveals six distinct subpopulations.
(A) Gating strategy to sort basal cells (BCs) from Trp63CreERT2; Rosa26lox-STOP-lox-tdTomato adult tracheas for single cell RNA sequencing (scRNA-Seq). (B) IF of cytospin of sorted tdTom airway BCs stained with canonical BC markers KRT5 and TRP63. Scale bar: 50 μm. (C) Expression levels [log2(TPM +1)] of cluster-distinct genes (rows) in each epithelial cell (columns).
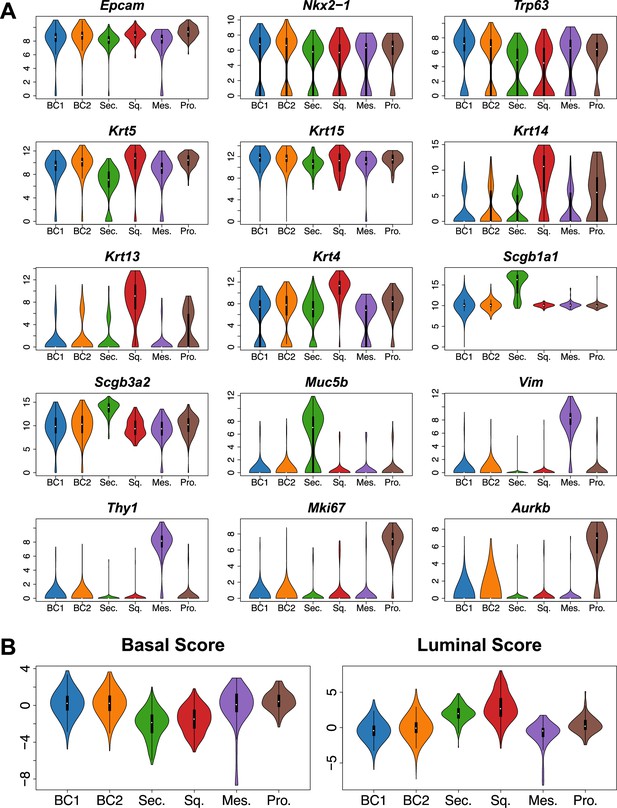
Mouse tracheal BC subpopulations exhibit distinct gene expression patterns.
(A) Violin plots of single cell RNA sequencing (scRNA-Seq) clusters for general epithelial marker Epcam, respiratory epithelial lineage Nkx2-1 marker, canonical BC markers Trp63, Krt5, Krt15, squamous markers Krt14, Krt13, Krt4, secretory markers Scgb1a1, Scgb3a2, Muc5b, mesenchymal markers Vim, Thy1, cell cycle markers Mki67, Aurkb. (B) Violin plots of scRNA-Seq clusters for the per single cell Basal/Luminal scores (integrated z-scores, computed using Stouffer’s method on per-gene z- scores) based on manually curated gene lists (Basal: Trp63, Krt5, Snai2, Egfr, Krt15, Jag2, Dll1, Ngfr, Dapl1, Gpr87, Dlk2, Bcam; Luminal: Krt8, Scgb1a1, Scgb3a2, Muc1, Krt13, Krt4, Lypd2, Notch2, Notch3, Perp, Ly6d, Ly6e, Lypd3, Hes1).
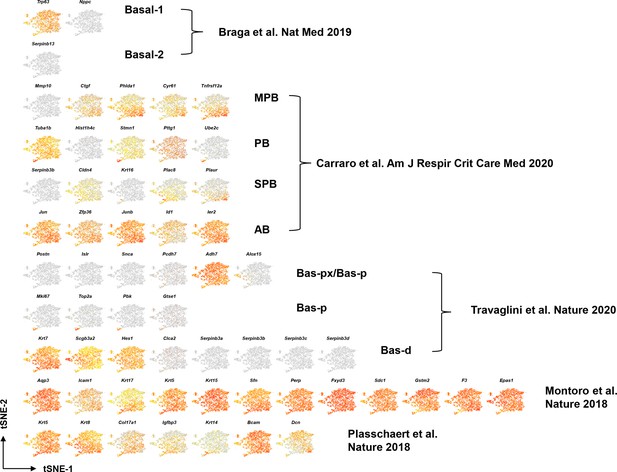
tSNE visualization of airway basal cell (BC) single cell RNA sequencing (scRNA-Seq) for BC markers identified from previous publications in mice and humans, colored by expression [log2(TPM +1)] of each marker gene.
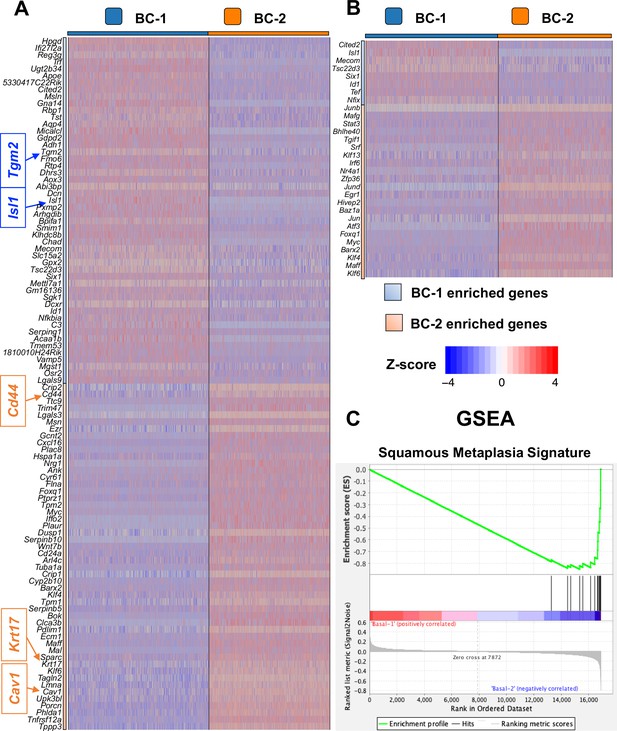
Basal cell (BC) subtypes showing unique gene expression signatures.
(A) Top 50 genes significantly enriched in BC-1 and BC-2 showing marked differences in expression between these two clusters. (B) Transcription factors differentially enriched in BC-1 or BC-2 clusters. Relative expression levels of genes (row-wise Z-score of mean log2(TPM +1)). (C) GSEA showing that BC-2 cluster exhibit strong correlation with squamous metaplasia signature.
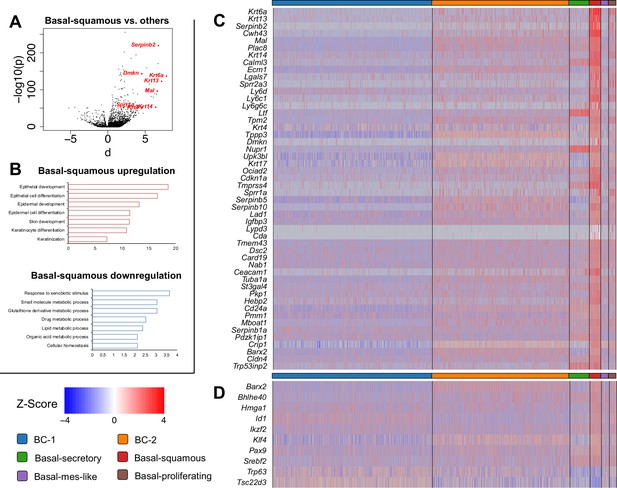
Basal-squamous cluster showing unique gene expression signature compared to the other five basal cell (BC) clusters.
(A) Volcano plot showing differentially expressed in the Basal-squamous cluster vs the other BCs (squamous-associated marker genes depicted in red dots). (B) Enriched KEGG gene sets significantly upregulated or downregulated in the Basal-squamous cluster. (C) Basal-squamous cluster gene signature. Top 50 genes ranked by fold change significantly enriched in the Basal-squamous cluster compared to all other BC clusters. (D) Transcription factors selectively upregulated or downregulated in the Basal-squamous cluster. Relative expression levels of genes (row-wise Z-score of mean log2(TPM +1)).
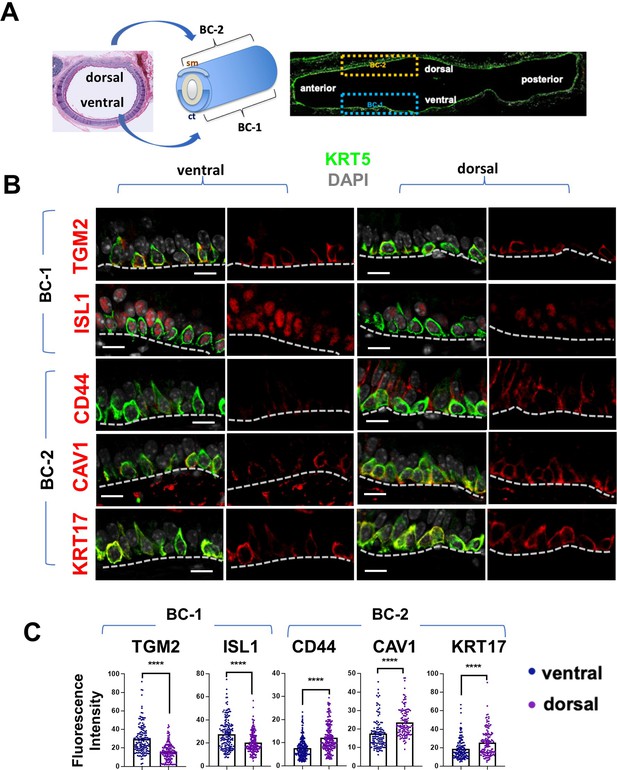
BC-1 and BC-2 reside in distinct niches of the adult tracheal epithelium.
(A) Schematic representation of the differential distribution of BC-1 and BC-2 markers in basal cells along the dorsal-ventral axis of the adult trachea (diagram: sm, smooth muscle; ct, cartilage). (B) Immunofluorescence of BC-1 and BC-2 markers co-labeled with KRT5 in serial sections of the adult trachea showing differential enrichment in ventral or dorsal epithelium, respectively. (C) Quantification of the fluorescence intensity of BC-1 and BC-2 markers confirms the significant differences in expression between ventral and dorsal BCs. Graphs: Each dot represents the average fluorescence intensity value of a single KRT5 + cell analyzed (already clarified in methods: Fluorescence intensity and area (μm2) for each cell was measured by Zen 2.3 lite software.); bars represent the mean ± SEM of 8–15 paired fields counted from 5 to 9 sections; n=3 animals (See also Figure S6). Student’s t-test, ****p<0.0001. Scale bars, 10 μm.
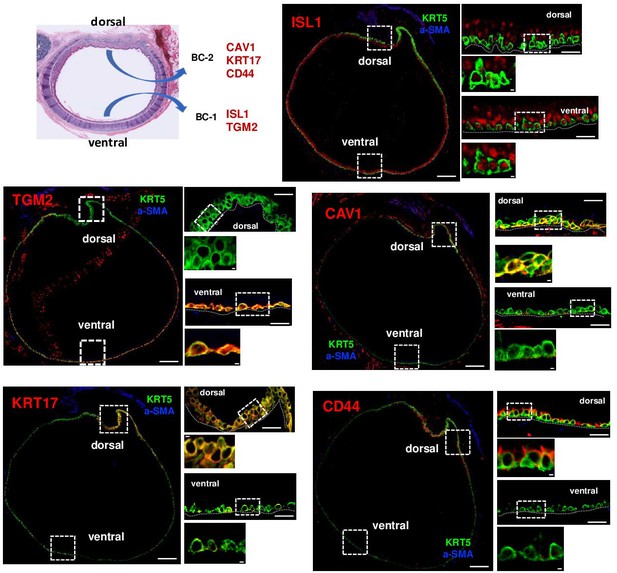
BC-1 and BC-2 populations reside in regionally distinct niches of the adult tracheal epithelium.
Serial cross-sections of adult mouse trachea immunostained for BC-1 and BC-2 markers, KRT5 and a-SMA (identifies smooth muscle layer in dorsal trachea). Differential enrichment of BC-1 and BC-2 markers in KRT5 + cells from ventral and dorsal tracheal epithelium, respectively. Smaller panels are enlargement from boxed areas. Scale bars: largest panels of overview: 100 μm; mid-size panels: 20 μm; smallest insets: 2 μm.
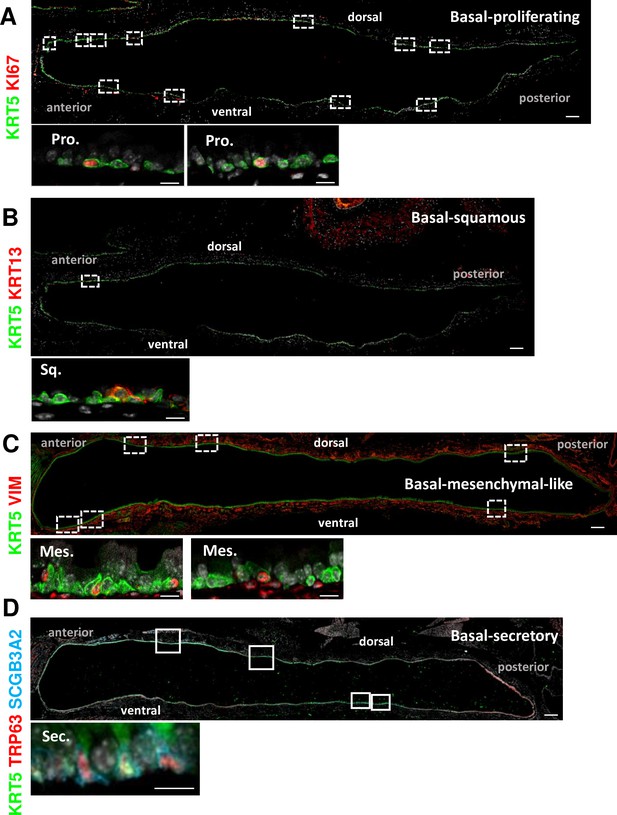
Basal cell (BC) subpopulations other than BC-1 and BC-2 are relatively rare and have no consistent spatial distribution in the adult tracheal epithelium.
Representative tile scan pictures of adult mouse tracheal sections immunostained for KRT5, TRP63 and representative markers of Basal-proliferating (A), Basal-squamous (B), Basal-mesenchyme-like (C), and Basal-secretory (D). High magnification images are representative of specific BC subpopulations depicted in boxed areas along the anterior-posterior or dorsal-ventral axis of the tracheal epithelium. Scale bars: large panels of overview: 200 μm; small panels: 10 μm.
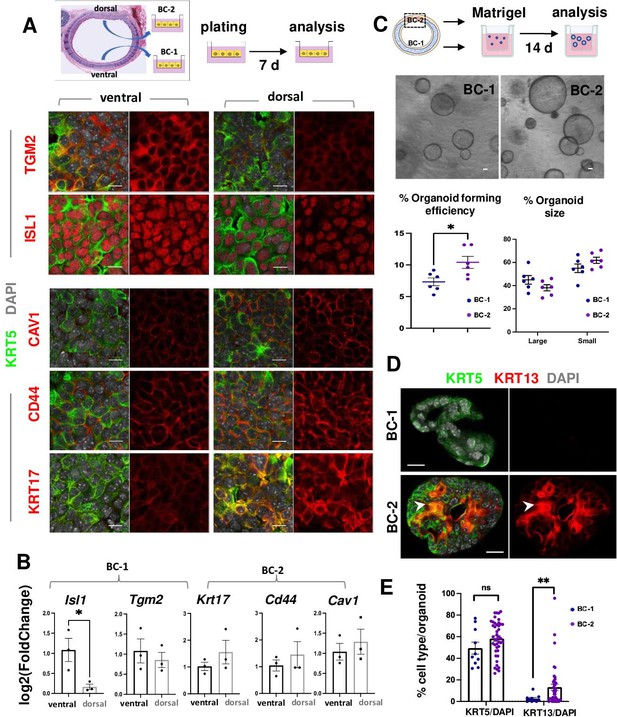
BC-1 and BC-2 differ in their ability to form organoids and activate a metaplastic program of differentiation in primary cultures.
(A) Strategy for isolation, expansion and analysis of BC-1 and BC-2 in submerged cultures. IF at day 7 showing preserved differential enrichment of BC-1 and BC-2 markers in cultures derived from ventral and dorsal BCs, respectively. (B) qPCR of day 7 cultures depicting differences in marker gene expression [log2(FoldChange)] between these subpopulations. Bars are mean ± SEM of 3 replicate cultures. (C) Experimental approach for functional analysis of BC-1 (ventral) and BC-2-derived (dorsal) cultures in organoid assays. Bright field images of BC-1 and BC-2-derived 3D-organoids at day 12. Dot plots: (left): Organoid-forming efficiency (percentage of organoid number / total seeding basal cell number); (right) percentage of small and large-sized day 12 organoids derived from BC-1 or BC-2 progenitors. Graphs are mean ± SEM of values from 6 independent cultures from each group. See methods for quantification details. (D) IF of KRT5 and KRT13 in day 14 organoids showing similar KRT5 signals but abundant KRT13 signals in BC-2-derived cultures compared to those from BC-1. (E) Percentage of KRT5 +or KRT13 + cells in total DAPI-labeled cells from BC-1 or BC-2-derived organoids. Bars represent mean ± SEM of values in each group (number of organoids: BC-1: n=10, BC-2: n=43). Student’s t-test, *p<0.05, **p<0.01, n.s., not significant. Scale bars:10μm.
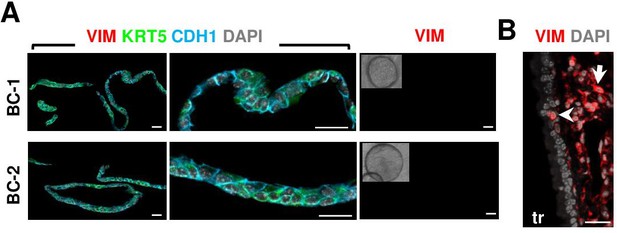
Absence of mesenchymal contamination in organoid cultures.
(A) IF of Vimentin (VIM), E-Cadherin (CDH1), and KRT5 in day 14 BC-1 (ventral) or BC-2 (dorsal)-derived organoids showing extensive CDH1 epithelial labeling and no evidence of VIM + cells in these cultures. Right panel: representative phase contrast image of organoids from each group stained for VIM. (B) Abundant VIM +staining in the mesenchyme of adult mouse trachea with a rare epithelial labeled cell (arrowhead) using the same antibody. Scale bars: 20 μm.
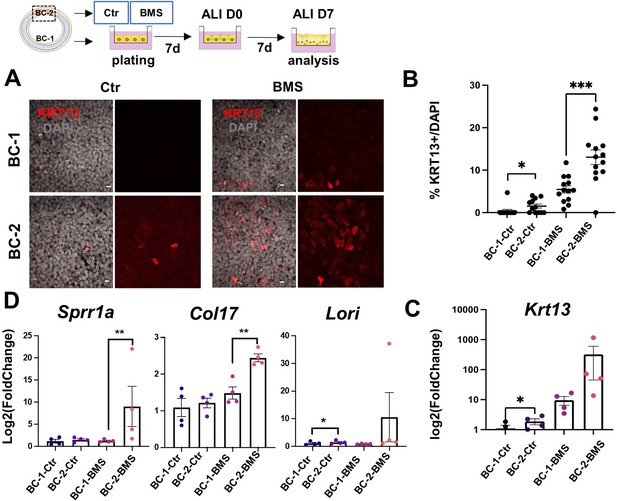
Differential induction of squamous-associated markers in retinoid-deficient BC-2-derived organotypic cultures.
Expansion and differentiation of BC-1 and BC-2 populations isolated from ventral or dorsal trachea, respectively, cultured in air-liquid-interface (ALI) in control or RAR antagonist (BMS493) conditions. (A) Increased KRT13 IF labeling in BC-2-derived cultures compared to BC-1 in both control and BMS-treated conditions. Scale bars: 10 μm. (B) Morphometric analysis of KRT13 + cells in ALI day 7 control or BMS-treated cultures. Each dot represents the percentage of KRT13 + cells in total cell population per field. Graph: mean ± SEM of more than 4 fields per batch, n=3 batches for each condition. (C–D) qPCR of Krt13, Sprr1a, Col17 and Loricrin in ALI day 7 control or BMS-treated cultures. Values [log2(foldchange)] are normalized to BC-1 controls. Bars are mean ± SE of n=4 replicates per culture condition. Student’s t-test, *p<0.05, **p<0.01, ***p<0.001.
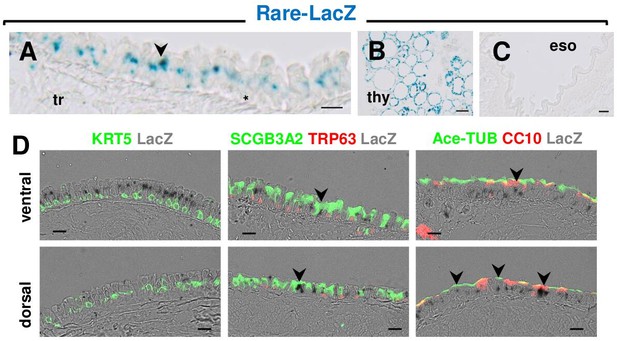
RAR signaling is active in ventral and dorsal luminal cells but not in basal cells (BCs) of the adult tracheal epithelium during homeostasis.
(A–C) X-gal staining of adult trachea (tr), thyroid (thy) and esophagus (eso) from RARE-LacZ reporter mice showing strong LacZ expression in the tracheal and thyroid cells (positive control) but not in esophageal cells (negative control). (D) Tracheal sections from RARE-LacZ adult mice showing LacZ signals (arrowheads) co-labeled with markers of secretory (SSCGB3A2, CC10) and multiciliated cells (Ace-a-TUBULIN) but not BCs (KRT5, TRP63). Scale bars: A: 15 μm, D: 20 μm.
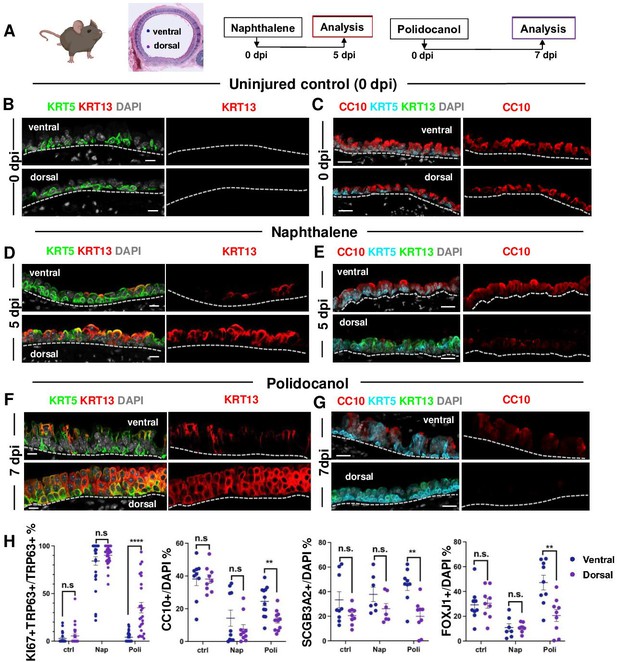
BC-2 and BC-1 have distinct behaviors during initiation of repair of the damaged epithelium in mouse models of injury.
(A) Diagram: strategy for induction of airway injury by Naphthalene (Nap, intraperitoneal) or Polidocanol (Poli, intratracheal) and analysis at 5dpi post-Nap or 7dpi post-Poli. (B–C) IF of tracheal sections from adult uninjured animals (0dpi, uninjured control, n=4 mice per group) showing typical dorsal-ventral distribution of KRT5 and CC10 (no KRT13 signals) in the basal and luminal layers, respectively. (D–G) Markedly distinct kinetics of repopulation of the airway epithelium by BCs from the ventral (BC-1) compared to dorsal (BC-2) regions at 5dpi (Naphthalene) or 7dpi (Polidocanol) (n=3 mice per group): extensive KRT13 expression in KRT5 + and luminal cells of the dorsal epithelium and low CC10 expression. (H) Morphometric analysis: percentage of proliferating (KI67+) BCs, and luminal (CC10+, SCGB3A2+, FOXJ1+) cells in ventral or dorsal epithelium of 5dpi or 7dpi (see also Figure S10, S11). For KI67 +TRP63+/TRP63+% (far left panel), each dot represents the percentage of proliferating BCs (KI67 +TRP63+) in total BC population (TRP63+) from each view. Graphs: mean ± SEM of 2–20 views per animal; 0dpi-control: n=4 animals; 5dpi-Nap: n=3 animals; 7dpi-Poli: n=3 animals. For CC10+/DAPI%, SCGB3A2+/DAPI%, FOXJ1+/DAPI% (right three panels), each dot represents the percentage of each lineage-committed cells in total epithelial population from each view. Graphs: mean ± SEM of 1–5 views per animal; 0dpi-control: n=4 animals; 5dpi-Nap: n=2–3 animals; 7dpi-Poli: n=3–4 animals. Student’s t-test, **p<0.01, ***p<0.001, ****p<0.0001, n.s., not significant. Scale bars:10μm.
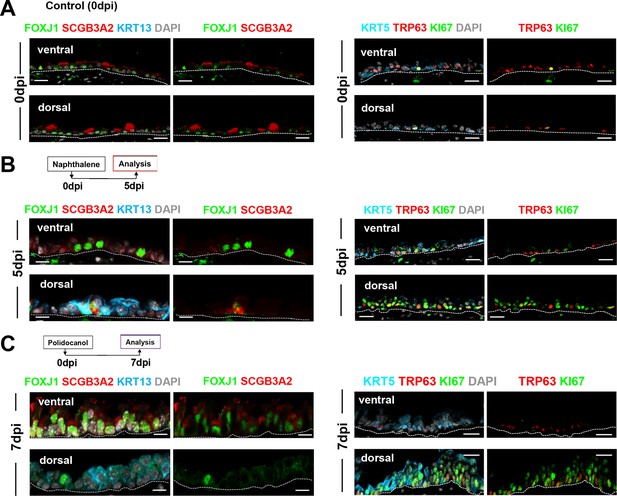
Distinct kinetics of repopulation between the ventral and dorsal BC-derived tracheal epithelium post-Naphthalene (5dpi) and post-Polidocanol (7dpi) injury in adult mice.
IF of representative areas showing ventral and dorsal distribution of markers of secretory (SCGB3A2), multiciliated (FOXJ1), BC (TRP63, KRT5), proliferation (KI67) and metaplastic (KRT13) cells. Extensive proliferation and expansion of KRT13 + cells at the costs of differentiation in dorsal BC-derived epithelium compared with ventral epithelium (see quantitative analysis in Figure 5H).
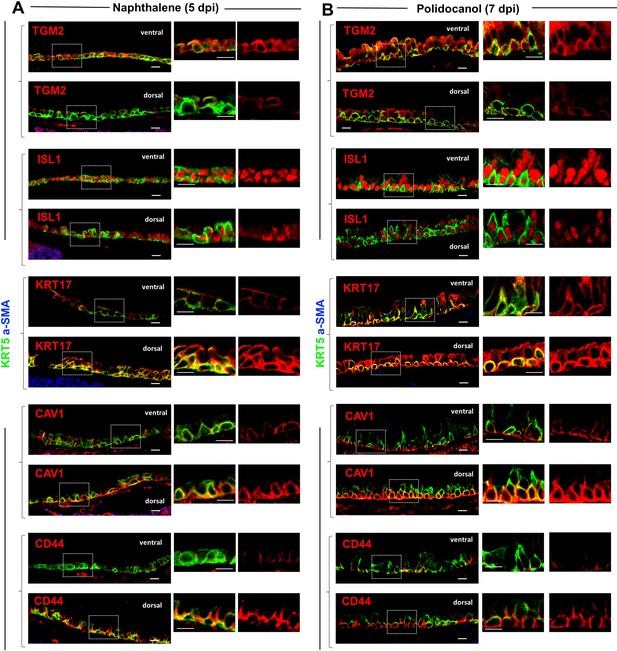
BC-1 and BC-2 markers maintain their differential ventral-dorsal enrichment in basal cells during repopulation of the airway epithelium post-injury.
Immunofluorescence of KRT5, a-SMA, BC-1 (TGM2, ISL1) and BC-2 (KRT17, CAV1, and CD44) markers in ventral and dorsal tracheal epithelium of 5dpi-Naphthalene or 7dpi-Polidocanol. Small panels are higher magnification images of the boxed areas depicting expression of each marker alone (right) or double-labeled with KRT5 (left). a-SMA identifies the smooth muscle layer in dorsal trachea (not displayed in all panels here). Scale bar: 10 μm.
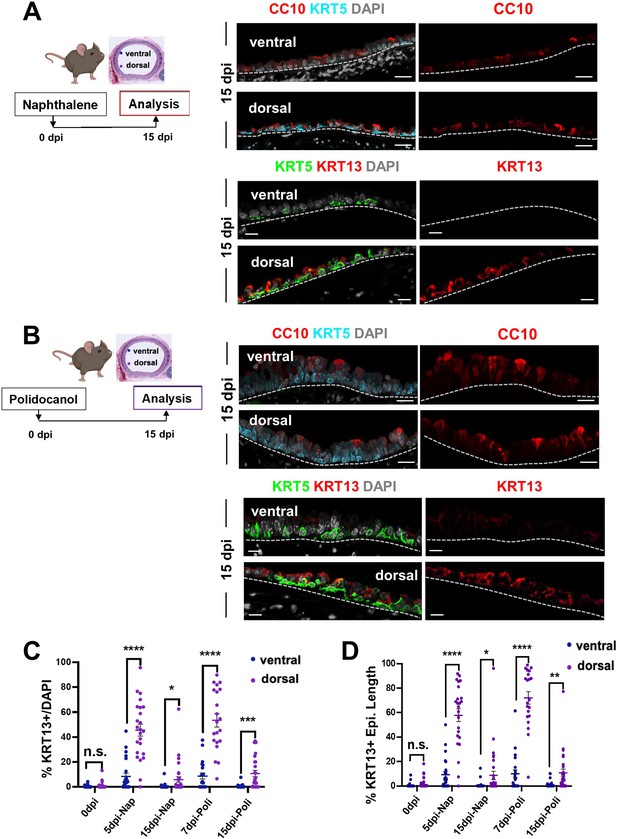
Regional differences in ventral-dorsal programs of repopulation are no longer evident at late stages of regeneration post injury.
(A–B) IF of KRT5 and CC10 in ventral and dorsal tracheal epithelium of 15dpi-Naphthalene or 15dpi-Polidocanol. (C–D) Morphometric analysis of KRT13 + cells in the ventral or dorsal adult tracheal epithelium of uninjured control mice (0dpi), and at early and late stages of regeneration (Naphthalene: 5dpi and 15dpi; Polidocanol: 7dpi and 15dpi). Graph (C): scattered dots representing % KRT13 + cells in total epithelial population from each field with mean ± SEM from n ≥ 21 paired fields (0dpi uninjured control n=59 from 14 sections, 4 animals; 5dpi-Nap n=24 from 9 sections, 3 animals; 15dpi-Nap n=36 from 10 sections, 3 animals; 7dpi-Poli n=21 from 9 sections, 3 animals; 15dpi-Poli n=28 from 9 sections, 3 animals) in different regions. Graph (D) Scattered dots representing % length of epithelium containing KRT13 + cells in total epithelium from each field, with mean ± SEM from n ≥ 21 paired views at the same tissue locations. Student’s t-test, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001; n.s., not significant. Scale bars, 10 μm.
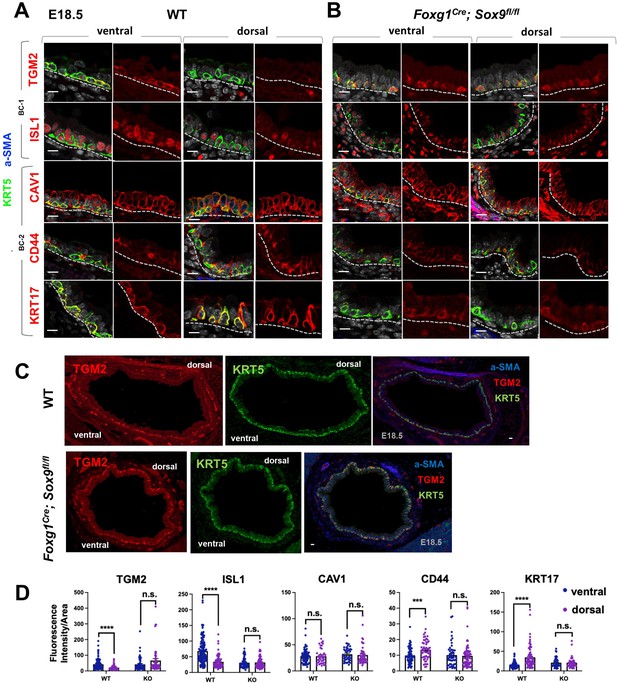
Basal cell heterogeneity is established during embryonic development.
(A–B) IF of BC-1 and BC-2 markers co-labeled with KRT5 in tracheal sections from E18.5 WT and Foxg1Cre; Sox9fl/fl littermates. BC-1 and BC-2 markers maintain the ventral-dorsal differential enrichment of adult WT trachea BCs (a-SMA marks the smooth muscle in dorsal trachea). This pattern is abolished in BCs from E18.5 mutant tracheas. (C) Broad view of a cross section from E18.5 WT and mutant tracheas depicting the TGM2 distribution in BCs also seen in (A), (B). (D) Quantification of Fluorescence intensity of markers in E18.5 ventral and dorsal BCs from WT and mutants. Bars represent the mean ± SEM of average fluorescence intensity values in a single KRT5 +BC (dots, already clarified in methods), n=3 animals per genotype. Student’s t-test, ***p<0.001, ****p<0.0001; n.s., not significant. Scale bars: 10 μm.
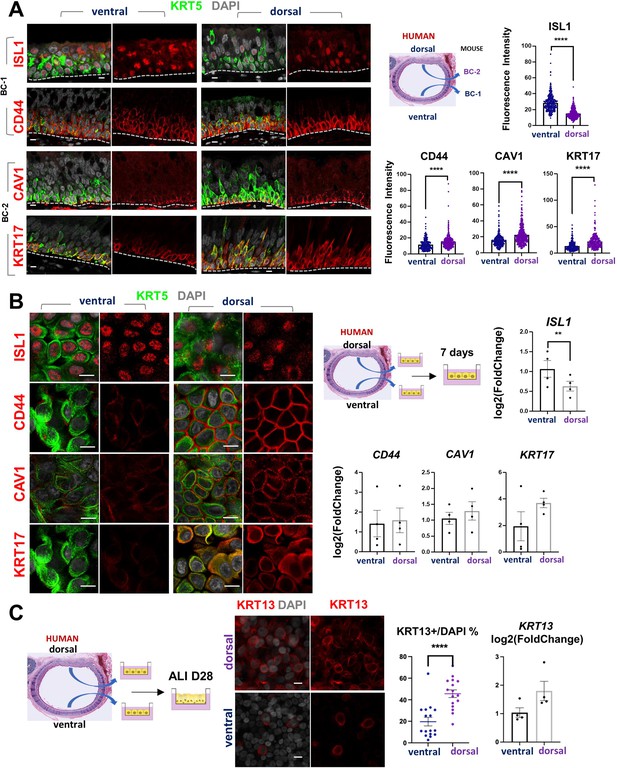
Airway basal cell spatial heterogeneity is conserved in human airways.
(A) IF of ISL1, CD44, CAV1 and KRT17 co-stained with KRT5 in adult human tracheal sections. Quantification of fluorescence intensity in ventral and dorsal BCs from paired regionally distinct areas. Bar graphs are mean ± SEM of the values from each BC (dots), n ≥ 227 BCs from 3 different human donors. (B) Differential enrichment of BC-1 and BC-2 markers in ventral and dorsal cultured human BCs, respectively. littermate control of BC-1 and BC-2 markers in 7 days submerged cultures of BCs isolated from ventral and dorsal sides of adult human donor tracheal epithelium. qPCR of ISL1, CD44, CAV1, and KRT17 expression in these cultures. Bar graphs are mean ± SEM log2(foldchange) values for each replicate culture (n=3). Data are normalized to ventral BCs. (C) IF of KRT13 in ALI day 28 cultures of human ventral and dorsal tracheal BCs. Scattered plot (left): percentage of KRT13 + cells per field in total ventral or dorsal BC population; mean ± SEM from 5 to 6 fields per cultures, n=3 batches. Bar graph (right): qPCR of KRT13 expression in ALI day 28 cultures from ventral and dorsal human BCs. Values (log2(foldchange)) are normalized to ventral BCs. Bars are mean ± SEM, dots representing values of each replicate. n=4 batches. Student’s t-test, **p<0.01, ****p<0.0001. Scale bars represent 10 μm.





