Retinopathy of prematurity: Metabolic risk factors
Abstract
At preterm birth, the retina is incompletely vascularized. Retinopathy of prematurity (ROP) is initiated by the postnatal suppression of physiological retinal vascular development that would normally occur in utero. As the neural retina slowly matures, increasing metabolic demand including in the peripheral avascular retina, leads to signals for compensatory but pathological neovascularization. Currently, only late neovascular ROP is treated. ROP could be prevented by promoting normal vascular growth. Early perinatal metabolic dysregulation is a strong but understudied risk factor for ROP and other long-term sequelae of preterm birth. We will discuss the metabolic and oxygen needs of retina, current treatments, and potential interventions to promote normal vessel growth including control of postnatal hyperglycemia, dyslipidemia and hyperoxia-induced retinal metabolic alterations. Early supplementation of missing nutrients and growth factors and control of supplemental oxygen promotes physiological retinal development. We will discuss the current knowledge gap in retinal metabolism after preterm birth.
Introduction
Preterm birth is common worldwide. In addition to increased mortality, the short- and long-term complications arising from the arrest of normal development after premature birth include retinopathy of prematurity (ROP) as well as delayed physical growth, vascular abnormalities (including intraventricular hemorrhage), pulmonary disease, sepsis, poor neurocognitive development, and metabolic dysregulation with increased risk of diabetes in young adulthood (Ramel and Rao, 2020; Ramel et al., 2013). In humans, retinal vascular development begins in the second trimester of pregnancy and is complete around term (Roth, 1977). In infants who are born extremely preterm, before 28 weeks of gestation, the retina is incompletely vascularized at birth. The lower the gestational age (GA) age at birth, the less developed the neural retina and the larger the area of peripheral avascularity. Promoting physiological retinal neurovascular development after preterm birth would be of great benefit in preventing blinding neovascular ROP.
In Phase I ROP, which starts immediately after preterm birth, physiological retinal vascular growth is inhibited. The first metabolic disruption postnatally is excess oxygen; even room air can increase oxygen saturation (SpO2) above that in utero (Hutter et al., 2010; Lara-Cantón et al., 2022). More problematic is supplemental oxygen, often given to preterm infants to overcome poor lung function to reduce mortality. Hyperoxia inhibits normal retinal vascular development by suppressing oxygen-regulated vascular and neural growth factors. A balance must be found, with individualization of oxygen supplementation based on the GA, postnatal age and sex to optimize retinal and systemic outcomes (Oei et al., 2017; Oei and Vento, 2019; Vento et al., 2013). In addition to disruption of oxygen, there are metabolic fuel imbalances caused by relative starvation, hyperglycemia, and inadequate supplies of specific amino acids and lipids as well as hormonal imbalances, all of which inhibit normal vascular growth.
In Phase II ROP, the avascular retina limits the delivery of both oxygen and nutrients causing hypoxia and fuel deficiency in the non-vascularized but slowly maturing retina. Retinal metabolism is also limited by a shortage of growth factors/nutrients normally provided by the mother during pregnancy which are missing after premature birth (Figure 1; Tomita et al., 2021b). Phase II ROP is driven by hypoxia and nutrient deficits that cause a massive release of vaso-formative factors triggering vision-threatening uncontrolled neovessel growth. Therefore, improving retinal vascularization during Phase I ROP will prevent the impairment in the vascular supply of oxygen and nutrients to meet the demand of growing neurons and prevents the progression to neovascularization of Phase II ROP.
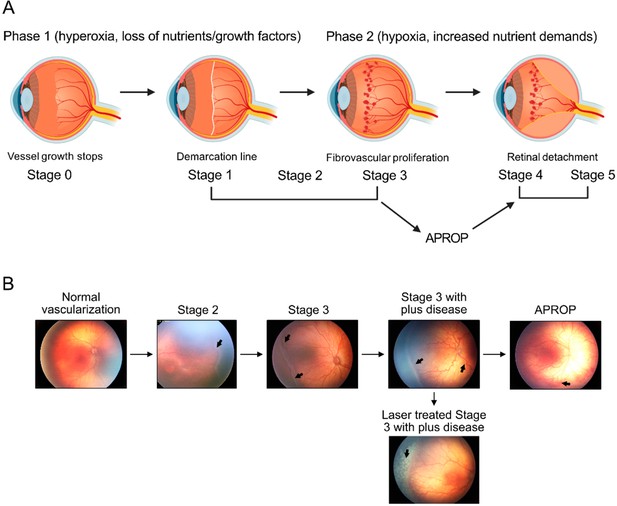
Schematics of ROP development (A) and illustration of human ROP development.
In ROP, hyperglycemia and hyperoxia causes retinal vessel growth cessation (Phase 1). As the neural retina matures, increasing nutrient and oxygen demand triggers retinal neovessel growth (Phase 2). Human neovascular ROP (Phase 2) develops through the following stages: stage 2 with ridge (arrow), stage 3 with neovascularization and hemorrhage (arrows), stage 3 with plus disease (dilation and tortuosity of vessels) (arrow), Aggressive posterior ROP (APROP) with central changes (arrow) is particularly pathological. Laser treatment (arrow) of stage 3 ROP is illustrated. Figure was reproduced from Figure 1 from Tomita et al., 2021b.
Retinal and choroidal vascular development
To promote normal inner retinal vascularization, it is important to understand major imbalances in the normal metabolic driving forces. Vascularization of the maturing neural retina is normally stimulated by increased energy demands that create a wave of fuel and oxygen deficits moving from the optic nerve to the periphery as the retina matures that stimulates vaso-formative factors at the wave front. At the leading edge of the wave, the vaso-formative factors stimulate the physiological outgrowth of the vasculature which relieves the hypoxia and nutrient deficiency and locally suppresses the production of vaso-formative factors. In front of the wave, there is further maturation, further deficits of nutrients and oxygen, and further expression of vaso-formative factors (in particular vascular endothelial growth factor, VEGF) moving the vascularization process forward (Chan-Ling et al., 1995; Joyal et al., 2018; Joyal et al., 2016; Pierce et al., 1996; Shih et al., 2003). In the incompletely developed retina of a preterm child, oxygen supplementation can cause pruning of immature formed inner retinal vessels and suppression of new physiological vessel growth (Phase I ROP) (Figure 2).
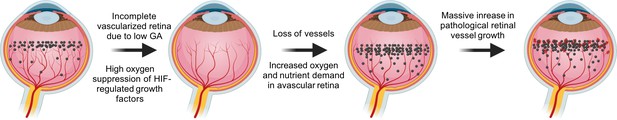
VEGF in the pathogenesis of ROP.
During normal retinal vascular development, growth factor like VEGF (black dots) is found anterior to the developing vasculature driving the normal retinal vessel development forward. After preterm birth, in Phase I ROP, hyperoxia suppresses HIF-regulated growth factor (VEGF) production, causing vaso-obliteration and vessel growth cessation. As the retina matures with increasing metabolic demand, the non-perfused peripheral retina becomes hypoxic and nutrient deprived and overproduces growth factors (VEGF). Neovascularization occurs in response to high levels of growth factors. Images were created using BioRender, adapted and modified from ‘retina’, ‘dots’ by BioRender.com (2022).
There are two vascular systems in the retina. The inner retina is supplied by three layers of interconnected vessels that develop as described above. The outer retina, particularly the retinal pigment epithelium (RPE) and photoreceptors is supplied by a vascular plexus, the choriocapillaris, the precursors of which emerge at the fourth week of gestation. Most of the choroidal vasculature matures during the third and fourth months of gestation (Anand-Apte and Hollyfield, 2010). VEGFA is essential to maintain choroidal structure and function (Kim et al., 2020). In rodent oxygen-induced retinopathy (OIR) (Figure 3), the retardation of choroidal vascular development starts from postnatal day 7 during hyperoxia exposure and persists (Kim et al., 2018; Shao et al., 2011), suggesting that nutrient and oxygen supply from the choroidal vascular system to the RPE and photoreceptors is also affected with oxygen supplementation.
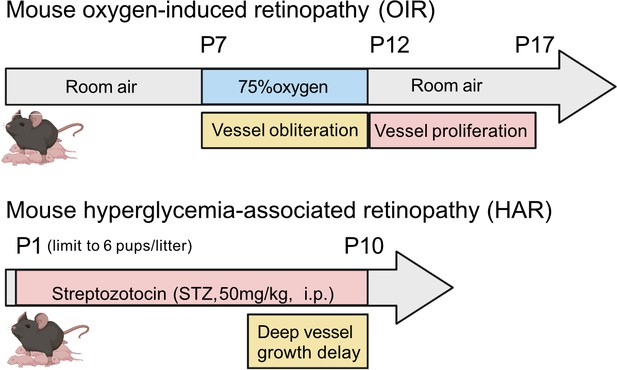
Schematics of mouse oxygen-induced retinopathy (OIR) and hyperglycemia-associated retinopathy (HAR).
In OIR, mouse pups and the nursing dam are exposed to 75% oxygen from postnatal day (P) 7 for 5 days causing vessel loss and cessation of vessel growth and returned to room air where the avascular retina becomes hypoxic and causes neovascularization. There is also a metabolic model of suppression of retinal vessel growth as seen in Phase I ROP. In HAR, mouse pups are given low dose streptozotocin (STZ 50 mg/kg) daily from P1 to P9, causing hyperglycemia which suppresses normal vascular development examined at P10. Images were created using BioRender, adapted and modified from “mouse” by BioRender.com (2022).
Oxygen and fuel in the mature and immature retina
Oxygen gradients in the retina
The normal mature vascularized retina is highly metabolically active and consumes oxygen avidly (Wangsa-Wirawan and Linsenmeier, 2003). Oxygen tension falls steeply from the choriocapillaris plexus to the photoreceptor inner segments, where SpO2 is close to zero, likely secondary to very high flux (Figure 4; Linsenmeier and Zhang, 2017). The SpO2 then increases gradually across the outer retina. In the inner retina, oxygen peaks are present close to inner retinal vessels (Cringle et al., 1991; Linsenmeier, 1986). Inner retinal oxygen tension is regulated through vaso-constriction of vessels to control blood flow (Riva et al., 1983). With high oxygen supplementation causing hyperoxia, the choroidal oxygen tension increases dramatically and a greater portion of the retina can then be supplied by the choroid (Cringle and Yu, 2018; Cringle et al., 1991; Linsenmeier and Yancey, 1989). Under conditions of hypoxia, the inner retina oxygen tension is regulated with increased retinal blood flow (Ahmed et al., 2001; Eperon et al., 1975; Palkovits et al., 2014; Wangsa-Wirawan and Linsenmeier, 2003). In the choroid however, hypoxia leads to a steep decrease in oxygen tension and causes a large decrease in photoreceptor oxygen consumption in dark-adapted retina but only mild changes are seen in light-adapted retina, which consumes less energy than in the dark (Joyal et al., 2018; Linsenmeier and Braun, 1992; Wangsa-Wirawan and Linsenmeier, 2003).
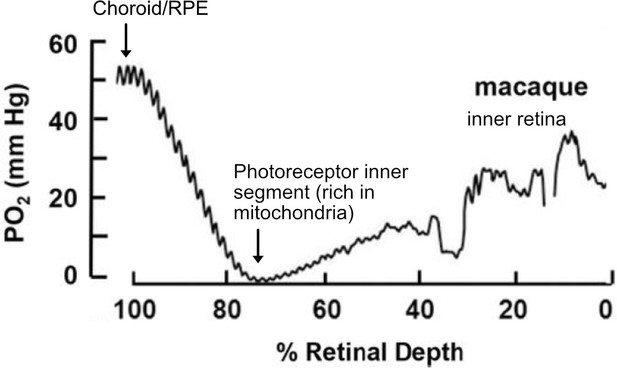
O2 profiles through the dark-adapted retina of rhesus monkey.
A retinal depth of zero is the vitreoretinal border and 100% is the RPE-choroid border. The lowest oxygen tension is found in the layer containing photoreceptor inner segments with very high mitochondrial density. Rising oxygen tension across the inner retinal layers is due to three layers of inner retinal vasculature. Figure was reproduced from Figure 1 from Linsenmeier and Zhang, 2017. License number 5416050680408.
Hyperoxia, hypoxia, and ROP
Supplemental oxygen, besides increasing the risk of ROP, increases the level of reactive oxygen species (ROS), exceeding the capacity of the immature antioxidant defense system in preterm infants. Excess ROS damages DNA, RNA, lipids, proteins, membranes, and organelles. In premature infants with ROP vs. no ROP, there are lower levels of the antioxidants superoxide dismutase and glutathione in mitochondria (Lynch et al., 2016; Oziebło-Kupczyk et al., 2006) and higher total levels of pro-oxidants and malondialdehyde (Banjac et al., 2018). Modulation of redox homeostasis may help control cellular damage and should be considered to prevent ROP and other complications of preterm birth.
Low levels of oxygen are also damaging. Hypoxia induces nitric oxide synthases (NOSs) and releases NO (Jung et al., 2000), which is a strong competitor of oxygen for cytochrome c oxidase in the electron transport chain. Cells also decrease protein synthesis and Na-K-ATPase activity (a major ATP consumer) to reduce ATP demand by modulating AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) complex 1 (mTORC1) under hypoxic conditions (Gusarova et al., 2011). These hypoxia-induced adaptive pathways have been examined in mouse OIR retinas. Hypoxia (via induction of Adora2a) promotes endothelial cell glycolytic enzyme expression (Liu et al., 2017). Inhibition of endothelial NOS (eNOS) and inducible NOS (iNOS) inhibits retinal neovascularization (Brooks et al., 2001; Ninchoji et al., 2021; Zhang et al., 2009). iNOS expressed in the avascular retina increases retinal neuronal cell death (Sennlaub et al., 2002). pNaKtide, which inhibits Na-K-ATPase ROS amplification, reduces retinal neovascularization by decreasing HIF and VEGF levels. mTOR inhibitors (rapamycin and everolimus) reduce retinal neovascular tuft formation and rapamycin decreases the activation of cyclin D1 (an important regulator of cell cycle progression) in OIR retinas (Jiang et al., 2020; Yagasaki et al., 2014).
Since Phase I ROP involves oxygen-induced suppression of VEGF, which directs physiological vascularization, another approach to prevent Phase I ROP is to stabilize the upstream control of VEGF and other oxygen-regulated factors, including hypoxia-induced factor 1 (HIF1). HIF is degraded with hyperoxia, decreasing physiological VEGF production and suppressing physiological vessel growth. Stabilization of HIF1 with prolyl hydroxylase inhibition (systemic Roxadustat) in hyperoxia-induced Phase I ROP (OIR) suggests that serine and one-carbon metabolism may protect against vaso-obliteration (Singh et al., 2019). However, Phase II ROP can potentially be exacerbated with the stabilization of HIF1. In Phase II ROP, hypoxia in the avascular retina leads to stabilization of HIF1 protein and increased VEGF expression.
Hyperoxia and hypoxia through HIF alter multiple metabolic pathways. Hypoxia-induced HIF stabilization not only increases VEGF production to trigger new vessel growth to deliver more oxygen, but also modulates mitochondrial function to adapt to low oxygen status (Solaini et al., 2010; Wheaton and Chandel, 2011). In addition to mediating oxygen effects on the retina, HIF also affects energy metabolism through glycolysis and mitochondrial oxidative phosphorylation (OXPHOS) (Solaini et al., 2010; Wheaton and Chandel, 2011). HIF induces the expression of glucose transporters and glycolytic enzymes to enhance glycolysis and also activates pyruvate dehydrogenase kinase 1 and inactivates pyruvate dehydrogenase (Kierans and Taylor, 2021; Kim et al., 2006; Mobasheri et al., 2005), thereby preventing the conversion of pyruvate to acetyl-CoA for mitochondrial function.
Taken together, changes in oxygen alter multiple metabolic pathways and modulation of these pathways may help to prevent pathological retinal angiogenesis. Control of oxygen in Phase I ROP is essential to prevent suppression of retinal vessel growth and the progression to Phase II ROP (Oei et al., 2017; Oei and Vento, 2019; Vento et al., 2013).
Fuels used in retina
Glucose metabolism in the retina
Glucose as photoreceptor fuel
Glucose is the major fuel source for the retina, particularly photoreceptors, but it is primarily metabolized through aerobic glycolysis rather than OXPHOS despite the high density of mitochondria in photoreceptors (Cohen and Noell, 1960; Joyal et al., 2018). Photoreceptors lack direct contact with blood vessels, but adjacent RPE cells which are in contact with the high flow choroidal vasculature preferentially pass glucose to them (Kanow et al., 2017) while RPE itself can use fatty acids, amino acids, succinate and lactate both from the blood and from the retina as fuel (Hurley, 2021). In animal studies, when RPE is genetically manipulated to consume glucose, thereby decreasing glucose transport from RPE to photoreceptors, photoreceptors die (Zhao et al., 2011). Disruption of rod photoreceptor glycolysis with rod-specific knockdown of the rate-limiting glycolytic enzyme hexokinase 2 decreases photoreceptor function and increases photoreceptor mitochondrial mass, suggesting that OXPHOS is increased under the metabolic stress of decreased glycolysis (Petit et al., 2018). There is additional evidence that photoreceptors use glucose primarily for aerobic glycolysis to produce lactate (Joyal et al., 2018). Photoreceptors express high levels of glycolytic enzymes favoring lactate production, which is mainly transported to RPE and Müller glia as energy sources instead of being metabolized in photoreceptors (Country, 2017).
Hyperglycemia in ROP
Although glucose is the major fuel of photoreceptors, excess glucose is damaging. Hyperglycemia in the early postnatal period strongly influences the incidence and severity of ROP (Blanco et al., 2006; Chavez-Valdez et al., 2011; Mohsen et al., 2014). Preterm infants are born with an immature gastrointestinal tract that limits enteral nutrition, which in combination with low body fat reserves and high metabolic demands, may result in inadequate nutrition (starvation) (Webbe et al., 2022). Starvation triggers insulin resistance, hepatic gluconeogenesis, and hyperglycemia (Whitfield and Hendrikson, 2006). Postnatal hyperglycemia is found in ~80% of premature infants with birth weights of less than 750 grams and ~45% with birth weights of less than 1000 grams (Binder et al., 1989).
Animal models of metabolic influences on Phase I ROP have increased our understanding of the impact of hyperglycemia and dyslipidemia on postnatal retinal vascular development. In the hyperglycemia models of Phase I ROP in neonatal (although not preterm) rodents, as opposed to preterm infants, animals are metabolically developed and not starved and the response to hyperglycemia may not be the same. Streptozotocin (STZ)-induced postnatal hyperglycemia only partially mimics preterm hyperglycemia which results from both insulin deficiency and insulin resistance (Salis et al., 2017) and immature metabolic pathways (Whitfield and Hendrikson, 2006). In the eye in both neonatal rats and mice, hyperglycemia attenuates retinal vessel growth, increases inflammation and disrupts glucose metabolism (Fu et al., 2018a; Kermorvant-Duchemin et al., 2013). Hyperglycemia increases retinal apoptosis. There are fewer photoreceptors and other cells in the inner neuronal layer even after the hyperglycemia is resolved. In mice, neonatal hyperglycemia (Figure 3) suppresses the expression of genes involved in metabolism and there is delayed growth of all major retinal neurons (Fu et al., 2022). Decreased retinal neuronal activity (and cell number) persist into adulthood (Fu et al., 2018a). In mice, neonatal hyperglycemia also exacerbates hypoxia-induced retinal neovessel growth (Cakir et al., 2020). These findings correspond to clinical characteristics of ROP with hyperglycemia-associated retinal vascular growth delay in Phase I and increased disease severity in Phase II suggesting the utility of these Phase I ROP models despite the limitations noted above.
Mitochondrial OXPHOS is necessary for eye function
Even though glucose is preferentially used as fuel by photoreceptors and glucose is primarily metabolized through aerobic glycolysis and not through mitochondrial OXPHOS, the very high density of mitochondria in the photoreceptor inner segment suggests that maintaining mitochondrial activity and OXPHOS is important to retinal function (Ball et al., 2022). There is genetic evidence of the importance of mitochondrial function in the eye. Leber hereditary optic neuropathy, affecting retinal ganglion cells is caused by a mutation in the MT-ND5 gene (m.13345G>A), which affects mitochondrial complex I (Engvall et al., 2021). Other mitochondrial genetic disorders often have eye manifestations such as increased autofluorescence, macular dystrophy, yellow subretinal deposits, and abnormal electroretinogram (ERG) signals (Birtel et al., 2022; Daruich et al., 2014; Rath et al., 2008; Yu-Wai-Man and Newman, 2017), suggesting a disruption in RPE and photoreceptors.
Mitochondria in isolated mouse rod photoreceptors generate substantial nicotinamide adenine dinucleotide phosphate (NADPH) using glutamine in the absence of extracellular metabolic substrates which may support cell function under nutrient shortage (Adler et al., 2014). In zebrafish, overexpression of mitochondrial Ca2+ uniporter in cone photoreceptors enhances the TCA cycle and accelerates recovery kinetics of the cone response to light (Hutto et al., 2020), suggesting that improving photoreceptor mitochondrial energy production to prevent retinal neural dysfunction may be feasible. This concept is supported by the evidence that defects in ERG signals of mice lacking a subunit of mitochondrial complex I in vivo can be rescued by modulating the extracellular environment ex vivo when retinas are supplemented with proper nutrients (Gospe et al., 2019).
As glucose is mainly used for aerobic glycolysis in the retina, not mitochondrial OXPHOS, mitochondria also use alternative energy sources, particularly during stress conditions (Chinchore et al., 2017; Casson et al., 2016; Joyal et al., 2018; Joyal et al., 2016; Lindsay et al., 2014; Rajala et al., 2016; Reading, 1965; Reidel et al., 2011; Wang et al., 1997). Two major unanswered questions are (I) what fuels other than glucose are used in mitochondrial OXPHOS and (II) can these fuels or other molecules be provided to improve retinal metabolism in preterm infants?
Lipids
There is disrupted fatty acid oxidation in ROP infants (Yang et al., 2020). Lipid deficiency (including short-chain and long-chain fatty acids) in photoreceptors causes retinal degeneration and abnormal retinal vessel growth (Joyal et al., 2016). The saturated fatty acid palmitate (C16) has been shown to be a fuel source for OXPHOS in photoreceptors (Joyal et al., 2016). Other lipids have not yet been assessed.
Hypertriglyceridemia is correlated with an increased risk for ROP although the association becomes non-significant after adjustment for gestational age and birth weight (Sinclair et al., 2018). Moreover, blood proteins involved in lipid metabolism are associated with ROP (Danielsson et al., 2022). More than being a source for energy production, many lipids are bioactive and are involved in the regulation of diverse biological functions. Dietary essential omega-3 and omega-6 fatty acids are partly oxidized, but importantly, they also serve as substrates for the production of a large family of signaling molecules with metabolic and immune regulatory activities. Deficiency of omega-3 docosahexaenoic acid (DHA) and omega-6 arachidonic acid (ARA) in preterm infants causes developmental delay (Hadley et al., 2016; Smith and Rouse, 2017). The levels of circulating DHA, ARA, as well as the signaling sphingolipid sphingosine-1-phosphate (S1P) are inversely correlated with the risk of developing ROP (Hellström et al., 2021b; Löfqvist et al., 2018; Nilsson et al., 2021; Table 1). Interestingly, ARA vs. DHA better preserves retinal metabolism in mice with postnatal hyperglycemia (Fu et al., 2022).
Summary of metabolic risk factors for ROP.
| Risk factors | Comparison | Outcomes | References |
|---|---|---|---|
| DHA | no ROP, mild or moderate ROP (stage 1–2), or severe ROP (stage 3 and type 1). | High serum DHA correlated with less severe ROP, only in infants with sufficiently high ARA levels | Hellström et al., 2021b |
| Enteral DHA vs. placebo | No difference in any stage of ROP, but DHA lowered the relative risk for severe ROP. | Bernabe-García et al., 2019 | |
| ARA | ROP vs. no ROP | Low serum ARA correlated with ROP development | Löfqvist et al., 2018 |
| DHA +ARA | Enteral DHA +ARA vs. no supplementation | DHA:ARA at 1:2 ratio lowered severe ROP (stage 3 and/or type 1). | Hellström et al., 2021a |
| Metabolites | ROP vs. no ROP | Higher levels of glycolytic intermediates (pyruvate, lactate), lower levels of TCA metabolites (citrate, aconitate, succinyl carnitine), higher malonyl carnitine (C3DC), glycine in ROP | Yang et al., 2022; Yang et al., 2020 |
| Insulin | No or mild ROP (1–2) vs. severe ROP (3-4) | Insulin exposure was a stronger predictor for severe ROP than hyperglycemia per se | Kaempf et al., 2011 |
| No or mild ROP vs severe ROP (needing treatment) | Blood glucose >150 mg/ml and insulin exposure associated with severe ROP | Lee et al., 2016 | |
| IGF-1 | No ROP, ROP (1,2, 3-4) | Low plasma IGF-1 correlated with high glucose levels and increased ROP severity | Cakir et al., 2020 |
| APN | No ROP stage vs. any ROP | Low serum APN correlated with ROP; serum APN positively correlated with serum DHA | Fu et al., 2015a |
| Plasma glucose tertiles and retinal vascular coverage in preterm infants | Low serum APN correlated with high glucose levels and delayed retinal vascularization | Fu et al., 2018a |
Peroxisomes may play a role in supplying appropriate lipids for optimal retinal mitochondrial metabolism. Although mitochondria are the primary organelle for fatty acid oxidation, peroxisomes uniquely oxidize very long-chain fatty acids which cannot be processed by mitochondria into shorter chains that can be used. Peroxisomal disorders are associated with retinal ganglion cell loss, and retinal degeneration (Chen et al., 2022; Das et al., 2021). In Zellweger syndrome with mutations in PEX genes involved in peroxisomal biogenesis, there is loss of retinal cells (photoreceptors, retinal ganglion cells) and diminished ERG signals. Gene defects in peroxisomal α- and β-oxidation may also cause attenuated light responses, decreased visual acuity, and blindness (Chen et al., 2022; Das et al., 2021). Although omega-3 long-chain polyunsaturated fatty acids are primarily obtained from dietary sources, there is some endogenous production from shorter-chain precursors. Peroxisomes contribute to the endogenous conversion of precursor omega-3 fatty acids to DHA (Chen et al., 2022; Das et al., 2021). Oral DHA ethyl ester supplementation improves visual function in patients with peroxisomal disorders (Noguer and Martinez, 2010). Moreover, peroxisomes are key in maintaining redox homeostasis (Cipolla and Lodhi, 2017; Fransen et al., 2012). Preserving retinal lipid homeostasis by modulating lipid metabolism and supplementing proper types of lipids would likely benefit retinal health. More work is necessary to determine the specific interventions needed.
Amino acids
There is currently very limited direct evidence that amino acids serve as mitochondrial fuel in the retina, although gene mutations in endogenous serine synthetic enzyme phosphoglycerate dehydrogenase (PHGDH) are associated with macular degeneration (Gantner et al., 2019; Scerri et al., 2017). Amino acid (arginine, glutamine) metabolism is altered in hyperglycemia- and dyslipidemia-associated retinal disorders in adults and in preterm infants (Paris et al., 2016; Rhee et al., 2018; Tomita et al., 2021a). However, there may be abnormal amino acid profiles in preterm infants associated with ROP, along with higher than normal steady-state plasma levels of glycolytic intermediates (pyruvate, lactate) and intermediates associated with fatty acid metabolism, and lower than normal plasma levels of TCA metabolites (citrate, aconitate, succinyl carnitine) (Yang et al., 2022). This study found perturbed metabolism of lipids, arginine, glycine, serine and threonine, alanine and aspartate and glutamate. As parenteral versus enteral nutrition during the neonatal period has a profound impact on the serum metabolome (Nilsson et al., 2022; Vanhaesebrouck et al., 2008), further validation of longitudinal nutritional management, serum metabolome and ROP risks is needed. In mice modeling hypoxia-induced severe ROP, retinal changes include increased metabolites associated with lipids, glycine and serine and threonine metabolism (Tomita et al., 2021a). However, it remains to be determined if these adaptations in fatty acid and amino acid metabolism during hypoxia are transient or whether they have a long-term impact.
Recycling pathways in ROP
Retinal health depends on well-coordinated uptake, recycling and processing of nutrients and metabolites between cell organelles and through the ‘cell death’ and autophagy pathways. The metabolic interactions among retinal mitochondria, peroxisomes, lysosomes as well as endoplasmic reticulum need to be investigated. Disturbance of function in any of these organelles causes metabolic and cellular stress. ROP is associated with increased redox imbalance and disruption of the antioxidant system (Banjac et al., 2018; Boskabadi et al., 2021; Kumar et al., 2008; Lynch et al., 2016; Oziebło-Kupczyk et al., 2006; Spierer et al., 2005), ultimately leading to cell death.
The common types of cell demise including apoptosis, autophagy, necrosis and senescence have been reported in ROP retinas (Beauchamp et al., 2001; Binet et al., 2020; Crespo-Garcia et al., 2021; Oubaha et al., 2016; Pesce et al., 2021; Sennlaub et al., 2002; Sprott et al., 2019). Targeting retinal apoptosis and necrosis protects against hypoxia-induced neurovascular damage in mouse OIR (Beauchamp et al., 2001; Grant et al., 2020; Narayanan et al., 2014; Sennlaub et al., 2002). Emerging evidence has shown that targeting senescence and autophagy protects the retina in animal models of hypoxia-induced retinopathy.
Senescence and ROP
Recently, it has been shown that senescent cells accumulate in neovascular tufts in proliferative mouse OIR. Pharmacological inhibition of cellular senescence promotes neovessel regression and normal revascularization (Binet et al., 2020; Crespo-Garcia et al., 2021; Oubaha et al., 2016). Targeting endothelial cell senescence might be a way to ameliorate retinal proliferation in Phase II ROP without suppressing normal retinal vascularization as occurs with anti-VEGF treatment.
Autophagy, and other recycling pathways
Autophagy, a process in which lysosomes degrade and recycle cellular components, is key in preserving retinal homeostasis and sustaining metabolic function. Defects in autophagy also interrupt the RPE degradation of the photoreceptor outer segment and the recycling of proteins and lipids (Villarejo-Zori et al., 2021). Restoring photoreceptor autophagy dysregulated by accumulated circulating lipids enhances mitochondrial function and inhibits pathological retinal angiogenesis in mice (Heckel et al., 2022). Dysregulated retinal autophagic markers are reported in rat OIR and a potential association between autophagy and necroptosis (not apoptosis) is also observed (Pesce et al., 2021). However, pharmaceutical inhibition of autophagy does not restore neural retinal function compromised in OIR (Pesce et al., 2021). Loss of autophagy protein 5 in endothelial cells impairs mitochondrial function, decreases mitochondrial ROS and reduces retinal neovascular tuft formation in mouse OIR (Sprott et al., 2019). Further investigations of the role of autophagic responses in controlling retinal pathology are needed.
Hormonal influence in ROP: insulin, IGF-1, adiponectin, FGF21
Insulin
Glucose IV infusion is commonly used in premature infants to provide calories. However, preterm infants may be unable to use excess glucose properly due to immature regulation systems including insufficient insulin secretion and insulin insensitivity (Salis et al., 2017). Exogenous insulin is sometimes given to control hyperglycemia, but its use is controversial as it is generally ineffective at controlling hyperglycemia and insulin use is associated with increased mortality (Beardsall et al., 2008) and ROP (Kaempf et al., 2011; Lee et al., 2016). In preterm infants, other metabolic regulators like insulin-like growth factor-1 (IGF-1), adiponectin (APN) and FGF21 may better targets to control hyperglycemia (Table 1).
Insulin-like growth factor-1 (IGF-1)
IGF-1 deficiency in ROP
Loss of hormones as well as essential nutrients normally provided in utero also delays retinal vascularization. IGF-1, mainly derived from the liver, regulates body and retina growth (Daughaday and Rotwein, 1989; Liegl et al., 2016). IGF-1 levels fall immediately after birth in preterm infants and remain low for many weeks. IGF-1 is required for normal vessel growth in mice (Hellstrom et al., 2001) and low IGF-1 levels suppress VEGF activation of endothelial cell proliferation (phase I ROP) (Hellstrom et al., 2001; Smith et al., 1999). Low systemic IGF-1 levels correlate with a high risk for neovascular ROP (Cakir et al., 2020; Hård et al., 2013; Hellgren et al., 2021; Hellström et al., 2003; Hellstrom et al., 2001; Jensen et al., 2017). Low IGF-1 levels also correlate with low weekly platelet counts and ROP progression (Cakir et al., 2018; Hellgren et al., 2021; Jensen et al., 2018). Experimental studies demonstrate that IGF-1 supplementation before high oxygen challenge decreases retinal vessel loss and subsequent hypoxia-induced neovascularization in mice OIR (Vanhaesebrouck et al., 2009). Low levels of plasma IGF1 correlate with high plasma glucose in extremely preterm infants (Cakir et al., 2020). Experimental investigation also shows that induction of postnatal hyperglycemia in mouse OIR exacerbates retinal neovascularization and attenuates normal retinal vascularization (Cakir et al., 2020). Meanwhile, liver-derived IGF1 is reduced and recombinant human IGF-1 treatment improves normal retinal vasculature (Cakir et al., 2020). This finding suggests that replacing IGF-1 is a feasible approach to treat and prevent ROP.
Prediction of ROP based on IGF-1 and growth
Because circulating IGF-1 levels correlate with body growth and postnatal weight gain, poor postnatal weight gain during the first weeks of life can be substituted for IGF-1 levels to predict the development of ROP. WINROP, the first ROP prediction algorithm and online monitoring tool, was based on sex, GA, and both weekly weight and IGF-1 levels of preterm infants (Löfqvist et al., 2009). Later, WINROP was found to function well using only weight gain, omitting IGF-1 blood sampling (Hellstrom et al., 2009). As even accurate weight gain may be difficult to measure routinely in preterm infants, recently, DIGIROP using GA at birth, sex, standardized birth weight and age at the first sign of ROP was developed to predict the risk for severe ROP and shows high predicative ability in a contemporary Swedish cohort without using either IGF-1 or weight gain (Pivodic et al., 2022).
Adiponectin (APN) and Fibroblastic Growth Factor 21 (FGF21)
In mice with hyperglycemia-associated retinopathy (Figure 3), modeling Phase I ROP with suppression of physiological retinal vascularization, adiponectin (APN) is induced in response to insulin shortage; APN supplementation promotes physiological retinal vessel growth and improves long-term neural retinal function (Fu et al., 2018a). In addition, serum levels of liver-derived fibroblast growth factor 21 (FGF21), which is a metabolic regulator of APN production and secretion (Holland et al., 2013; Talukdar et al., 2016), normally increases immediately after birth (Sánchez-Infantes et al., 2015). However, FGF21 levels are very low in premature infants (below the sensitivity of most assays) (Guasti et al., 2014; Mericq et al., 2014). FGF21 treatment suppresses hypoxia-induced retinal neovessel growth through APN and protects neurons in diabetic retinopathy in mice (Fu et al., 2017; Fu et al., 2018b). In a phase 2 a clinical trial of a long-acting Fc-FGF21 fusion protein (efruxifermin), the hepatic fat fraction was reduced in adult patients with non-alcoholic steatohepatitis (ClinicalTrials.gov NCT03976401) (Harrison et al., 2021). AKR-001, an Fc-FGF21 analog, increases insulin sensitivity and reduces circulating lipids in adult diabetic patients (Kaufman et al., 2020). FGF21 treatment may also be a promising approach in preterm infants to promote retinal maturation by modulating insulin sensitivity and lipid metabolism.
Retinal-cell-specific contribution to vessel growth
To better understand the retinal fuel demand in ROP, we also need to consider the retinal-cell-specific fuel preferences and metabolism as there are significant interactions among these cells. A schematic of retinal structure is shown in Figure 5.
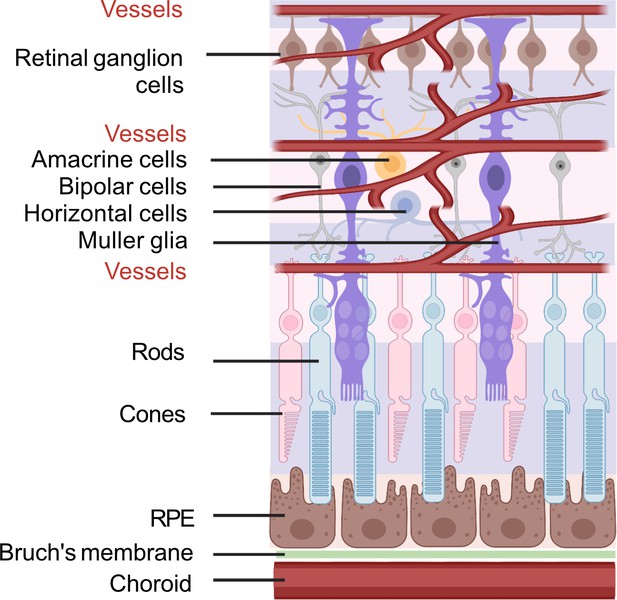
Schematic of retinal neuronal and vascular structure.
RGC, retinal ganglion cells, RPE, retinal pigment epithelium. Images were created using BioRender, adapted and modified from ‘eye’, ‘retinal cell’, ‘generic branching vessel’ by BioRender.com (2022).
Endothelial cells (EC)
Endothelial cell (EC) metabolism regulates proliferation and migration. EC glycolysis rather than OXPHOS generates ATP for vessel sprouting and loss of the rate-limiting enzyme in glycolysis 6-phosphofructo-2-kinase/fructose-2,6-biophosphatase 3 (PFKFB3) impairs tip cell formation (De Bock et al., 2013). Inhibition of EC glycolysis via targeting PFKFB3 or adenosine A2a receptor (ADORA2A) reduces retinal neovascularization in mice OIR (Liu et al., 2017; Schoors et al., 2014; Xu et al., 2014). Attenuation of the polyol pathway, which is induced under hyperglycemia, also decreases retinal neurovascular dysfunction in mouse OIR (Fu et al., 2015b; Fu et al., 2012). Increasing glycolysis by promoting glucose uptake during hyperoxia reduces retinal vessel loss and later neovascularization in rat OIR (Han et al., 2019). In addition to glycolysis, ECs rely on glutamine for vessel growth and blockade of glutamine use causes sprouting defects in physiological and pathological retinal angiogenesis (Huang et al., 2017). Loss of the endogenous serine synthetic enzyme PHGDH leads to EC death and impairs retinal angiogenesis (Vandekeere et al., 2018). Fatty acid oxidation and de novo lipogenesis also regulates EC proliferation and retinal vascular sprouting (Schoors et al., 2015; Wei et al., 2011). VEGF stimulation enhances the gene expression of fatty acid binding protein 4 (Fabp4) in ECs and loss of Fabp4 in EC decreases proliferation (Elmasri et al., 2009).
Retinal neurons
Growing evidence has shown that retinal neurons control vessel growth (Fu et al., 2020). Photoreceptor dysfunction predicts vascular abnormalities in human ROP infants and rat OIR (Akula et al., 2007; Fulton et al., 2009). Photoreceptor glucose and fatty acid metabolism controls both physiological and pathological retinal angiogenesis (Fu et al., 2018a; Joyal et al., 2016). Photoreceptor c-FOS, a master inflammation regulator, modulates retinal neovascularization in mice (Sun et al., 2017). Moreover, retinal ganglion cells (RGCs) also control retinal vessel growth through G protein-coupled receptor-91 (GPR91) (Sapieha et al., 2008) and neuronal guidance cue semaphorin 3 A (SEMA3A) (Joyal et al., 2011), and capillary degeneration in mice induced with ischemia-reperfusion injury (Ueda et al., 2010; Zheng et al., 2007). Loss of retinal neuronal/glial suppressor of cytokine signaling 3 (SOCS3), which inhibits inflammation and VEGF signaling, exacerbates retinal angiogenesis in mouse OIR (Sun et al., 2015). Müller glia-derived VEGF is one of the primary driving forces of retinal neovascularization and leakage (Becker et al., 2018; Le, 2017; Wang et al., 2010). Stimulation of the G-protein-coupled receptor 81 (GPR81) in Müller glia with lactate induces angiogenic factor production and governs retinal vascularization (Madaan et al., 2019). Müller glial NETRIN-4, an axonal guidance molecule, increases VEGF release under hypoxic condition and NETRIN-4 stimulates proliferation in bovine retinal endothelial cells (Lange et al., 2012).
Microglia
Microglia are unique immune cells in the central nervous system necessary for homeostasis of the local microenvironment. Microglia control retinal vascular stability in normal and disease conditions (Arnold and Betsholtz, 2013; Davies et al., 2006). In mouse OIR, microglia are the predominant myeloid cells in neovascular tufts (Boeck et al., 2020). A study of the association between microglial status and retinal vessel growth in mouse OIR found that an increased number of microglia (mostly the activated amoeboid phenotype) in the superficial vascular layer correlates with increased superficial retinal vessels in OIR. However, an increase in microglia (mostly the quiescent ramified phenotype) in the deep vascular layer does not correlate with deep retinal vessels. Loss of microglia before and immediately after hyperoxia leads to more subsequent retinal neovascularization (Liu et al., 2022). These findings suggest a role for microglia in modulating retinal neovascularization.
In summary, in ROP eyes, there are shifts in glucose, lipid and amino acid metabolism, as well as disruptions in redox balance associated with cell death. Further studies regarding the long-term impact of metabolic imbalance on the neurovascular retina are needed. Metabolic crosstalk between different retinal cell types should be examined. RPE and Müller glia metabolically support photoreceptors. RPE and Müller glia have fuel preference that spare glucose for photoreceptors. Metabolic and molecular interaction among retinal organelles also need to be further examined to better understand their involvement in controlling retinal cell homeostasis.
Gut microbiota and retinal development
At birth, the neonatal gastrointestinal tract is a sterile environment but quickly becomes colonized by microbes, that is, fungi, bacteria, archaea, protozoa, and viruses. The mother exposes the fetus to IgG and microbial antigens prenatally, and supplies the child with antibodies and metabolites through breast milk postnatally to protect against invasive pathogens and promote a beneficial microbiome (Brodin, 2022). Factors that influence the colonization process include GA (Fouhy et al., 2019), cesarian delivery (Fouhy et al., 2019), feeding type (mother’s own milk/donor milk/formula/parenteral nutrition) (Dahlgren et al., 2019; Kumbhare et al., 2022; Parra-Llorca et al., 2018; Piñeiro-Ramos et al., 2021), and exposure to antibiotics (Arboleya et al., 2015; Gibson et al., 2016). It is now widely accepted that the gut microbiome plays crucial roles in maintaining health homeostasis, and if disturbed (dysbiosis), can contribute to the pathogenesis of several diseases, including retinal disease (Rinninella et al., 2018), which has led to the concept of a ‘gut-retina axis’. Recent evidence also suggests that an imbalance in the neonatal gut microbiome can affect the clinical course of ROP.
In a pilot study, Skondra et al. found that infants with type 1 ROP compared with controls with no ROP had enrichment of stool Enterobacteriaceae at 28 weeks’ PMA (Skondra et al., 2020). The change in the gut microbiome was accompanied by alterations in microbial metabolic pathways, including amino acid biosynthesis, which was suggested to be related to infant IGF-1 expression. Interestingly, using a mouse model to investigate the effect of the gut microbiome on retinal gene expression, the signaling pathways of IGF-1, VEGF, and HIF-1, among others, were affected by the presence of a functional microbiome (Zhang et al., 2022). Recently, Westway et al. found that infants diagnosed with ROP (stage 1 or greater) had a lower taxonomic microbial diversity than infants with no ROP at admission (Westaway et al., 2022). Furthermore, ROP was significantly associated with an enrichment of bacteria from the Gram-positive genus Staphylococcus.
Gut microbes interact and communicate with their host through an elaborate crosstalk involving metabolites and other signaling molecules. One such mechanism is through the release of short-chain fatty acids (SCFAs) produced by anaerobic fermentation of human milk oligosaccharides (HMOs) by certain intestinal bacteria. SCFAs from gut microbiota affect host inflammation and glucose and lipid metabolism and contribute to microglia maturation and function in mice (Erny et al., 2015). Levels of SCFAs in stool collected on day 14 and 28 from infants born <28 weeks’ GA were not associated with the risk of any level of ROP (Frazer et al., 2022). However, most of the gut SCFAs are effectively absorbed by colonocytes and used as energy or further transported to the systemic circulation. Thus, quantification of circulatory SFCAs in preterm infants at risk of ROP could shed further light on gut metabolic activity and host interaction in relation to disease development. We conclude that targeting the microbiome and the gut-retina axis through pre- and probiotics may be a new therapeutic avenue in the prevention of ROP.
Current treatments in practice or in clinical trial
Inhibiting neovascularization in Phase II ROP
Laser photocoagulation
Current clinical treatments to control neovascularization in Phase II ROP include laser photocoagulation and anti-VEGF therapy. Both therapies target the avascular retina to limit the production of factors induced by hypoxia and fuel insufficiency that cause pathological retinal neovascularization. Laser therapy ablates the more avascular peripheral retina but also causes permanent destruction of the peripheral retina (Clark and Mandal, 2008).
Anti-VEGF treatment
VEGF is a key growth factor controlling vascular and neuronal development. Increased levels of hypoxia-induced VEGF are a significant factor contributing to the neovascularization of Phase II ROP. Anti-VEGF therapy which quickly suppresses neovascularization avoids some of the adverse effects of laser therapy such as retinal scarring but also has its own adverse effects. Inhibition of VEGF using Bevacizumab, Ranibizumab, Conbercept and Aflibercept show efficacy in ROP (Table 2; Cheng et al., 2018; Mintz-Hittner et al., 2011; Stahl et al., 2018; Wallace et al., 2018). The BEAT-ROP trial found that intravitreal bevacizumab (0.625 mg or 50% of the adult dose) monotherapy is effective in preventing ROP progression (Mintz-Hittner et al., 2011). However, intravitreal injections of anti-VEGF drugs leak into the systemic circulation lasting up to 2 months after a single injection (Kong et al., 2015; Sato et al., 2012; Hartnett et al., 2022; Wu et al., 2015). The persistent anti-VEGF effect can suppress physiological vascular growth locally in the eye, but also systemically, creating safety concerns in the developing preterm infant. There may be increased neurological damage in preterm infants with the use of anti-VEGF drugs (Arima et al., 2020). Neural retinal damage persists even after the vascular pathology has resolved (Hansen et al., 2017). Efforts have been made to determine a minimal dose to maintain durable suppression of retinal neovascularization while avoiding suppression of plasma VEGF (Cheng et al., 2018; Stahl et al., 2018). Intravitreal Bevacizumab (as low as 0.031 mg) can result in good retinal structural outcomes. However, multiple treatments are needed in many eyes (Wallace et al., 2018). Intravitreal ranibizumab is effective in controlling acute ROP at low doses (0.12 mg and 0.2 mg). Superior vascularization of the peripheral retina is found with 0.12 mg of ranibizumab (Stahl et al., 2018). Intravitreal Conbercept at a low dose (0.15 mg) is effective for Zone II Stage 2/3+RIO and no adverse ocular outcomes were observed during the follow-up period until 90 weeks postmenstrual age (Cheng et al., 2018). A recent Phase 3 trial of intravitreal Aflibercept (0.4 mg) versus laser therapy showed that treatment success is similar between the two groups and less rescue treatment is required in the Aflibercept-treated group (Stahl et al., 2022). However, the drug persists in systemic circulation for at least 8 weeks post intravitreal injection.
Summary of current or potential treatments for Phase II ROP.
| Drug & dose | Sample size | Outcomes | References |
|---|---|---|---|
| Anti-VEGF (intravitreal) | |||
| Bevacizumab (0.625 mg) | 150 infants (BW <1500 g, GA <30 weeks) | Benefits zone I not zone II posterior stage 3+ROP | Mintz-Hittner et al, N Engl J Med. 2011 Feb 17;364(7):603–15. |
| Bevacizumab (0.25 mg, 0.125 mg, 0.063 mg, 0.031 mg) | 61 infants (mean BW = 709 g, mean GA = 24.9 weeks) | ROP regression by 6 months corrected age and very good retinal structure | Wallace et al, Ophthalmology. 2018 December; 125(12): 1961–1966. |
| Ranibizumab (0.12 mg and 0.2 mg) | 19 infants (mean GA = 36.4 weeks) | Required no rescue therapy; systemic VEGF levels not suppressed. | Stahl et al, JAMA Pediatr. 2018 Mar 1;172(3):278–286. |
| Conbercept (0.15 mg) | 20 infants (mean BW = 1297.5 g, mean GA = 28.6 weeks) | Complete regression of retinopathy and retinal vascularization to zone III | Cheng et al, Sci Rep. 2018 Jul 16;8 (1):10732. |
| Aflibercept (0.4 mg) | 118 infants (GA <32 weeks) | Rescue treatment required in 4.8% aflibercept group vs 11.1% with laser. Serious adverse event rates were similar. | Stahl et al, JAMA. 2022 Jul 26;328(4):348–359. |
| Dexamethasone | |||
| Eye drop (1 mg/ml, 1 drop daily) | 48 premature infants | Reduced laser ablation | Öhnell et al, Ophthalmol Retina. 2022 Feb;6 (2):181–182. |
| Antenatal systemic dexamethasone | 63 infants (mean BW = 981 g, mean GA = 27.8 weeks) | Decreased incidence of ROP of stage 2 or higher | Higgins et al, Arch Ophthalmol. 1998 May;116(5):601–5. |
| ≤1.8 mg/kg (low cumulative) or >1.8 mg/kg (high cumulative) body weight (via bolus intravenous infusion) | 115 infants (BW ≤1250 g, GA ≤32 weeks) | No association between dexamethasone and severe ROP incidence | Cuculich et al, Biol Neonate. 2001 Jan;79(1):9–14. |
| 0–0.9 mg/kg or 0–0.73 mg/kg (accumulative, systemic) | 74 infants (GA <28 weeks) | Higher dose was associated with severe ROP | Pediatr Neonatol. 2022 May;63(3):220–226 |
| Platelets | |||
| Low Platelets | 202 preterm infants (GA <34 weeks) | Less incidence of ROP with higher platelet count | Cakir et al, JCI Insight. 2018 Oct 4;3 (19):e99448 |
| Platelet transfusion | 136 infants (Mean GA = 25.3 weeks, mean BW = 782 g) | Less incidence of ROP | Faheem et al, Annals of R.S.C.B., 2021, 25 (6): 5442–5448 |
Steroid treatment for ROP
In addition to VEGF, inflammatory mediators such as tumor necrosis factor-alpha (TNFα) are also involved in the development and progression of ROP (Connor et al., 2007). Dexamethasone greatly decreases retinal Tnfα expression in mice with hypoxia-induced retinopathy (Yossuck et al., 2001). Pretreatment with dexamethasone (0.5 mg/kg subcutaneously) before a high oxygen challenge reduces retinal neovascularization in mouse OIR (Yossuck et al., 2000). An observational cohort study reported that antenatal dexamethasone administration in mothers seems to be associated with a decreased incidence of ROP (stage 2 or higher) in preterm infants with very-low birth weight and low gestational age (Higgins et al., 1998). A recent retrospective study examining premature infants who received topical dexamethasone eye drops before potential laser treatment found that fewer progressed to severe ROP requiring treatment. (Öhnell et al., 2022). Currently, a clinical trial titled “Pharmacokinetics and Safety of Dexamethasone Eye Drops in Preterm Infants” (ClinicalTrials.gov Identifier: NCT05387941) is investigating efficacy of topical dexamethasone treatment before florid neovascularization to prevent progression. An experimental study shows that systemic dexamethasone treatment (0.5 mg/kg subcutaneously) before but not after high oxygen exposure inhibits retinal neovascularization (Yossuck et al., 2000), suggesting that the timing of treatment is critical. The outcomes of dexamethasone treatment appear to be influenced by the dose and route of administration (Cuculich et al., 2001; Tao, 2022). Further optimization of the dose, route and timing of administration is needed.
Platelets and ROP
An experimental (and clinical) study found that platelet depletion increases and platelet transfusion decreases hypoxia-induced retinal neovascularization in OIR mice (Cakir et al., 2018). In the same study, retinal VEGF-A expression was found to be induced with platelet depletion and decreased with platelet transfusion and that clinically, platelet deficiency is associated with severe ROP (Cakir et al., 2018). Other studies have also shown this association (Jensen et al., 2018; Parrozzani et al., 2021; Şahinoğlu Keşkek et al., 2020; Seliniotaki et al., 2022). A hospital based prospective study found that platelet transfusion protects against ROP development (Faheem et al., 2021). A correlation was found between platelet count and serum VEGF-A, platelet-derived growth factor (PDGF-BB), and brain-derived neurotrophic factor (BDNF) from serum samples taken on the same day in preterm infants (Hellgren et al., 2021). Taken together, platelet transfusion may be a promising therapeutic approach for ROP prevention and treatment.
Preventing vessel loss in Phase I ROP
Oxygen control
Oxygen supplementation is commonly used to treat preterm infants with poor lung development to increase their survival. However, the correct balance between high oxygen supplementation to decrease mortality and lower oxygen to prevent Phase I ROP remains unknown (Askie et al., 2011). Several studies examined the risk of ROP and the survival rate with lower vs. higher SpO2 with target ranges of: (70–90% vs. 88–98%; Tin et al., 2001), (≤92% vs.>92%; Anderson et al., 2004), (83–90% vs. >90–98%; Chow et al., 2003). The Neonatal Oxygenation Prospective Meta-analysis (NeOProM) Collaboration with about 5000 preterm infants (GA <28 weeks) given oxygen supplementation during the entire postnatal period showed that there is increased mortality associated with a lower SpO2 target range (85–89% vs. 91–95%). There was no significance difference in the primary outcome disability including bilateral blindness reported (Askie et al., 2018). However, in this study, timing of different SpO2 ranges for Phase I and Phase II was not evaluated. A recent study comparing biphasic (85–92% when GA <34 weeks, 95% when GA ≥34 weeks) vs. static (91–95%) SpO2 showed that biphasic SpO2 decreases incidence and severity of ROP without increasing mortality (Shukla et al., 2019). This suggests that a biphasic approach of oxygen supplementation may optimize the balance between ROP and mortality.
IGF-1
A recent clinical trial of IGF-1 supplementation during early life (rhIGF-1/rhIGFBP-3, 250 mcg/kg/24 hours, continuous intravenous infusion from <24 hr of birth to postmenstrual age 29 weeks, ClinicalTrials.gov Identifier: NCT01096784) found decreased severe bronchopulmonary dysplasia and less severe intraventricular hemorrhage (Ley et al., 2019). Better understanding of the appropriate IGF-1 dose in ROP prevention could be achieved from the ongoing trial using SHP607 (recombinant protein complex of IGF-1/IGFBP3, continuous intravenous infusion of SHP607 250 mcg/kg/24 hr and 400 mcg/kg/24 hours from birth to postmenstrual age 29 weeks+6 days, ClinicalTrials.gov Identifier: NCT03253263).
Lipid supplementation
Supplementation of nutrients lacking after premature birth also protects against the incidence and severity of ROP. Both DHA and ARA are considered conditionally essential fatty acids for preterm infants and increasing evidence points to health benefits with their supplementation. Low blood DHA and ARA levels in premature infants correlate with ROP progression (Fu et al., 2015a; Lapillonne et al., 2010; Löfqvist et al., 2018). The Mega Donna Mega trial with enteral intake of DHA (50 mg/kg/day) and ARA (100 mg/kg/day) provided within 3 days after birth until postmenstrual age 40 weeks versus no supplementation increases circulating DHA and ARA levels and reduces severe ROP by 50% (Hellström et al., 2021a). The double-blind parallel clinical trial with enteral DHA supplementation at 75 mg/kg/day versus high oleic sunflower oil to preterm infants for 14 days lowers the incidence of stage 3 ROP (Bernabe-García et al., 2019). The DIAMOND (DHA Intake And Measurement Of Neural Development, ClinicalTrials.gov Identifier: NCT00753818) study show that supplementing DHA:ARA at 1:2 ratio in formula to healthy, term infants from the first two weeks of birth improves visual acuity at one year of age. However, further increasing DHA does not generate additional visual improvement (Birch et al., 2010). Meta-analysis of dietary DHA-supplemented formula vs. DHA-free formula to preterm infants improves visual acuity at 2 and 4 months of corrected age (SanGiovanni et al., 2000).
However, the results of DHA supplementation to prevent ROP in preterm infants are not always consistent and parenteral supplementation of lipids may differ from enteral administration (Nilsson et al., 2019). Intravenous (parenteral) fat emulsion containing fish oil versus soybean and olive oil reduces severe ROP requiring laser therapy in very-low-birth-weight infants (Beken et al., 2014; Pawlik et al., 2011; Pawlik et al., 2014). But in another study, a parenteral lipid emulsion containing fish oil (SMOFlipid) versus olive oil-based (Clinoleic) emulsion only marginally increases circulating DHA levels, reduces ARA levels and has no significant impact on the incidence and severity of ROP (Najm et al., 2017). The outcomes may have been affected by the different period of lipid emulsion delivery ranging from 2 to 28 days (Najm et al., 2017) and by reduced ARA levels, as low postnatal ARA levels strongly predict ROP development (Löfqvist et al., 2018). Further investigation of lipid components, route and time of supplementation, as well as the sex differences should be considered to optimize the nutrient supply for best clinical outcomes.
Tools for future retinal metabolic studies
Advanced technologies make it feasible to investigate detailed metabolic status in the eye. Single-cell transcriptomics and spatial transcriptome profiling are useful in preclinical retinal studies. Metabolomics, lipidomics, and proteomics of blood and retinas have been applied to clinical and experimental studies to detect metabolic biomarkers for ROP. The development and application of these ‘omics’ approaches at the single cell level (Li et al., 2021; Perkel, 2021; Seydel, 2021) should expand our understanding of cell-specific activity. Although Seahorse XF analysis measuring oxygen consumption rate and extracellular acidification rate in a closed and rapidly depleted system has been used for metabolic investigation in cell culture, in tissue samples this technique is restricted by the limited nutrient and oxygen supply which affects readout. A microfluidics flow system providing a continuous supply of nutrients and oxygen, to maintain tissue viability and functionality for a much longer period of time has added to our understanding of retinal metabolism (Bisbach et al., 2020; Rountree et al., 2016; Tsantilas et al., 2021). Organ-on-a-chip technology which can evaluate human tissue and can be used to assess physical and biochemical stimuli, may be a better system than conventional 3D cell culture systems in vitro.
Conclusion
In summary, our current knowledge of the impact of metabolic disruption such as lipid deficiency, starvation and hyperglycemia on the immature retina in preterm infants is limited. Improving retinal development at an early stage may help prevent ROP before progression to vision-threatening neovascularization. A better understanding of substrate use and metabolic shifts in the neonatal period will help determine how to promote retinal neuronal and vascular maturation by supplementing proper nutrients under hyperglycemic, hyperoxic, and hypoxic conditions. Metabolic modulation to normalize concentrations of naturally occurring growth factors like IGF-1, APN and FGF21 might also be more physiological interventions. Compared to pharmaceutical interventions, modulation and normalization of nutrient supplementation is relatively safe for fragile premature infants. Understanding the correlation between nutrient shortage after premature birth and retinal development will help find effective approaches for disease prevention at an early stage.
References
-
Mitochondria contribute to NADPH generation in mouse rod photoreceptorsThe Journal of Biological Chemistry 289:1519–1528.https://doi.org/10.1074/jbc.M113.511295
-
Rod photoreceptor function predicts blood vessel abnormality in retinopathy of prematurityInvestigative Ophthalmology & Visual Science 48:4351–4359.https://doi.org/10.1167/iovs.07-0204
-
Developmental anatomy of the retinal and choroidal vasculatureEncyclopedia of the Eye 48:8–9.https://doi.org/10.1016/B978-0-12-374203-2.00169-X
-
Retinopathy of prematurity and pulse oximetry: a national survey of recent practicesJournal of Perinatology 24:164–168.https://doi.org/10.1038/sj.jp.7211067
-
Intestinal microbiota development in preterm neonates and effect of perinatal antibioticsThe Journal of Pediatrics 166:538–544.https://doi.org/10.1016/j.jpeds.2014.09.041
-
Pro-Oxidants and antioxidants in retinopathy of prematurityActa Clinica Croatica 57:458–463.https://doi.org/10.20471/acc.2018.57.03.08
-
Early insulin therapy in very-low-birth-weight infantsThe New England Journal of Medicine 359:1873–1884.https://doi.org/10.1056/NEJMoa0803725
-
Role of thromboxane in retinal microvascular degeneration in oxygen-induced retinopathyJournal of Applied Physiology 90:2279–2288.https://doi.org/10.1152/jappl.2001.90.6.2279
-
Enteral docosahexaenoic acid and retinopathy of prematurity: a randomized clinical trialJPEN. Journal of Parenteral and Enteral Nutrition 43:874–882.https://doi.org/10.1002/jpen.1497
-
Insulin infusion with parenteral nutrition in extremely low birth weight infants with hyperglycemiaThe Journal of Pediatrics 114:273–280.https://doi.org/10.1016/s0022-3476(89)80797-8
-
The diamond (DHA intake and measurement of neural development) study: a double-masked, randomized controlled clinical trial of the maturation of infant visual acuity as a function of the dietary level of docosahexaenoic acidThe American Journal of Clinical Nutrition 91:848–859.https://doi.org/10.3945/ajcn.2009.28557
-
Reduced severity of oxygen-induced retinopathy in eNOS-deficient miceInvestigative Ophthalmology & Visual Science 42:222–228.
-
M-Type pyruvate kinase isoforms and lactate dehydrogenase A in the mammalian retina: metabolic implicationsInvestigative Ophthalmology & Visual Science 57:66–80.https://doi.org/10.1167/iovs.15-17962
-
The effect of oxygen on vasoformative cell division. Evidence that “ physiological hypoxia ” is the stimulus for normal retinal vasculogenesisInvestigative Ophthalmology & Visual Science 36:1201–1214.
-
Dysfunctional peroxisomal lipid metabolisms and their ocular manifestationsFrontiers in Cell and Developmental Biology 10:982564.https://doi.org/10.3389/fcell.2022.982564
-
Peroxisomal dysfunction in age-related diseasesTrends in Endocrinology and Metabolism 28:297–308.https://doi.org/10.1016/j.tem.2016.12.003
-
Treatment of retinopathy of prematurityEarly Human Development 84:95–99.https://doi.org/10.1016/j.earlhumdev.2007.11.007
-
Glucose catabolism of rabbit retina before and after development of visual functionJournal of Neurochemistry 5:253–276.https://doi.org/10.1111/j.1471-4159.1960.tb13363.x
-
Intraretinal oxygen tension in the rat eyeGraefe’s Archive for Clinical and Experimental Ophthalmology = Albrecht von Graefes Archiv Fur Klinische Und Experimentelle Ophthalmologie 229:574–577.https://doi.org/10.1007/BF00203324
-
Regulation of oxygen tension in the mammalian retina during systemic hyperoxia is species dependentAdvances in Experimental Medicine and Biology 1072:241–244.https://doi.org/10.1007/978-3-319-91287-5_38
-
Peroxisomal disorders and their mouse models point to essential roles of peroxisomes for retinal integrityInternational Journal of Molecular Sciences 22:4101.https://doi.org/10.3390/ijms22084101
-
Microglia and macrophages are increased in response to ischemia-induced retinopathy in the mouse retinaMolecular Vision 12:467–477.
-
The effect of arterial PO2 on relative retinal blood flow in monkeysInvestigative Ophthalmology 14:342–352.
-
Host microbiota constantly control maturation and function of microglia in the CNSNature Neuroscience 18:965–977.https://doi.org/10.1038/nn.4030
-
Effect of platelet transfusion on retinopathy of prematurity- hospital based prospective studyAnnals of the Romanian Society for Cell Biology 25:5442–5448.
-
Perinatal factors affect the gut microbiota up to four years after birthNature Communications 10:1517.https://doi.org/10.1038/s41467-019-09252-4
-
Role of peroxisomes in ROS/RNS-metabolism: implications for human diseaseBiochimica et Biophysica Acta 1822:1363–1373.https://doi.org/10.1016/j.bbadis.2011.12.001
-
Aldose reductase deficiency reduced vascular changes in neonatal mouse retina in oxygen-induced retinopathyInvestigative Ophthalmology & Visual Science 53:5698–5712.https://doi.org/10.1167/iovs.12-10122
-
Dietary ω-3 polyunsaturated fatty acids decrease retinal neovascularization by adipose-endoplasmic reticulum stress reduction to increase adiponectinThe American Journal of Clinical Nutrition 101:879–888.https://doi.org/10.3945/ajcn.114.099291
-
Deficiency of aldose reductase attenuates inner retinal neuronal changes in a mouse model of retinopathy of prematurityGraefe’s Archive for Clinical and Experimental Ophthalmology = Albrecht von Graefes Archiv Fur Klinische Und Experimentelle Ophthalmologie 253:1503–1513.https://doi.org/10.1007/s00417-015-3024-0
-
Photoreceptor glucose metabolism determines normal retinal vascular growthEMBO Molecular Medicine 10:76–90.https://doi.org/10.15252/emmm.201707966
-
Targeting neurovascular interaction in retinal disordersInternational Journal of Molecular Sciences 21:E1503.https://doi.org/10.3390/ijms21041503
-
Retinal degenerative and hypoxic ischemic diseaseDocumenta Ophthalmologica. Advances in Ophthalmology 118:55–61.https://doi.org/10.1007/s10633-008-9127-8
-
Serine and lipid metabolism in macular disease and peripheral neuropathyThe New England Journal of Medicine 381:1422–1433.https://doi.org/10.1056/NEJMoa1815111
-
Photoreceptors in a mouse model of leigh syndrome are capable of normal light-evoked signalingThe Journal of Biological Chemistry 294:12432–12443.https://doi.org/10.1074/jbc.RA119.007945
-
Blocking endothelial apoptosis revascularizes the retina in a model of ischemic retinopathyThe Journal of Clinical Investigation 130:4235–4251.https://doi.org/10.1172/JCI127668
-
Elevated FGF21 leads to attenuated postnatal linear growth in preterm infants through GH resistance in chondrocytesThe Journal of Clinical Endocrinology and Metabolism 99:E2198–E2206.https://doi.org/10.1210/jc.2014-1566
-
Hypoxia leads to na,K-atpase downregulation via ca(2+) release-activated ca(2+) channels and AMPK activationMolecular and Cellular Biology 31:3546–3556.https://doi.org/10.1128/MCB.05114-11
-
Enhancing retinal endothelial glycolysis by inhibiting UCP2 promotes physiologic retinal vascular development in a model of retinopathy of prematurityInvestigative Ophthalmology & Visual Science 60:1604–1613.https://doi.org/10.1167/iovs.19-26553
-
The neural retina in retinopathy of prematurityProgress in Retinal and Eye Research 56:32–57.https://doi.org/10.1016/j.preteyeres.2016.09.004
-
Nutrition, insulin-like growth factor-1 and retinopathy of prematuritySeminars in Fetal & Neonatal Medicine 18:136–142.https://doi.org/10.1016/j.siny.2013.01.006
-
Antenatal dexamethasone and decreased severity of retinopathy of prematurityArchives of Ophthalmology 116:601–605.https://doi.org/10.1001/archopht.116.5.601
-
Role of glutamine and interlinked asparagine metabolism in vessel formationThe EMBO Journal 36:2334–2352.https://doi.org/10.15252/embj.201695518
-
Retina metabolism and metabolism in the pigmented epithelium: a busy intersectionAnnual Review of Vision Science 7:665–692.https://doi.org/10.1146/annurev-vision-100419-115156
-
Causes and mechanisms of intrauterine hypoxia and its impact on the fetal cardiovascular system: a reviewInternational Journal of Pediatrics 2010:401323.https://doi.org/10.1155/2010/401323
-
Retinal energy demands control vascular supply of the retina in development and disease: the role of neuronal lipid and glucose metabolismProgress in Retinal and Eye Research 64:131–156.https://doi.org/10.1016/j.preteyeres.2017.11.002
-
Hyperglycemia, insulin and slower growth velocity may increase the risk of retinopathy of prematurityJournal of Perinatology 31:251–257.https://doi.org/10.1038/jp.2010.152
-
Regulation of glycolysis by the hypoxia-inducible factor (HIF): implications for cellular physiologyThe Journal of Physiology 599:23–37.https://doi.org/10.1113/JP280572
-
Oxygen-Induced retinopathy and choroidopathy: in vivo longitudinal observation of vascular changes using OCTAInvestigative Ophthalmology & Visual Science 59:3932–3942.https://doi.org/10.1167/iovs.18-24320
-
Retinal VEGFA maintains the ultrastructure and function of choriocapillaris by preserving the endothelial PLVAPBiochemical and Biophysical Research Communications 522:240–246.https://doi.org/10.1016/j.bbrc.2019.11.085
-
Pharmacokinetics of bevacizumab and its effects on serum VEGF and IGF-1 in infants with retinopathy of prematurityInvestigative Ophthalmology & Visual Science 56:956–961.https://doi.org/10.1167/iovs.14-15842
-
Antioxidant levels in cord blood of low birth weight newbornsIndian Pediatrics 45:583–585.
-
Insulin, hyperglycemia, and severe retinopathy of prematurity in extremely low-birth-weight infantsAmerican Journal of Perinatology 33:393–400.https://doi.org/10.1055/s-0035-1565999
-
RhIGF-1/rhigfbp-3 in preterm infants: a phase 2 randomized controlled trialThe Journal of Pediatrics 206:56–65.https://doi.org/10.1016/j.jpeds.2018.10.033
-
Igf-1 in retinopathy of prematurity, a CNS neurovascular diseaseEarly Human Development 102:13–19.https://doi.org/10.1016/j.earlhumdev.2016.09.008
-
Effects of light and darkness on oxygen distribution and consumption in the cat retinaThe Journal of General Physiology 88:521–542.https://doi.org/10.1085/jgp.88.4.521
-
Effects of hyperoxia on the oxygen distribution in the intact cat retinaInvestigative Ophthalmology & Visual Science 30:612–618.
-
Oxygen distribution and consumption in the cat retina during normoxia and hypoxemiaThe Journal of General Physiology 99:177–197.https://doi.org/10.1085/jgp.99.2.177
-
Retinal oxygen: from animals to humansProgress in Retinal and Eye Research 58:115–151.https://doi.org/10.1016/j.preteyeres.2017.01.003
-
Retinal microglia protect against vascular damage in a mouse model of retinopathy of prematurityFrontiers in Pharmacology 13:945130.https://doi.org/10.3389/fphar.2022.945130
-
The relationship of novel plasma proteins in the early neonatal period with retinopathy of prematurityInvestigative Ophthalmology & Visual Science 57:5076–5082.https://doi.org/10.1167/iovs.16-19653
-
Serum fibroblast growth factor 21 levels are inversely associated with growth rates in infancyHormone Research in Paediatrics 82:324–331.https://doi.org/10.1159/000367922
-
Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurityThe New England Journal of Medicine 364:603–615.https://doi.org/10.1056/NEJMoa1007374
-
A prospective study on hyperglycemia and retinopathy of prematurityJournal of Perinatology 34:453–457.https://doi.org/10.1038/jp.2014.49
-
Influence of human milk and parenteral lipid emulsions on serum fatty acid profiles in extremely preterm infantsJPEN. Journal of Parenteral and Enteral Nutrition 43:152–161.https://doi.org/10.1002/jpen.1172
-
Sphingolipidomics of serum in extremely preterm infants: association between low sphingosine-1-phosphate levels and severe retinopathy of prematurityBiochimica et Biophysica Acta. Molecular and Cell Biology of Lipids 1866:158939.https://doi.org/10.1016/j.bbalip.2021.158939
-
Visual follow-up in peroxisomal-disorder patients treated with docosahexaenoic acid ethyl esterInvestigative Ophthalmology & Visual Science 51:2277–2285.https://doi.org/10.1167/iovs.09-4020
-
Dexamethasone eye drops for the treatment of retinopathy of prematurityOphthalmology. Retina 6:181–182.https://doi.org/10.1016/j.oret.2021.09.002
-
Senescence-associated secretory phenotype contributes to pathological angiogenesis in retinopathyScience Translational Medicine 8:362ra144.https://doi.org/10.1126/scitranslmed.aaf9440
-
The estimation of selected parameters in antioxidant system in red blood cells in ROP screening of premature infantsKlinika Oczna 108:413–415.
-
Regulation of retinal oxygen metabolism in humans during graded hypoxiaAmerican Journal of Physiology. Heart and Circulatory Physiology 307:H1412–H1418.https://doi.org/10.1152/ajpheart.00479.2014
-
Preterm gut microbiome depending on feeding type: significance of donor human milkFrontiers in Microbiology 9:1376.https://doi.org/10.3389/fmicb.2018.01376
-
Fish-oil fat emulsion supplementation reduces the risk of retinopathy in very low birth weight infants: a prospective, randomized studyJPEN. Journal of Parenteral and Enteral Nutrition 38:711–716.https://doi.org/10.1177/0148607113499373
-
An imbalance in autophagy contributes to retinal damage in a rat model of oxygen-induced retinopathyJournal of Cellular and Molecular Medicine 25:10480–10493.https://doi.org/10.1111/jcmm.16977
-
Regulation of vascular endothelial growth factor by oxygen in a model of retinopathy of prematurityArchives of Ophthalmology 114:1219–1228.https://doi.org/10.1001/archopht.1996.01100140419009
-
Neonatal hyperglycemia and diminished long-term growth in very low birth weight preterm infantsJournal of Perinatology 33:882–886.https://doi.org/10.1038/jp.2013.77
-
Hyperglycemia in extremely preterm infantsNeoReviews 21:e89–e97.https://doi.org/10.1542/neo.21-2-e89
-
Characterisation of the macular dystrophy in patients with the A3243G mitochondrial DNA point mutation with fundus autofluorescenceThe British Journal of Ophthalmology 92:623–629.https://doi.org/10.1136/bjo.2007.131177
-
BookProtein biosynthesis and the hexose monophosphate shuntin the developing normal and dystrophic retinaIn: Graymore CN, editors. Biochemistry of the Retina. NY: Academic Press. pp. 73–90.
-
Proteomic profiling of a layered tissue reveals unique glycolytic specializations of photoreceptor cellsMolecular & Cellular Proteomics 10:M110.https://doi.org/10.1074/mcp.M110.002469
-
Laser doppler velocimetry study of the effect of pure oxygen breathing on retinal blood flowInvestigative Ophthalmology & Visual Science 24:47–51.
-
Retinal vascular development in premature infantsAmerican Journal of Ophthalmology 84:636–640.https://doi.org/10.1016/0002-9394(77)90377-4
-
Impact of platelet count in retinopathy of prematurityTurkish Journal of Ophthalmology 50:351–355.https://doi.org/10.4274/tjo.galenos.2020.01058
-
Circulating FGF19 and FGF21 surge in early infancy from infra- to supra-adult concentrationsInternational Journal of Obesity 39:742–746.https://doi.org/10.1038/ijo.2015.2
-
The succinate receptor GPR91 in neurons has a major role in retinal angiogenesisNature Medicine 14:1067–1076.https://doi.org/10.1038/nm.1873
-
Serum concentrations of bevacizumab (avastin) and vascular endothelial growth factor in infants with retinopathy of prematurityAmerican Journal of Ophthalmology 153:327–333.https://doi.org/10.1016/j.ajo.2011.07.005
-
Association of platelet deficiency with severe retinopathy of prematurity: a reviewActa Paediatrica 111:2056–2070.https://doi.org/10.1111/apa.16472
-
Inducible nitric oxide synthase mediates retinal apoptosis in ischemic proliferative retinopathyThe Journal of Neuroscience 22:3987–3993.https://doi.org/10.1523/JNEUROSCI.22-10-03987.2002
-
Single-Cell metabolomics hits its strideNature Methods 18:1452–1456.https://doi.org/10.1038/s41592-021-01333-x
-
Choroidal involution is a key component of oxygen-induced retinopathyInvestigative Ophthalmology & Visual Science 52:6238–6248.https://doi.org/10.1167/iovs.10-6742
-
Selective stimulation of VEGFR-1 prevents oxygen-induced retinal vascular degeneration in retinopathy of prematurityThe Journal of Clinical Investigation 112:50–57.https://doi.org/10.1172/JCI17808
-
Docosahexaenoic acid and the preterm infantMaternal Health, Neonatology and Perinatology 3:22.https://doi.org/10.1186/s40748-017-0061-1
-
Hypoxia and mitochondrial oxidative metabolismBiochimica et Biophysica Acta 1797:1171–1177.https://doi.org/10.1016/j.bbabio.2010.02.011
-
Endothelial-specific deficiency of ATG5 (autophagy protein 5) attenuates ischemia-related angiogenesisArteriosclerosis, Thrombosis, and Vascular Biology 39:1137–1148.https://doi.org/10.1161/ATVBAHA.119.309973
-
Inflammatory signals from photoreceptor modulate pathological retinal angiogenesis via c-fosThe Journal of Experimental Medicine 214:1753–1767.https://doi.org/10.1084/jem.20161645
-
Postnatal administration of systemic steroids increases severity of retinopathy in premature infantsPediatrics and Neonatology 63:220–226.https://doi.org/10.1016/j.pedneo.2021.09.005
-
Pulse oximetry, severe retinopathy, and outcome at one year in babies of less than 28 weeks gestationArchives of Disease in Childhood. Fetal and Neonatal Edition 84:F106–F110.https://doi.org/10.1136/fn.84.2.f106
-
An analysis of metabolic changes in the retina and retinal pigment epithelium of aging miceInvestigative Ophthalmology & Visual Science 62:20.https://doi.org/10.1167/iovs.62.14.20
-
Influence of duration of parenteral nutrition on retinopathy of prematurityArchives of Disease in Childhood. Fetal and Neonatal Edition 93:F170.https://doi.org/10.1136/adc.2007.128991
-
Oxygen saturation after birth in preterm infants treated with continuous positive airway pressure and air: assessment of gender differences and comparison with a published nomogramArchives of Disease in Childhood. Fetal and Neonatal Edition 98:F228–F232.https://doi.org/10.1136/archdischild-2012-302369
-
New insights into the role of autophagy in retinal and eye diseasesMolecular Aspects of Medicine 82:101038.https://doi.org/10.1016/j.mam.2021.101038
-
Glucose metabolism in pig outer retina in light and darknessActa Physiologica Scandinavica 160:75–81.https://doi.org/10.1046/j.1365-201X.1997.00030.x
-
Retinal oxygen: fundamental and clinical aspectsArchives of Ophthalmology 121:547–557.https://doi.org/10.1001/archopht.121.4.547
-
Nutrition for the micro preemie: beyond milkSeminars in Fetal & Neonatal Medicine 27:101344.https://doi.org/10.1016/j.siny.2022.101344
-
De novo lipogenesis maintains vascular homeostasis through endothelial nitric-oxide synthase (eNOS) palmitoylationThe Journal of Biological Chemistry 286:2933–2945.https://doi.org/10.1074/jbc.M110.193037
-
Hypoxia. 2. hypoxia regulates cellular metabolismAmerican Journal of Physiology. Cell Physiology 300:C385–C393.https://doi.org/10.1152/ajpcell.00485.2010
-
Endothelial PFKFB3 plays a critical role in angiogenesisArteriosclerosis, Thrombosis, and Vascular Biology 34:1231–1239.https://doi.org/10.1161/ATVBAHA.113.303041
-
Anti-Angiogenic effects of mammalian target of rapamycin inhibitors in a mouse model of oxygen-induced retinopathyBiological & Pharmaceutical Bulletin 37:1838–1842.https://doi.org/10.1248/bpb.b14-00487
-
Targeted blood metabolomic study on retinopathy of prematurityInvestigative Ophthalmology & Visual Science 61:12.https://doi.org/10.1167/iovs.61.2.12
-
Comparative analysis reveals novel changes in plasma metabolites and metabolomic networks of infants with retinopathy of prematurityInvestigative Ophthalmology & Visual Science 63:28.https://doi.org/10.1167/iovs.63.1.28
-
Dexamethasone and critical effect of timing on retinopathyInvestigative Ophthalmology & Visual Science 41:3095–3099.
-
Dexamethasone alters TNF-alpha expression in retinopathyMolecular Genetics and Metabolism 72:164–167.https://doi.org/10.1006/mgme.2000.3124
-
Inherited eye-related disorders due to mitochondrial dysfunctionHuman Molecular Genetics 26:R12–R20.https://doi.org/10.1093/hmg/ddx182
-
Suppression of retinal neovascularization by the iNOS inhibitor aminoguanidine in mice of oxygen-induced retinopathyGraefe’s Archive for Clinical and Experimental Ophthalmology = Albrecht von Graefes Archiv Fur Klinische Und Experimentelle Ophthalmologie 247:919–927.https://doi.org/10.1007/s00417-009-1066-x
-
Mtor-Mediated dedifferentiation of the retinal pigment epithelium initiates photoreceptor degeneration in miceThe Journal of Clinical Investigation 121:369–383.https://doi.org/10.1172/JCI44303
-
Retinal ischemia and reperfusion causes capillary degeneration: similarities to diabetesInvestigative Ophthalmology & Visual Science 48:361–367.https://doi.org/10.1167/iovs.06-0510
Article and author information
Author details
Funding
National Eye Institute (R01EY032492)
- Zhongjie Fu
National Eye Institute (R01EY017017)
- Zhongjie Fu
- Lois EH Smith
National Eye Institute (R01EY030904)
- Lois EH Smith
Boston Children's Hospital (1U54HD090255)
- Lois EH Smith
Massachusetts Lions Eye Research Fund (77426)
- Zhongjie Fu
Massachusetts Lions Eye Research Fund (73735)
- Lois EH Smith
Boston Children's Hospital
- Zhongjie Fu
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Acknowledgements
We sincerely thank Dr. James Hurley from University of Washington for his valuable suggestions on this review. The research was funded by NIH R01EY032492, R01EY017017, Boston Children’s Hospital (OFD/BTREC/CTREC Faculty Career Development Grant 97906, Pilot Grant 92214, and Ophthalmology Foundation 85010), Mass Lions Eye Foundation 77426 (ZF); NIH R01EY017017, R01EY030904, BCH IDDRC (1U54HD090255), Mass Lions Eye Foundation 73735 (LEHS); The Swedish Research Council (DNR# #2020–01092), Government grants under the ALF agreement ALFGBG-717971, The Wallenberg Clinical Scholars (AH).
Copyright
© 2022, Fu et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 1,433
- views
-
- 279
- downloads
-
- 22
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Citations by DOI
-
- 22
- citations for umbrella DOI https://doi.org/10.7554/eLife.80550






